Abstract
Purpose
Mutations in the fibroblast growth factor (FGF) receptor can result in strabismus, but little is known about how FGFs affect extraocular muscle structure and function. These were assessed after short-term and long-term exposure to exogenously applied FGF2 to determine the effect of enhanced signaling.
Methods
One superior rectus muscle of adult rabbits received either a series of three injections of 500 ng, 1 µg, or 5 µg FGF2 and examined after 1 week, or received sustained treatment with FGF2 and examined after 1, 2, or 3 months. Muscles were assessed for alterations in force generation, myofiber size, and satellite cell number after each treatment.
Results
One week after the 5 µg FGF2 injections, treated muscles showed significantly increased force generation compared with naïve controls, which correlated with increased myofiber cross-sectional areas and Pax7-positive satellite cells. In contrast, 3 months of sustained FGF2 treatment resulted in decreased force generation, which correlated with decreased myofiber size and decreased satellite cells compared with naïve control and the untreated contralateral side.
Conclusions
FGF2 had distinctly different effects when short-term and long-term treatments were compared. The decreased size and ability to generate force correlated with decreased myofiber areas seen in individuals with Apert syndrome, where there is sustained activation of FGF signaling. Knowing more about signaling pathways critical for extraocular muscle function, development, and disease will pave the way for improved treatment options for strabismus patients with FGF abnormalities in craniofacial disease, which also may be applicable to other strabismus patients.
Keywords: extraocular muscles, fibroblast growth factor, myogenic growth factors, strabismus
Strabismus is a common and serious eye disorder, affecting about 2% to 6% of the population worldwide.1–3 Although some of these patients can be treated with prescription glasses and/or patching, many of these patients require strabismus surgery for definitive treatment of their eye misalignment via surgical manipulation of the extraocular muscles. The success of strabismus surgery can be highly variable, with failure rates as high as 50% to 80% in long-term studies.4–8 There are a few limited alternatives for strabismus treatment in addition to surgery. Botulinum toxin and, more recently, bupivacaine are pharmacologic agents that are injected into extraocular muscles, to either weaken or strengthen over or underacting extraocular muscles.9,10 However, studies have not shown that these agents are more successful than strabismus surgery, unless used in conjunction with strabismus surgery.11
There is a need to discover effective therapies for strabismus. Neurotrophic growth factors are pharmacologic agents that have been studied as a potential treatment for strabismus. Mostly in research settings, neurotrophic growth factors, such as insulin-like growth factor I and II (IGFI, IGFII), brain-derived neurotrophic factor, and glial cell-line derived neurotrophic factor (GDNF), have been studied as a possible treatment for strabismus with the potential for more effective weakening or strengthening for overacting or underacting extraocular muscles. Because neurotrophic factor levels were decreased in strabismic extraocular muscle samples from human subjects compared with age-matched controls, the reintroduction of these factors has been proposed as a possible treatment.12,13 These neurotrophic factors have varying effects on extraocular muscle contraction properties, force generation, and relaxation rates, as well as extraocular muscle cross-sectional areas.14–19 Sustained local administration of IGFI on extraocular muscle partially reversed eye misalignment in a non-human primate model of strabismus.20 It is possible that the abrupt changes in the visual world and as well as the direct changes to muscle tension resulting from strabismus surgery or toxin administration trigger compensatory mechanisms that drive the oculomotor system back to presurgical ocular misalignment. We propose that the sustained local release of growth factors on extraocular muscle may allow the oculomotor system sufficient time to overcome the mechanisms that essentially push the system back to the “pretreatment” condition21,22 and would also allow for slow adaptation of the visual and ocular motor systems to changes in eye alignment to result in increased treatment success. The mechanisms of neurotrophic growth factor effects on extraocular muscle contraction and histology and strabismus are still not well-understood. However, because these neurotrophic factors are normally retrogradely and/or anterogradely transported within the axons of their innervating cranial motor neurons,23–26 it is likely that they not only have a direct muscle effect, but are likely to alter the physiologic properties of the motor neurons as well.
Fibroblast growth factor (FGF) is a promising candidate to treat at least some forms of strabismus. FGFs, especially FGF2 (basic FGF), have been studied extensively in limb skeletal muscle for their essential role in muscle precursor cell renewal, muscle repair, regeneration, and reinnervation, as well as muscle maintenance.27–30 FGF2 is normally transported anterogradely by motor axons, and this transport is upregulated after motor nerve injury.23 FGF signaling mediates muscle satellite cell activation and proliferation during development and in response to injury in limb skeletal muscle.31–36 The role of FGF signaling in the extraocular muscle is unclear, although one study showed that zebrafish extraocular muscle regeneration was impaired by blocking FGF signaling after injury.37 Studies in another craniofacial muscle, the laryngeal muscle thyroarytenoid, showed a significant regenerative response to FGF2 when injected locally after denervation.38,39 This finding suggests that FGF2 signaling, through its exogenous addition into the extraocular muscles, would have the potential to modify muscle force generation, possibly through precursor cell proliferation and alteration in muscle size, similar to the role of FGF2 in limb skeletal muscle.
The premise of this study was to analyze the effects of FGF2 on extraocular muscle force generation and morphology and to investigate possible mechanisms of action by studying the effects of FGF signaling on extraocular muscle precursor cells.
Methods
Adult New Zealand white rabbits used for these studies were purchased from Bakkam Rabbitry (Viroqua, WI). The rabbits were singly housed under the care of Resource Animal Resources in the AALAC-approved facility at the University of Minnesota. All studies were approved by the Institutional Animal Care and Use Committee. All experiments followed animal use guidelines of the National Institutes of Health and ARVO.
All surgery and muscle injections were performed after ketamine:xylazine anesthesia (10 mg/kg:2 mg/kg intramuscularly) under sterile conditions. One cohort of rabbits received injections of selected doses of FGF2 in a randomly selected superior rectus muscle, with the contralateral side receiving an injection of a similar volume of saline only. FGF2 (Peprotech, Inc., Rocky Hill, NJ) was prepared as a solution in 100 µL sterile isotonic saline at the following doses: 500 ng (3 rabbits), 1 µg (5 rabbits), and 5 µg (5 rabbits). Using sterile technique, a small incision was made through the conjunctiva anterior to the location of the superior rectus muscle. The superior rectus muscle was exposed, and the superior oblique muscle was cut and allowed to retreat into the orbit. Using a 30G needle, the saline or FGF2 plus saline was slowly injected into the body of the superior rectus muscle. The needle was left in place for 30 seconds to minimize leakage. The conjunctiva was closed, and a tobramycin/dexamethasone ointment (Tobradex, Alcon, Forth Worth, TX) was placed in the conjunctiva cul-de-sac to minimize inflammation. Each animal received a sequential series of injections over the course of 3 days, and all were examined 1 week after the first injection. An additional five rabbits received a sequential series of injections of 5 µg FGF2 once per day over the course of 3 days, and were examined 2 weeks after the first injection.
