Highlights
-
•
Rate of gastrostomy tube down trended during the study period.
-
•
Thousands of patients are still undergoing gastrostomy tube placement.
-
•
Gastrostomy tube is associated with longer hospital stay.
-
•
Gastrostomy tube is associated with a higher odds of discharge to nursing facility.
Keywords: Neurodegenerative diseases, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Gastrostomy tube, Palliative care
Abstract
Introduction
Dysphagia causing aspiration pneumonia is a common complication in the advanced stages of neurodegenerative disorders. Historically, physicians attempted to prevent this complication with gastrostomy tube (GT) placement. Its use is supported in amyotrophic lateral sclerosis (ALS), not supported in Alzheimer’s disease (AD), and without disease-specific guidelines in Parkinson’s disease (PD).
Method
The rate of GT placement in these three populations over two decades, from 1990 to 2010, was calculated using a binomial regression model with the data extracted using diagnosis and procedural codes from a national database. The median length-of-stay (LOS) and discharge destinations were compared.
Results
The rate of GT placement was 6.0% lower annually in AD, 3.4% in PD, and 0.2% in ALS (all p ≤ 0.007). The analysis of hospital LOS and discharge destination showed 3.2 to 5.5 days longer LOS with GT placement in all groups (all p ≤ 0.01), and three to four times lower odds of going home with GT placement in AD and PD groups (OR 0.28, 95% CI 0.14–0.55, and OR 0.22, CI 0.11–0.42 respectively), while unchanged in ALS group (OR 1.1, 95% CI 0.6–1.9).
Conclusion
Despite the downward trend of GT placement over two decades, thousands of AD and PD patients still underwent GT placement annually, and this was associated with longer LOS in all groups and increased likelihood of being discharged to a nursing facility in AD and PD. Further research is necessary to understand the effects of GT on physician practices and patient expectations in advanced AD and PD.
1. Introduction
Severe dysphagia leading to aspiration pneumonia is a common cause of death in advanced neurodegenerative disorders. Almost half of early Alzheimer’s Disease (AD) patients and nearly 90% of advanced AD patients exhibit dysphagia [1]. In Parkinson’s Disease (PD), rates of dysphagia range from 35% to 82% in cross-sectional studies, more prevalent in advanced stages [2]. In amyotrophic lateral sclerosis (ALS), about 80% of patients will have dysphagia at the end stage of disease [3].
In the past, it seemed logical to prevent aspiration pneumonia with gastrostomy tube (GT) placement in these populations. However, studies have shown no improvement in the risk of aspiration, no survival benefit, or nutritional benefit in older adults with dementia [4], [5], [6]. For example, scintigraphic studies have demonstrated the aspiration of gastric contents in patients with GT [7]. In contrast, in ALS, which is mostly a non-dementing neurodegenerative process, GT placement has been shown to prolong life for approximately 1.5 years [8], [9]. The data for the PD population is less clear, and no disease-specific guidelines exist.
In this study, we sought to assess the trend of GT placement among hospitalized patients with AD, PD, and ALS. In addition, we examined indirectly the burden of health care utilization associated with GT placement by analyzing the length of stay (LOS) and discharge destination.
2. Methods
We conducted a secondary analysis of a national administrative database, the National Hospital Discharge Survey (NHDS).
2.1. Data source
The NHDS is a publicly available, de-identified database of inpatient utilization records collected by the National Center for Health Statistics (NCHS). Data is collected annually from 542 hospitals selected by national probability sampling to represent 4 different population levels in 50 states and the District of Columbia [10], [11]. These hospitals are non-federal, short-stay (duration < 30 days) hospitals. Participating hospitals must have a minimum of 6 beds. NHDS was collected from 1965 to 2010. We extracted the International Classification of Diseases, 9th revision, Clinical Modification (ICD9-CM) codes, procedural codes, LOS, and discharge destination for 2 recent decades between 1990 and 2010 for this study. More recent data (i.e. after 2010) is excluded because of the increasing use of GT placement for the intestinal infusion of levodopa in PD patients [12]. The database provided sample weights, which were applied to all calculations to generate nationally representative estimates. The Institutional Review Board waived the necessity for board review because the database was de-identified, publicly available, and posed no risk to subjects.