A second cohort of rabbits was treated with a sustained release pellet containing FGF2 (Peprotech, Inc.) to release 2 µg/day over a period of 90 days. The pellets were custom-made by Innovative Research of America (Sarasota, FL) and came with verification of their release profile. Four rabbits received unilateral placebo pellets to assess whether the surgical placement of the pellets was sufficient to alter muscle force development. The rabbits were anesthetized with ketamine:xylazine, as above. One FGF2-containing pellet was placed between the sclera and the superior rectus muscle on a randomly selected side, and the contralateral side received a placebo pellet made of just the carrier material but no FGF2. The pellets are quite sticky and do not move from the original implantation site.17,40,41 The conjunctiva was closed and tobramycin/dexamethasone ointment placed in the conjunctival cul-de-sac. One set of control rabbits (n = 4) received placebo pellets on both the right and left superior rectus muscles, and force generation was examined similar to the FGF2-treated muscles.
At the end of 1 week for the injected muscles and after 1, 2, or 3 months for the pellet-implanted muscles, the rabbits were deeply anesthetized, and both superior rectus muscles were dissected out from the sclera to as far back in the apex as possible. In addition, six surgically naïve control superior rectus muscles were examined for force production in a similar manner. Muscle force was assessed in these muscles using our standard in vitro procedure using the Aurora Scientific Isolated Muscle System (Aurora, Ontario, Canada).16 In brief, the superior rectus muscles were attached to a lever arm using a suture, which in turn is connected to a calibrated force transducer. During the programmed stimulation protocol, the muscles were immersed in a double-walled glass tissue bath, which contained oxygenated Ringer's solution maintained at 30 °C with a circulating water bath. In brief, the muscles were stimulated using two flanking electrodes. Isometric length–tension curves were produced after stimulation at supramaximal intensity, and the resting length was varied over a range of 0.5 to 10.0 g. Once the optimal length–tension curves were generated, to ensure that a maximal isometric twitch force was generated, the actual testing was performed at this calculated optimal preload. Two minutes of rest were provided between each stimulus. Force generation was measured at single pulse (twitch) (0.5 second pulse duration), followed by 10, 20, 40, 100, 150, and 200 Hz stimulations with a 500 ms train duration. Forces measured in grams were converted to mN/cm2 by dividing muscle mass (g) by the product of muscle length (cm) times a muscle density of 1.056 g/cm3.16 These values were averaged for each stimulation frequency for an average of six rabbits per treatment type for both the treated muscles and for the superior rectus muscles on the contralateral side. All analyses were performed masked relative to the treatment until data were graphed.
All the superior rectus muscles were subsequently embedded in tragacanth gum and frozen using 2-methylbutane chilled in liquid nitrogen. All blocks were stored at −80 °C until sectioned at 12 µm using a Leica cryostat. Slides were air-dried and stored at −30 °C. One set of slides was stained with hematoxylin and eosin using standard protocols and was used for measurement of the cross-sectional areas. A second set of slides was stained for the presence of either Pax7. For immunostaining of Pax7, slides were rinsed in 0.01 M PBS followed by an incubation in 3% hydrogen peroxide in 0.1 M phosphate buffer to remove endogenous peroxidase. The slides were then rinsed in PBS and blocked in 10% normal horse serum in antibody body consisting of 0.01 M PBS containing 0.1% Triton X-100. Without rinsing, the sections were blocked for avidin/biotin nonspecific binding using the Vector kit (SP-2001; Vector Laboratories, Burlingame, CA) following the package directions. After a PBS rinse, the slides were incubated with an antibody to Pax7 (1:50 in antibody buffer; Hybridoma Bank Pax7c; University of Iowa, Iowa City, IA) for 1 hour at room temperature. After a PBS rinse, the slides were incubated with secondary antibody using the Elite mouse IgG kit (PK-1602, Vector Laboratories) following the package directions. After a PBS rinse, the slides were incubated in diaminobenzidine with heavy metal intensification, rinsed in PBS, dehydrated in a series of increasing alcohol concentrations, incubated in xylene, and coverslipped with Permount mounting medium (Fisher Scientific, Waltham, MA).
All morphometric analyses were performed masked relative to the treatment of the rabbits from which the slides were prepared. Morphometric analyses were performed using Bioquant software (Bioquant, Nashville, TN) and a Leica microscope. For cross-sectional areas, three sections approximately 1.50, 1.75, and 2.00 mm from the beginning of muscle fibers at the insertional ends were counted. Fibers were traced manually from number coded slides, and a minimum of 200 myofibers in both orbital and global layers were measured. The averages of the means from each slide were determined per each rabbit muscle analyzed, and the means from each rabbit were used to determine overall mean for a specific treatment paradigm. For Pax7 counts similarly, the number of myofibers in a given area was determined and the number of Pax7-positive nuclei was determined. This allowed measurement of Pax7-positive nuclei/myofiber calculated as a percent for both orbital and global layers, with similar numbers as described for fiber areas. Means from each rabbit were used to determine overall means per muscle per animal and were used to generate overall means for any given treatment type and duration.
Graphing and statistical analyses were performed using GraphPad Prism software (GraphPad, San Diego, CA). Data are presented at mean ± SEM. ANOVAs were performed, followed by Tukey's post hoc multiple comparison tests for any data that were significantly different, defined as P < 0.05.
Results
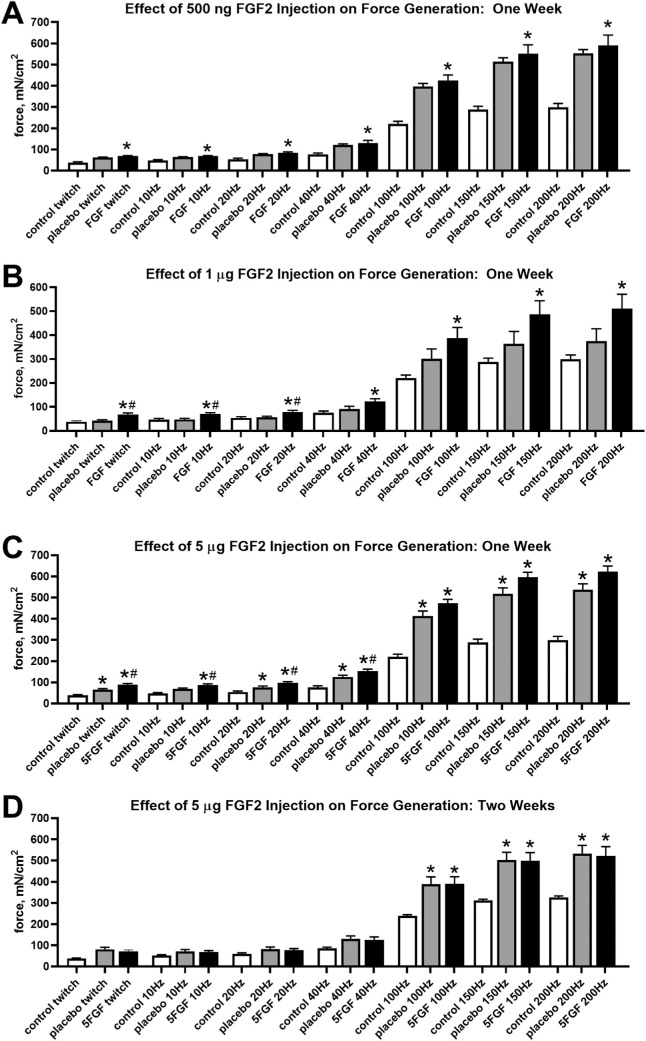
Force generation was examined after direct injection of FGF2 into one superior rectus muscle, at doses of either 500 ng, 1 µg, or 5 µg (Fig. 1). Direct injection of 500 ng of FGF2 did not result in a significant alteration in force between the treated muscles compared with the saline-injected contralateral superior rectus muscles at any of the stimulation frequencies (Fig. 1A). However, a 500 ng FGF2 injection resulted in significantly increased force compared with naïve control rabbits at all stimulation frequencies (Fig. 1A). These were differences of 82.4% (P < 0.008), 43.0% (P < 0.01), 58.7% (P < 0.02), 71.5% (P < 0.04), 92.6% (P < 0.02), 91.3% (P < 0.03), and 97.3% (P < 0.03), at each of the stimulation frequencies, respectively.
Figure 1.
Force generation (mN/cm2) was quantified for the superior rectus muscles of naïve control, placebo injection control, and FGF2-treated muscles one week after (A) 500 ng FGF2 injections for 3 consecutive days, (B) 1 µg FGF2 injections for 3 consecutive days, (C) 5 µg FGF2 injections for 3 consecutive days, and (D) 2 weeks after injection of 5 µg FGF2 injections for 3 consecutive days. * Statistical difference from naïve control superior rectus muscle. # Statistical difference from contralateral saline-injected control superior rectus muscle.
After treatment with 1 µg FGF2 for three sequential days, force, in mN/cm2, was significantly increased in the FGF2-treated muscles compared with the naïve control muscles (Fig. 1B). These treatments significantly increased force generation by 56.5%, 48.5%, 47.6%, 62.1%, 75.9%, 69.2%, and 71.1% (all P < 0.01), at each of the stimulation frequencies respectively. When the forces generated by the FGF2-treated muscles were compared with the forces generated by the superior rectus on the contralateral side, these forces were only significantly different at the lowest 3 stimulation frequencies, with a 59.6%, 46.8%, and 42.8% increase in force (all P < 0.01), respectively (Fig. 1B).
Direct injection of 5 µg FGF2 for three sequential days resulted in the most significant differences in generated force of the three injected dose series. Compared with naïve control superior rectus muscles, all treated muscles showed a significant increase in force at all stimulation frequencies (Fig. 1C). These represented force increases of 124%, 83.1%, 87.6%, 108.8%, 103.8%, 93.7%, and 96% (P < 0.001 for all comparisons), at each of the stimulation frequencies respectively. In addition, the superior rectus muscles injected with FGF2 generated significantly more force than the contralateral superior rectus muscles at the following stimulation frequencies: twitch (39.2% more force; P < 0.01), 10 Hz (31.2% more force; P < 0.01), 20 Hz (32.4% more force; P < 0.006), and 40 Hz (27.6% more force; P < 0.016). It should be noted that the muscles contralateral to the FGF2-treated muscles showed a significant increase in force compared with the naïve control muscles at all stimulation frequencies except 10 Hz, with increases of 60.9% (P < 0.03), 41.7% (P < 0.03), 64.6% (P < 0.003), 75.3% (P < 0.003), 67.2% (P < 0.01), and 66.7% (P < 0.01), at each of the stimulation frequencies, respectively. A separate group of rabbits was injected with 5 µg FGF2 and assessment of force was examined 2 weeks after the first injection (Fig. 1D). There were no significant differences in the normalized force generated between the 5 µg FGF2-injected superior rectus muscles and the contralateral saline injected muscles 2 weeks after treatment. Surprisingly, both these muscles produced significantly increased forces compared with the naïve control superior rectus muscles, but only at the highest three stimulation frequencies of 100 Hz, 150 Hz, and 200 Hz (P < 0.001).
One week after FGF2 treatment, the dose–response to the three concentrations of FGF2 was calculated with all the controls pooled (Fig. 2). After 1 µg FGF2 injections, force generation was significantly increased over naïve controls (P < 0.01). After 5 µg FGF2 injections, force generation was significantly increased compared with naïve controls and compared with saline-injected muscles contralateral to the FGF2-treated muscles (P < 0.0001). There was also a significant increase in force generation when treatment with 1 µg FGF2 was compared with treatment with 5 µg FGF2 (P < 0.039).
Figure 2.
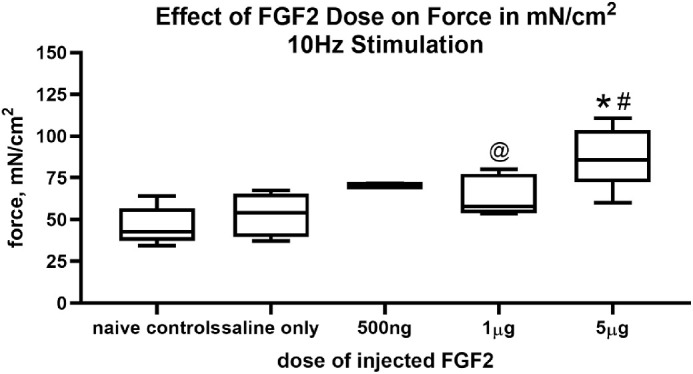
Dose response of force generation for the 10 Hz stimulation frequency after the injection of saline only, 500 ng, 1 µg, or 5 µg FGF2 compared with naïve control superior rectus muscles. * Statistical difference from naïve control superior rectus muscle. # Statistical difference from saline injected superior rectus muscle. @ Significant difference from 5 µg FGF2.