2.2. Definitions and variables
AD, PD, and ALS cases were identified using ICD9-CM codes: 331.0, 332.0, and 335.20 respectively; these codes were validated in a previous study [13]. Other common causes of GT placement in this population, such as acute ischemic and hemorrhagic stroke cases were excluded, using ICD9-CM codes: 434.11, 434.91, 436, 430, and 431; these are also validated from previous studies [14], [15]. The annual incidence of GT placement in these patients was identified using ICD9-procedure codes: 43.11, 43.19, and 44.32 [16]. Current Procedure Terminology (CPT) codes including 432.46, 436.53, 437.50, 438.30, 438.32, 443.72, 443.73, and 743.50 were not used, as these did not generate any data. The mean age and sex of each diagnosis group with GT placement were compared. For the LOS, less than 1 day was counted as 0.5 days. Discharge destination included home, short-term facility, long-term facility, and others: left against medical advice, alive but disposition not stated, dead, or not reported.
2.3. Analysis
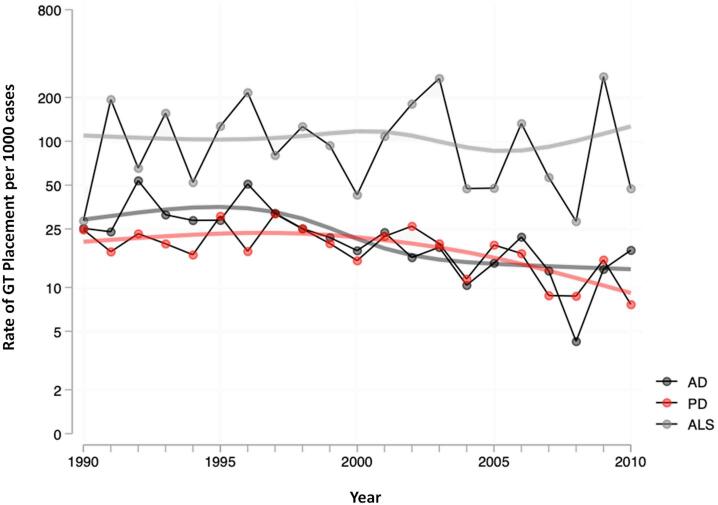
Rates of annual GT placement in AD, PD, and ALS were calculated as the number of GT placements per thousand cases per year. The results were plotted on the log-binomial regression model to evaluate trends. According to the Bayesian information criterion, the spline model was a better characterization of the trend, compared to the linear model (Fig. 1) Additionally, years were aggregated into 5-year intervals to examine overall trends in placement across disease groups. Analysis of LOS and discharge destination was conducted using data from the most recent 5 years. LOS (in days) and discharge destination were compared between patients with- versus without-GT placement in these disease groups. All results were age and sex-adjusted.
Fig. 1.
Yearly trends in gastrostomy (GT) placement. Rate of GT placement in each diagnosis group are shown in log-binomial regression model. Smoothed lines reflect a restricted cubic spline fit to year.
3. Results
3.1. General characteristics
A total of 78,562 discharges were identified using the codes and applying the exclusion criteria. Age and gender characteristics are shown in Table 1. Cases for AD were 44,078, PD were 33,168, and ALS were 1316. Mean age (standard deviation, SD), in years, of AD, PD, and ALS patients were 82.0 (8.0), 77.2 (9.3), and 65.2 (14.1), and males were 32.9%, 52.4%, and 56.5% respectively.
Table 1.