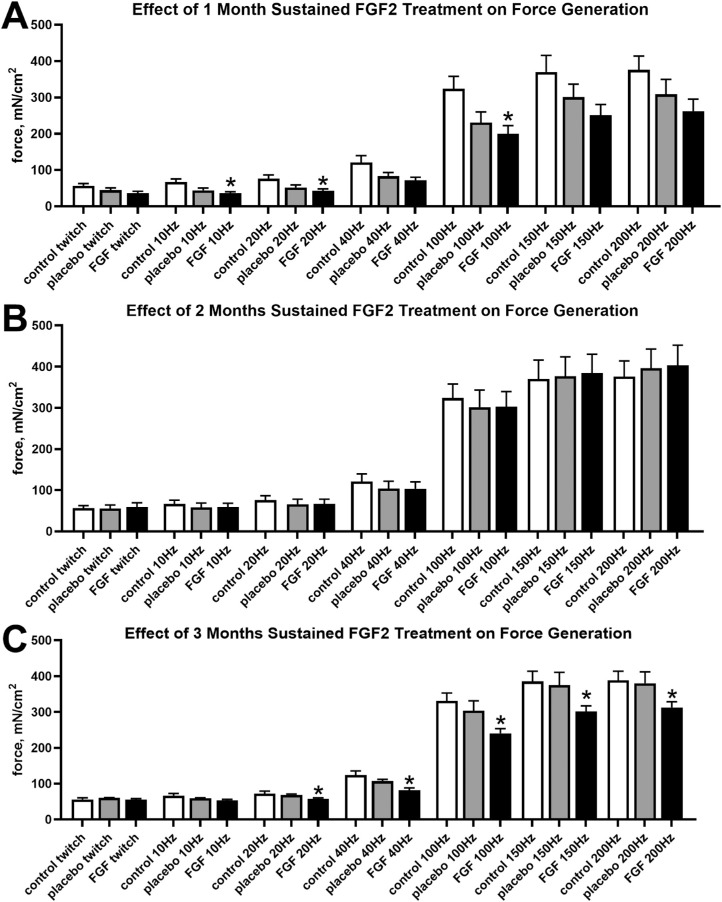
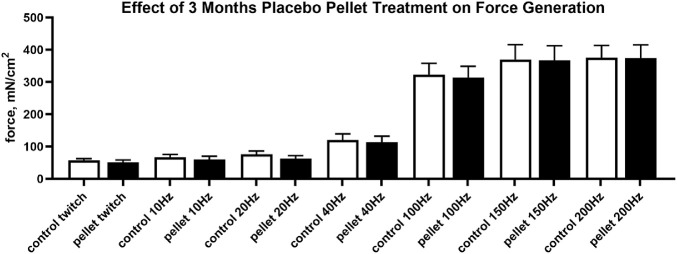
As a result of the significant changes seen with the short-term FGF2 studies, sustained treatment with FGF2 was performed. Force generation was compared between FGF2 treated muscles, placebo-treated superior rectus muscles on the contralateral side, and naïve control superior rectus muscles. In contrast with the results with short-term treatments after FGF2 injection, 1 month of sustained treatment resulted in significantly decreased force generation compared with naïve control superior rectus muscles (Fig. 3A) at 10 Hz, 20 Hz, and 100 Hz, with decreased forces of 46.5% (P < 0.02), 42.6% (P < 0.03), and 38.2% (P = 0.035), respectively. Although all the other stimulation frequencies resulted in a trend toward decreased force generation after 1 month of FGF2 treatment compared with naïve control muscles, none were significantly different (twitch, 36.2% decrease [P < 0.07]; 40 Hz, 40.5% decrease [P < 0.06]; 150 Hz, 32.2% decrease [P < 0.11]; and 200 Hz, 30.3% decrease [P < 0.13]). As seen in other studies of long-term neurotrophic factor treatment,16 after 2 months of sustained treatment there were no significant differences between any of the muscles at a given stimulation frequency (Fig. 3B). However, 3 months of sustained FGF2 treatment resulted in significantly decreased force generation between FGF2-treated and naïve control superior rectus muscles at the four highest stimulation frequencies (Fig. 3C). Decreased forces were significantly different at 40 Hz (33.9% decrease [P < 0.02]), 100 Hz (27.3% decrease [P < 0.035]), 150 Hz (21.8% decrease [P < 0.046]), and 200 Hz (19.4% decrease [P < 0.048]). In addition, a series of rabbits received unilateral or bilateral placebo-only pellet implantation, and there were no significant differences seen at any of the stimulation frequencies (Fig. 4; unilateral data not shown).
Figure 3.
Force generation (mN/cm2) was quantified for the superior rectus muscles of naïve control, placebo-pellet treated control, and FGF2-pellet treated muscles (A) after 1 month, (B) after 2 months, and (C) after 3 months. * Statistical difference from naïve control superior rectus muscle. # Statistical difference from contralateral superior rectus muscle.
Figure 4.
Effects of 3 months of bilateral placebo pellet treatment on force generation (mN/cm2) in the superior rectus muscles.