Patient characteristics, rates, and outcomes of GT placement.
| AD | PD | ALS | |
|---|---|---|---|
| General Characteristics | |||
| Number of cases | 44,078 | 33,168 | 1316 |
| Age (SD) | 82.0 (8.0) | 77.2 (9.3) | 65.2 (14.1) |
| Male (%) | 32.9 | 52.4 | 56.5 |
| Five-year aggregated rates of GT placement per 1000 cases | |||
| 1990–1994 | 32.2 | 19.7 | 114 |
| 1995–1999 | 31.2 | 24.6 | 124 |
| 2000–2004 | 17.5 | 18.5 | 128.3 |
| 2005–2009 | 13.7 | 13.8 | 111.7 |
| Length of Hospital Stay, Median number of days (IQR) | |||
| without GT | 4 (3.6) | 4 (3.8) | 4.6 (3.8) |
| with GT | 7.4 (7.0) | 9.5 (12.6) | 7.8 (19.7) |
| Discharge destination (%) | |||
| Without GT | |||
| Home | 39.0 | 44.4 | 61.4 |
| Short-term facility | 6.2 | 6.7 | 0.9 |
| Long-term facility | 42.2 | 34.4 | 13.8 |
| Other | 12.2 | 13.7 | 23.6 |
| With GT | |||
| Home | 14.1 | 16 | 63.7 |
| Short-term facility | 12.3 | 11.1 | 0 |
| Long-term facility | 55 | 73.9 | 27.7 |
| Other | 11.8 | 5.2 | 9.6 |
3.2. Trends
From the regression model of the analyzed cohort, the rate of GT placement in AD and PD patients decreased each year compared to the previous year; 6.0% per year in AD patients (p < 0.001), 3.4% per year in PD patients (p < 0.001) (Fig. 1). The rate in ALS patients also decreased but at a much lesser degree, 0.2% per year (p = 0.007). This was also demonstrated with 5-year aggregate data (Table 1). In AD, the rate dropped from 32.2 to 13.7 per thousand cases when comparing 1990–1994 aggregate to 2005–2009 aggregate (p < 0.001). Similarly, in PD, the rate dropped from 19.2 to 13.8 per thousand cases when comparing 1990–1994 aggregate to 2005–2009 aggregate (p < 0.001). In contrast, for ALS, the rate dropped only non-significantly from 114.0 to 111.7 per thousand cases when comparing the same 5-year aggregate data (p = 0.49).
3.3. Length of stay
Median LOS was higher in all groups with GT placement (Table 1). The median LOS for patients without versus with GT placement, respectively, was 4 versus 7.4 days in AD (Wilcoxon rank-sum test, p < 0.001), 4 versus 9.5 days in PD (p < 0.001), and 4.6 versus 7.8 days in ALS (p = 0.01)
3.4. Discharge destination
Without GT placement, 39.0% of AD patients, 44.4% of PD patients, and 61.4% of ALS patients were discharged home (Table 1). Among AD and PD patients, the odds of being discharged to home were three to four times lower with GT placement (OR 0.28, 95% CI 0.14–0.55 in AD, and OR 0.22, CI 0.11–0.42 in PD, all p values <0.001). In contrast, in ALS patients, the odds of being discharged to home remained largely unchanged regardless of GT placement (OR 1.1, 95% CI 0.6–1.9).
4. Discussion
We found a decreasing rate of GT placement in AD and PD patients and a relatively unchanged rate in ALS patients over two-decades. Despite the downward trend in the rate of GT placement in AD and PD population, it is noteworthy that even in the later years of the study period, thousands of patients were still undergoing GT placement annually, despite lack of evidence for any benefit of GT in these populations.
The level of evidence and guidelines differ regarding GT placement for these conditions. Most well-studied is the dementia population, in which published reports demonstrate no improvement in risk of aspiration, survival, or nutritional status with GT placement [4], [5], [6]. Therefore, the American Geriatric Society (AGS) has recommended against GT placement in advanced dementia [17]. In contrast, in ALS, GT placement has been shown to prolong life, [8], [9] guiding current practice. While there are no controlled trials, observational data in PD suggest no survival benefit [18], [19]. As the vast majority of advanced PD patients develop dementia, they may be treated under this general guideline (i.e. against GT placement).