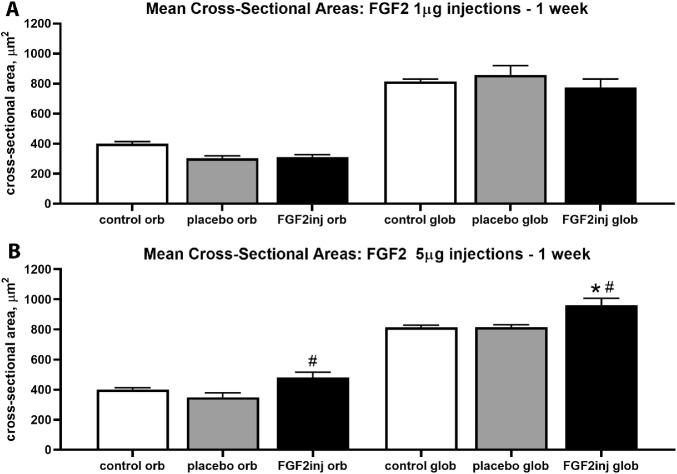
The potential bases for these changes in force between short-term and long-term treatment were investigated further by measuring the mean cross-sectional areas after each of these treatments. For the superior rectus muscles injected with 1 µg FGF2, there were no significant differences in the mean myofiber cross-sectional areas compared with naïve control muscles or when the treated and contralateral sides were compared (Fig. 5). For the superior rectus muscles injected with 5 µg FGF2 and examined after 1 week, the one-way ANOVA showed a significant difference (P < 0.0134; F = 8.495); thus, a post hoc Tukey's multiple comparisons test was performed. For the superior rectus muscles treated with injections of 5 µg FGF2, the mean cross-sectional areas of the orbital layer fibers were 27.4% significantly larger than the orbital layers fibers on the contralateral side (P < 0.009) (Fig. 5). The FGF2-treated orbital fibers were 16.02% larger than the naïve control myofibers, but this difference was not significant. The mean cross-sectional areas of the global layer fibers in the FGF2-treated muscles were significantly larger than both the naïve control global layer fibers, at 16.5% (P < 0.01), and the global fibers on the side contralateral to treatment, at 17.7% (P < 0.0.005). Thus, short-term injection of FGF2 into the superior rectus muscle resulted in a rapid mean increase in the mean cross-sectional areas of the treated myofibers.
Figure 5.
Mean cross-sectional areas (µm2) were determined one week after (A) 1 µg FGF2 and (B) 5 µg FGF2 injections. * Statistical difference from naïve control superior rectus muscle. # Statistical difference from saline injected superior rectus muscle.
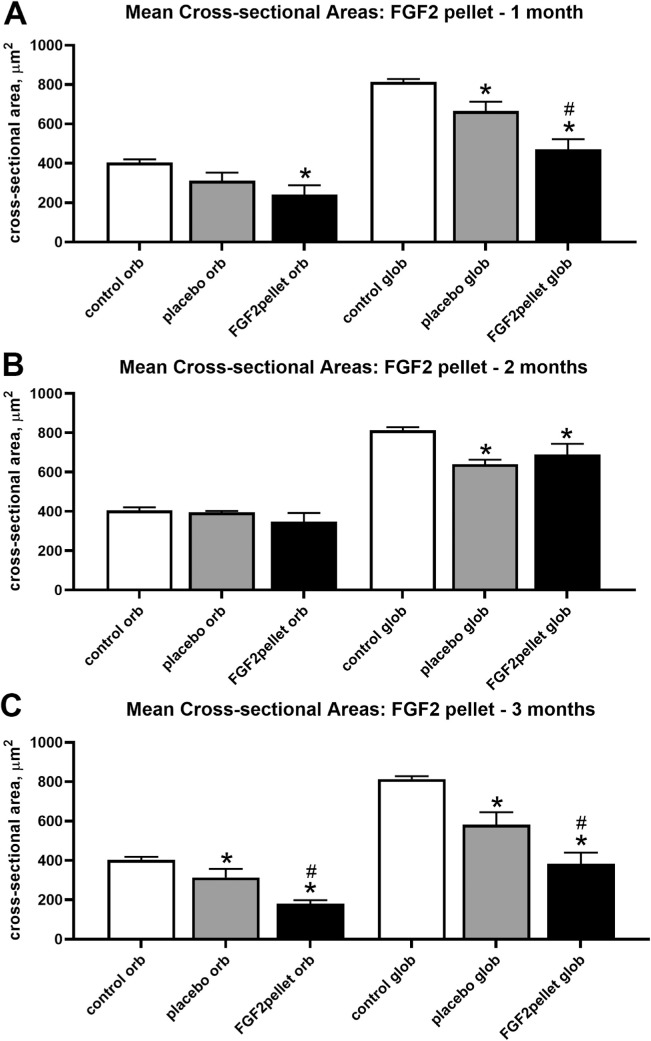
A very different picture was seen in the presence of sustained pellet FGF2 treatment (Figs. 6, 7). The one-way ANOVA for the 1-month time period was significant (1 month, P < 0.0132; F = 7.27); thus, a post hoc Tukey's multiple comparison test was performed. After 1 month of sustained FGF2 pellet treatment, the mean cross-sectional areas of both the orbital and global layers were significantly decreased compared with the naïve control superior rectus muscles (Figs. 6, 7), with a 40.7% decrease in the orbital layers and a 58.0% decrease in the global layers (P < 0.0074 and P < 0.0001, respectively). The mean cross-sectional areas of the global fibers on the contralateral side to the FGF2 treatment were also significantly smaller than the naïve control fibers, showing an 18% decrease (P < 0.02). The global layer fibers of the FGF2-treated superior rectus muscles were also significantly smaller than those in the superior rectus muscle on the contralateral side, showing a 29.2% decrease (P < 0.0085). After 2 months of FGF2 pellet treatment, the mean cross-sectional areas of only the global layers were significantly decreased compared with the naïve control superior rectus muscles, with a 15.4% decrease (P < 0.02) (Fig. 7), and the mean cross-sectional areas on the contralateral side were also significantly decreased compared with the naïve control fibers at a 21.3% decrease (P < 0.003). In contrast with 1 month after treatment, there was no significant difference between the treated and contralateral myofiber cross-sectional areas. The one-way ANOVA for the 3-month time period was significant (3 months, P < 0.0001; F = 30.16); thus, a post hoc Tukey's multiple comparison test was performed. After 3 months of FGF2 pellet treatment, both the orbital and global layer myofiber cross-sectional areas were significantly smaller than both the naïve control muscles and the fibers on the contralateral side (Figs. 6, 7). After the 3 months of pellet treatment, the orbital layer fibers were 55.4% (P < 0.0001) smaller than the naïve fiber areas and 22.3% (P < 0.012) smaller than the orbital myofibers on the contralateral side. The orbital fiber areas on the side contralateral to treatment were not significantly different from those in the naïve control muscles (P < 0.067). A similar picture was seen for the global layer fibers, which were significantly 52.9% smaller than the naïve control fibers (P < 0.0001) and significantly 34.2% smaller than the global fibers on the contralateral side (P < 0.024). In addition, the global layer fibers on the side contralateral to the FGF2 treatment were 28.4% smaller than those in the naïve control muscles (P < 0.005). To summarize, prolonged FGF2 treatment—particularly after either 1 month or 3 months—resulted in an imbalance in the mean myofiber cross-sectional areas on the treated side compared with the side contralateral to the treatment.
Figure 6.