The analyses also showed that GT placement was associated with a longer hospital stay in all disease groups, and the longest in the PD group. A potential explanation is that PD patients are prone to rapid deconditioning due to decreased ambulation and overall immobility while in the hospital. Furthermore, peri-procedural ‘nothing by mouth’ (NPO) state may lead to missed doses of PD medications, which have a visible effect on the patient’s clinical condition. Increased LOS among AD and ALS patients can be partially explained by the common practice of 48–72 h of inpatient observation after GT placement, although this can vary depending on institutional policy, physician practices, or both. The longer hospital stay after GT placement may also be due to diligent monitoring of post-procedural complications, which can include gastric perforation, perontinitis, and death [4].
Another factor in healthcare utilization is the increased use of long-term facilities after being discharged with GT. Since dysphagia is generally a sign of advanced disease, once discharged to these facilities, patients will likely remain there. The short- and long-term financial ramifications of GT placement in AD and PD are unknown, but expectedly, extend beyond the hospitalization, particularly when accounting for long term nursing care. Our data showed that AD and PD patients were three to four times less likely to be discharged home with GT placement than without. This is different from the ALS patients, in whom the odds of being discharged to home did not change regardless of GT placement. This might be due to ALS patients having a much lower incidence of dementia, potentially making the careless challenging at home. Since the results were adjusted for age and sex, these factors did not account for this difference. However, it is worth mentioning that AD and PD patients were significantly older than ALS patients, making ALS patients easier to care for at home. In addition, ALS patients who undergo invasive procedures were previously shown to have more financial support than those who do not, allowing them to set up care at home [20]. This difference in outcome can also be due to the long-established, widely available multidisciplinary ALS clinics, which provide education to patients and caregivers about management options and prognosis, perhaps including early and effective goals of care planning.
There are limitations in our study worth mentioning. We conducted a retrospective, secondary analysis of an administrative database, which does not provide clinical details required to verify the certainty of diagnosis. Specifically, ICD9-CM code for PD may not be specific to idiopathic PD and sometimes may include other parkinsonian syndromes. However, we tried to account for this by excluding codes for atypical parkinsonian disorders, including dementia with Lewy Body (331.82) and other diseases of basal ganglia (progressive supranuclear palsy, corticobasal syndrome) (333.0). Also, previous studies have successfully used this database to study populations with similar neurological disorders [13], [21], [22], and the predictive value is acceptable. Furthermore, the database lacked details about the severity of PD motor symptoms, dementia, dysphagia, and other clinical factors. We were also unable to determine whether patients were on dopaminergic therapy and if GT was placed for medication administration. This, in our experience, is a rare occurrence. Given the retrospective nature of this study, causality cannot be established. Our study examined ‘all-comers’ with a given disorder, irrespective of disease severity. The AD and PD populations were older than the ALS patients, but the sex- and age-adjusted analyses did not show that age affected the outcome. Compared to AD and PD, the number of ALS cases were expectedly significantly fewer, so the data should be interpreted with caution. For ALS cases, we also could not identify subtypes of ALS, so it is unclear how patients with bulbar onset ALS differed from the collective ALS cohort. The major strength of the study is that we used a large database with millions of patients to examine yearly trends without the bias of socioeconomic or demographic factors. It is possible that patients in these disease groups could have had GT placement for another indication, but this was addressed by the exclusion of common indications for GT – such as stroke – in this age population.
We believe that appropriate expectation and discussion between physicians, patients/caregivers addressing the goals of care may help inform the post-GT trajectory, and potentially decrease the likelihood of unnecessary or undesired nursing home placement. Studies in oncologic populations have shown that earlier goals of care discussion and palliative care consultation were associated with improved quality of life, symptom management, and reduced health care utilization cost [23], [24]. However, such discussions often do not occur because of physicians’ reluctance to address this issue [25]. Misconceptions about the perceived benefits of GT placement are common and may contribute to the continued use of GT in advanced neurodegenerative diseases. For example, a survey examining GT-related perceptions of 173 physicians found that the most common physician expectations from GT placement were nutritional benefit (93%), medication administration (58%), prolonging life (49%), and preventing aspiration pneumonia (44%) [26].
In 2014, AGS made the recommendation against GT placement in patients with advanced dementia [17]. Therefore, physicians, patients, and caregivers should consider early discussions regarding goals of care, and mutually make informed decisions that impact them directly, and the society indirectly. Further studies should explore patient and caregiver awareness about risks of GT placement, and physicians’ knowledge and practices.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
CRediT authorship contribution statement
Duk soo Kim: Conceptualization, Methodology, Writing - original draft. Richard N. Jones: Formal analysis. Theresa I. Shireman: Supervision, Writing - review & editing. Benzi M. Kluger: Supervision, Writing - review & editing. Joseph H. Friedman: Supervision, Writing - review & editing. Umer Akbar: Conceptualization, Methodology, Supervision, Writing - review & editing.
References
- 1.Seçil Y., Arıcı Ş., İncesu T.K., Gürgör N., Beckmann Y., Ertekin C. Dysphagie dans la maladie d’Alzheimer. Neurophysiol. Clin. 2016;46:171–178. doi: 10.1016/j.neucli.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Kalf J.G., de Swart B.J.M., Bloem B.R., Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Park. Relat. Disord. 2012;18:311–315. doi: 10.1016/j.parkreldis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Onesti E., Schettino I., Gori M.C., Frasca V., Ceccanti M., Cambieri C., Ruoppolo G., Inghilleri M. Dysphagia in amyotrophic lateral sclerosis: impact on patient behavior, diet adaptation, and riluzole management. Front. Neurol. 2017;8:94. doi: 10.3389/fneur.2017.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finucane T.E., Christmas C., Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA. 1999;282:1365–1370. doi: 10.1001/jama.282.14.1365. [DOI] [PubMed] [Google Scholar]
- 5.Sampson E.L., Candy B., Jones L. Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst. Rev. 2009 doi: 10.1002/14651858.CD007209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg L.S., Altman K.W. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin. Interv. Aging. 2014;9:1733–1739. doi: 10.2147/CIA.S53153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole M.J., Smith J.T., Molnar C., Shaffer E.A. Aspiration After percutaneous gastrostomy: assessment by Tc-99m labeling of the enteral feed. J. Clin. Gastroenterol. 1987;9:90–95. [PubMed] [Google Scholar]
- 8.Burkhardt C., Neuwirth C., Sommacal A., Andersen P.M., Weber M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS One. 2017;12:1–12. doi: 10.1371/journal.pone.0177555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui F., Sun L., Xiong J., Li J., Zhao Y., Huang X. Therapeutic effects of percutaneous endoscopic gastrostomy on survival in patients with amyotrophic lateral sclerosis: a meta-analysis. PLoS One. 2018;13:1–13. doi: 10.1371/journal.pone.0192243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.C. Dennison, R. Pokras, Design and operation of the National Hospital Discharge Survey: 1988 redesign, 2000. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=11261241. [PubMed]
- 11.N. Center For Health Statistics, National Hospital Discharge Survey 2009 Public Use Data File Documentation, (2009). http://www.cdc.gov/nchs/nhds.htm.