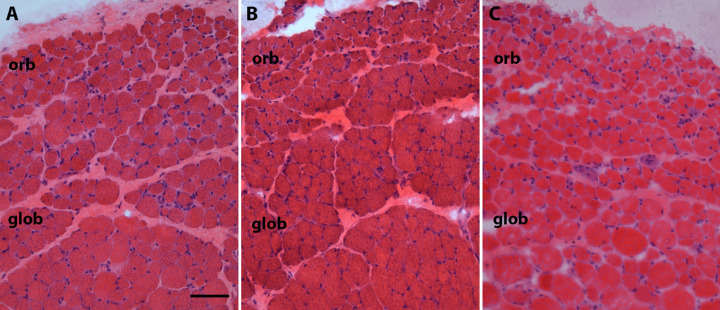
Photomicrograph of hematoxylin and eosin stained representative sections of superior rectus muscles from (A) naïve control, (B) placebo-pellet treated control, and (C) FGF2-containing sustained release pellets 3 months after treatment. Please note the heterogeneity of the myofibers including very small fibers in C. orb. orbital layer; glob, global layer. Bar is 50 µm.
Figure 7.
Mean cross-sectional areas (µm2) were determined after treatment with sustained release FGF2 pellets after (A) 1 month, (B) 2 months, and (C) 3 months. * Statistical difference from naïve control superior rectus muscle. # Statistical difference from pellet-treated superior rectus muscle.
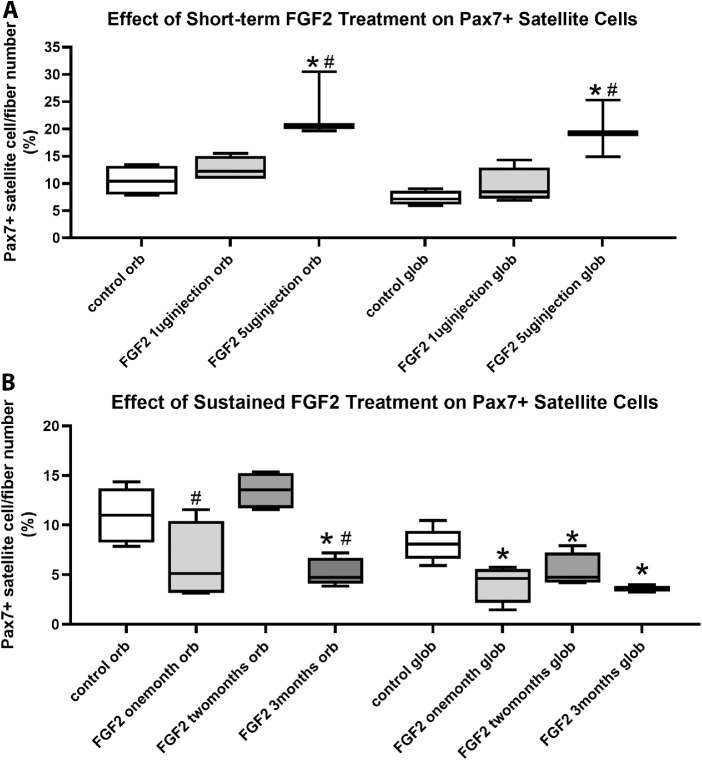
Decreased myofiber cross-sectional areas suggest that short-term or long-term exposure of the muscles to FGF2 would affect the Pax7-positive satellite cells within the extraocular muscle, which are more numerous in the extraocular muscles compared with limb skeletal muscles.42 Pax7-positive cell number as a percent of myofiber number was determined in all the treated superior rectus muscles compared with the contralateral untreated superior rectus muscles, as well as naïve control muscles (Figs. 8, 9). In the superior rectus muscles that received injections of 1 µg FGF2, no significant differences were seen in the number of Pax7-positive cells per myofiber number in either the orbital or global layers compared with naïve controls (Fig. 9) or the muscles on the contralateral side (data not shown). For the superior rectus muscles injected with 5 µg FGF2 and examined after 1 week, the one-way ANOVA showed a significant difference (P < 0.0033; F = 12.69); thus, a post hoc Tukey's multiple comparisons test was performed. In the superior rectus muscles treated with injections of 5 µg FGF2, the Pax7-positive cell population was significantly increased in both the orbital and global layers compared with both naïve controls (orbital, 123% increase [P < 0.0048]; global, 170.7% increase [P < 0.0033]) (Fig. 9) and to the muscles that had received the 1 µg FGF2 dosing (orbital, 85% increase [P < 0.013]; global, 107.5% increase [P < 0.01]) (Fig. 9). In the superior rectus muscles that received injections of 5 µg FGF2, after two weeks no significant differences were seen in the number of Pax7-positive cells per myofiber number in either the orbital or global layers compared with Pax7-positve cells on the contralateral side (data not shown).
Figure 8.
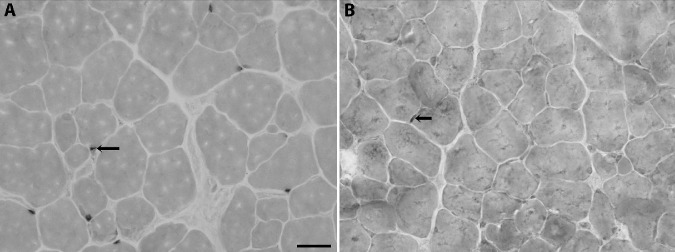
Photomicrograph of representative sections from superior rectus muscles immunostained for Pax7 3 months after treatment with either (A) a placebo pellet or (B) sustained release of FGF2. Bar is 20 µm. Arrows indicate Pax7-positive cells.
Figure 9.
Quantification of Pax7-positive muscle satellite cells from (A) short-term treatment with either 1 µg or 5 µg FGF2 and (B) long-term treatment with sustained release FGF2 pellets after 1 month, 2 months, and 3 months. * Statistical difference from naïve control superior rectus muscle. # Statistical difference from pellet-treated superior rectus muscle.