- 12.Olanow C.W., Kieburtz K., Odin P., Espay A.J., Standaert D.G., Fernandez H.H., Vanagunas A., Othman A.A., Widnell K.L., Robieson W.Z., Pritchett Y., Chatamra K., Benesh J., Lenz R.A., Antonini A. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13:141–149. doi: 10.1016/S1474-4422(13)70293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akbar U., Dham B., He Y., Hack N., Wu S., Troche M., Tighe P., Nelson E., Friedman J.H., Okun M.S. Incidence and mortality trends of aspiration pneumonia in Parkinson's disease in the United States, 1979–2010. Park. Relat. Disord. 2015;21:1082–1086. doi: 10.1016/j.parkreldis.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein L.B. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 15.McCormick N., Bhole V., Lacaille D., Avina-Zubieta J.A. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teno J.M., Mitchell S.L., Gozalo P.L., Dosa D., Hsu A., Intrator O., Mor V. Hospital characteristics associated with feeding tube placement in nursing home residents with advanced cognitive impairment. JAMA. 2010;303:544–550. doi: 10.1001/jama.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Geriatrics Society feeding tubes in advanced dementia position statement, J. Am. Geriatr. Soc. 62 (2014) 1590–1593. https://doi.org/10.1111/jgs.12924. [DOI] [PubMed]
- 18.Yamazaki Y., Kobatake K., Hara M., Katagiri M., Matsumoto M. Nutritional support by “conventional” percutaneous endoscopic gastrostomy feeding may not result in weight gain in Parkinson’s disease. J. Neurol. 2011;258:1561–1563. doi: 10.1007/s00415-011-5971-7. [DOI] [PubMed] [Google Scholar]
- 19.Marois C., Amador M.D.M., Payan C., Lacomblez L., Bonnet A.-M., Degos B., Corvol J.-C., Vidailhet M., Le Forestier N., Mesnage V., Grabli D. Outcome of gastrostomy in parkinsonism: a retrospective study. Parkinsonism Relat. Disord. 2017;43:110–113. doi: 10.1016/j.parkreldis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Andersen P.M., Kuzma M., Keller J., Aho H.E.A., Ciecwierska K., Szejko N., Vázquez C., Böhm S., Badura G., Thomas L., Petri S., Linse K., Hermann A., Semb O., Stenberg E., Nackberg S., Dorst J., Uttner I., Cristin A., Albert H., Dorothée C.L. Therapeutic decisions in ALS patients : cross - cultural differences and clinical implications. J. Neurol. 2018;265:1600–1606. doi: 10.1007/s00415-018-8861-4. [DOI] [PubMed] [Google Scholar]
- 21.Altman K.W., Yu G.-P., Schaefer S.D. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch. Otolaryngol. Head Neck Surg. 2010;136:784. doi: 10.1001/archoto.2010.129. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald P.W., Stern M.E., Rosen T., Clark S., Flomenbaum N. Trends in short-stay hospitalizations for older adults from 1990 to 2010: implications for geriatric emergency care. Am. J. Emerg. Med. 2014;32:311–314. doi: 10.1016/j.ajem.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Temel J.S., Greer J.A., Muzikansky A., Gallagher E.R., Admane S., Jackson V.A., Dahlin C.M., Blinderman C.D., Jacobsen J., Pirl W.F., Billings J.A., Lynch T.J. Early palliative care for patients with metastatic non–small-cell lung cancer. N. Engl. J. Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 24.Scibetta C., Kerr K., Mcguire J., Rabow M.W. The costs of waiting: implications of the timing of palliative care consultation among a cohort of decedents at a Comprehensive Cancer Center. J. Palliat. Med. 2016;19(1):69–75. doi: 10.1089/jpm.2015.0119. [DOI] [PubMed] [Google Scholar]
- 25.Jones L., Harrington J., Barlow C.A., Tookman A., Drake R., Barnes K., King M. Advance care planning in advanced cancer: Can it be achieved? An exploratory randomized patient preference trial of a care planning discussion. Pall. Supp. Care. 2011;9:3–13. doi: 10.1017/S1478951510000490. [DOI] [PubMed] [Google Scholar]
- 26.Hanson L.C., Garrett J.M., Lewis C., Phifer N., Jackman A., Carey T.S. Physicians' expectations of benefit from tube feeding. J. Palliat. Med. 2008;11(8):1130–1134. doi: 10.1089/jpm.2008.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]