The Pax7-positive cell population was also examined in the superior rectus muscles that had received 1 month, 2 months, or 3 months of sustained FGF2 treatment (Figs. 8, 9). The one-way ANOVA showed a significant difference (P < 0.0008; F = 9.848); thus, a post hoc Tukey's multiple comparisons test was performed. After 1 month of sustained treatment, there was a significant decrease in the percentage of myofibers with an associated Pax7-positive cell compared with the naïve controls in the global layer (49% decrease; P < 0.0047) and a trend that was not significant in the orbital layer (43.5% decrease; P = 0.058). After 2 months of sustained FGF2 treatment, there were no significant differences between the number of Pax7-positive cells in either the orbital or global layers compared with the naïve control muscles (orbital, P = 0.48; global, P = 0.06). However, after 3 months of sustained FGF2 treatment, both the orbital and global layers showed a significant reduction in the numbers of Pax7-positive cells compared with naïve controls (orbital, 52.2% decrease [P < 0.0128]; global, 56% decrease [P < 0.0008]) (Figs. 8 and 9). Pax7-positive cells were significantly lower in the orbital layer at both 1 month and 3 months compared with 2 months of FGF2 sustained treatment (2 months vs 1 month, 53.9% increase [P < 0.007]; 2 months vs 3 months, 61% decrease [P < 0.0016]). To summarize, short-term treatment with FGF2 resulted in an increase in the Pax7-positive population of satellite cells, whereas sustained FGF2 treatment resulted, by the end of 3 months, in a significant decrease in these muscle precursor cells.
Discussion
Exogenous addition of FGF2 to the extraocular muscles of adult rabbits had two distinctly different effects based on the duration of treatment. Short-term treatment resulted in an increase in force generation, which was associated with increases in satellite cell numbers and myofiber cross-sectional areas. In contrast, extended treatment of the extraocular muscles resulted in an overall decrease in muscle force generation, decreased myofiber size, and decreased Pax7-positive satellite cells. These differential effects of FGF2 on extraocular structure and function were unexpected.
FGF2 has well-described effects on development and regeneration of limb skeletal muscle.35,43 During regeneration in crush injured skeletal muscle, FGF2 expression was shown to increase,28 levels of FGF2 increased during regeneration in dystrophic muscle,27,44 and FGF2 levels also increased in animal models of skeletal muscle hypertrophy.45 In these studies, increased FGF2 levels resulted in the maintenance of or an increase in the overall skeletal muscle mass in injury and disease. These studies correlated with those that showed that loss of FGF receptor signaling resulted in decreased skeletal muscle mass.46 In addition, the exogenous addition of FGF2 into both limb and thyroarytenoid muscles resulted in increased muscle mass.39,47 We hypothesized that treatment with FGF2 would result in increased muscle size and, concomitantly, increased muscle force production.48 In the short term, this hypothesis proved to be true, with force generation increasing by almost 100% at the highest dose of treatment, depending on the stimulation frequency. These changes in force generation capacity correlated well with known effects of FGF2 on increasing muscle size and satellite cell proliferation.31,39,47 More surprising were the effects of sustained treatment of FGF2 on the superior rectus muscles, where force-generating capacity, myofiber cross-sectional areas, and the number of Pax7-positive muscle precursor cells were all significantly decreased as the duration of exposure to FGF2 increased. There are several hypotheses that could explain this significant difference in response with duration of exposure. FGF2 is known to stimulate the proliferation of satellite cells while also inhibiting myofiber differentiation.35,49 It may be that, in the continued presence of high levels of FGF2 and delayed differentiation of the myogenic precursor cells in the extraocular muscle, these precursor cells could not survive or integrate into the existing myofibers as a part of the normal process of myofiber remodeling.50 Studies of changes in FGF isoform levels at different time points after injury suggested there was likely to be a temporal sequence necessary for proper myofiber maintenance,29 which would have been disrupted owing to the sustained level of FGF2 above normal. It would also be interesting to test intermittent periodic injections for efficacy in this system.
It is important to note the compensatory changes in the muscles on the side contralateral to the FGF2 treatment, which speaks to how important it is to have naïve control specimens for statistical analyses. These types of compensatory changes in the yoked but untreated muscles is a common phenomenon in the ocular motor system after surgical or growth factor interventions and is consistent with Hering's law.40,41,51,52 In addition, we have found that these types of fiber size imbalances are usually associated with the development of strabismus in our non-human primate disease models.20,41
FGFs and Extraocular Muscle
Despite extensive studies in limb skeletal muscle, little is known about the role of FGF2 in extraocular muscle development or maintenance of normalcy in the adult. Using a zebrafish injury model, FGF was important for normal muscle regeneration after injury of the lateral rectus muscle; FGF was also important for maintaining activated caspase 3 expression after injury to the lateral rectus muscles in these fish.37 FGF was shown to increase within extraocular muscles after recession surgery in a rabbit model of strabismus.53 There is also only one study that demonstrated, using in situ hybridization, that FGFR2 is specifically expressed on the extraocular muscles in human fetal specimens.54 Despite few studies of normal expression patterns, FGF signaling has been implicated in a number of syndromes where strabismus is common.55,56
Strabismus in Common FGFR-Associated Craniosynostosis Syndromes
Despite this rather significant dearth of information about the normal expression levels of FGF isoforms and their receptors, the known FGF receptor mutations that result in craniosynostosis syndromes support the view that FGF signaling is critical for normal formation of both the bony orbit57 and the extraocular muscles.58,59 Apert syndrome is one of the most common craniosynostosis syndromes, with a high rate of strabismus in the affected individuals, and is caused by one of two mutations in the gene for FGFR2.60 Other FGFR2-related craniosynostosis diseases are also highly associated with strabismus.55,56 The three most common craniosynostosis syndromes, namely, Crouzon, Apert, and Pfeiffer syndromes, are all thought to be caused by a gain-of-function mutation in FGFR2 that results in increased ligand affinity and prolonged FGF signaling in the affected tissues.61,62 Individuals with Crouzon syndrome have a reported prevalence of strabismus that ranges in the literature from 39% to 75%.63,64 A majority of patients with Pfeiffer syndrome have mutations in the gene for FGFR1 or FGFR2.65,66 One study reports a prevalence of strabismus of 55% in individuals with Pfeiffer syndrome.67 In clinical practice, it is clear that patients with FGFR2-related craniosynostosis syndromes have a high rate of strabismus,68 which can be difficult to manage.69 The similarity between changes in the superior rectus muscles after sustained FGF2 treatment in the present experiment with those seen in muscles from individuals with Apert syndrome59 sheds light on the potential biological mechanism and functional consequences of this aberrant signaling in the extraocular muscles from these syndromic individuals.
Other Neurotrophic Factors
A number of myogenic growth factors have been described in the literature as involved in muscle development, and they are also upregulated during muscle regeneration. These include, but are not limited to, FGF2, hepatocyte growth factor, epidermal growth factor, cardiotrophin-1, GDNF, and ciliary neurotrophic factor.70 The treatment of denervated or atrophic muscle by one or more of these factors prevented decreases or restored muscle size and/or force generation.70 Either single growth factors or a cocktail of several growth factors, including cardiotrophin-1, IGFI, GDNF, and brain-derived neurotrophic factor, were injected into the orbits of chicks, and these treatments resulted in altered force generation of the extraocular muscles examined.15,71 Contrasting effects of different neurotrophic factors on extraocular muscle function demonstrates the complexity with which these act on structure and function. Using similar approaches, sustained IGF-1 treatment resulted in increased extraocular muscle force generation in rabbit superior rectus muscles,17 whereas GDNF resulted in decreased force generation.72 The roles played by the altered signaling of these varied neurotrophic factors in the development and maintenance of strabismus are not understood, but gene studies of muscles from strabismic individuals compared with age-matched controls have implicated a number of these factors as playing a role in producing or maintaining eye misalignment.12,13
In summary, extraocular muscles treated with FGF2 had a completely different response to short-term injections compared with sustained exposure over a period of 3 months. These responses correlated with increased or decreased myofiber cross-sectional areas. The effects of sustained FGF2 signaling in these studies suggest a potential mechanism for why strabismus may be a common finding in craniosynostosis syndromes where FGFR mutations result in abnormal and prolonged signaling of FGF.
Acknowledgments
Supported by EY15313 and EY11375 from the National Eye Institute and an unrestricted grant to the Department of Ophthalmology at the University of California San Diego from Research to Prevent Blindness, Inc.
Disclosure: J.C. Rudell, None; L.K. McLoon, None
References
- 1. Friedman DS, Repka MX, Katz J, et al.. Prevalence of amblyopia and strabismus in white and African American children aged 6 and 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009; 116: 2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Repka MX, Lum F, Burugapalli B.. Strabismus, strabismus surgery, and reoperation rate in the United States: analysis from the IRIS registry. Ophthalmology. 2018; 125: 1646–1653. [DOI] [PubMed] [Google Scholar]
- 3. Hashemi H, Pakzad R, Heydarian S, et al.. Global and regional prevalence of strabismus: a comprehensive systematic review and meta-analysis. Strabismus. 2019; 27: 54–65. [DOI] [PubMed] [Google Scholar]
- 4. Keskinbora KH, Pulur NK.. Long-term results of bilateral medial rectus recession for congenital esotropia. J Pediatr Ophthalmol Strabismus. 2004; 41: 351–355. [DOI] [PubMed] [Google Scholar]
- 5. Ekdawi NS, Nusz KJ, Diehl NN, Mohney BG.. Postoperative outcomes in children with intermittent exotropia from a population-based cohort. J AAPOS. 2009; 13: 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louwagie CR, Diehl NN, Greenberg AE, Mohney BG.. Long-term follow-up of congenital esotropia in a population-based cohort. J AAPOS. 2009; 13: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pineles SL, Ela-Dalman N, Zvansky AG, Yu F, Rosenbaum AL.. Long-term results of surgical management of intermittent exotropia. J AAPOS. 2010; 14: 298–304. [DOI] [PubMed] [Google Scholar]
- 8. Mohan K, Sharma SK.. Long-term motor and sensory outcomes after surgery for the nonaccommodative component of partially refractive accommodative esotropia. J AAPOS. 2018; 22: 356–360. [DOI] [PubMed] [Google Scholar]
- 9. Scott AB. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981; 79: 734–770. [PMC free article] [PubMed] [Google Scholar]
- 10. Scott AB, Miller JM, Shieh KR.. Bupivacaine injection of the lateral rectus muscle to treat esotropia. J AAPOS. 2009; 13: 119–122. [DOI] [PubMed] [Google Scholar]
- 11. Rowe FJ, Noonan CP.. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev . 2017; 3: CD006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altick AL, Feng CY, Schlauch K, Johnson LA, von Bartheld CS. Differences in gene expression between strabismic and normal human extraocular muscles. Invest Ophthalmol Vis Sci. 2012; 53: 5168–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agarwal AB, Feng CY, Altick AL, et al.. Altered protein composition and gene expression in strabismic human extraocular muscles and tendons. Invest Ophthalmol Vis Sci. 2016; 57: 5576–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McLoon LK, Christiansen SP.. Increasing extraocular muscle strength with insulin-like growth factor II. Invest Ophthalmol Vis Sci. 2003; 443: 3866–3872. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, von Bartheld CS.. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci. 2004; 45: 3538–3545. [DOI] [PubMed] [Google Scholar]
- 16. Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK.. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006; 47: 2461–02467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLoon LK, Anderson BC, Christiansen SP.. Increasing muscle strength as a treatment for strabismus: Sustained release of insulin-like growth factor-I in rabbit extraocular muscle. J AAPOS. 2006; 10: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson KR, Stevens SM, McLoon LK.. Prolongation of relaxation time in extraocular muscles with brain derived neurotropic factor in adult rabbit. Invest Ophthalmol Vis Sci. 2016; 57: 5834–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleuriet J, Willoughby CL, Kueppers RB, Mustari MJ, McLoon LK.. Eye alignment changes caused by sustained GDNF treatment of an extraocular muscle in infant non-human primates. Sci Rep. 2020; 10(1): 11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLoon LK, Christiansen SP, Ghose GM, Das VE, Mustari MJ.. Improvement of eye alignment in adult strabismic monkeys by sustained IGF-1 treatment. Invest Ophthalmol Vis Sci. 2016; 57: 6070–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pullela M, Degler BA, Coats DK, Das VE.. Longitudinal evaluation of eye misalignment and eye movements following surgical correction of strabismus in monkeys. Invest Ophthalmol Vis Sci. 2016; 57: 6040–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pullela M, Agaoglu MN, Joshi AC, et al.. Neural plasticity following surgical correction of strabismus in monkeys. Invest Ophthalmol Vis Sci. 2018; 59: 5011–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Otsuka H, Matsuda S, Fujita H, et al.. Localization of basic fibroblast growth factor (bFGF)-like immunoreactivity in neural circuits innervating the gastrocnemius muscle, with reference to the direction of bFGF transport. Arch Histol Cytol. 1993; 56: 207–215. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Butowt R, Rind HB, von Bartheld CS. GDNF increases the survival of developing oculomotor neurons through a target-derived mechanism. Mol Cell Neurosci. 2003; 24: 41–56. [DOI] [PubMed] [Google Scholar]
- 25. Steljes TP, Kinoshita Y, Wheeler EF, Oppenheim RW, von Bartheld CS. Neurotrophic factor regulation of developing avian oculomotor neurons: differential effects of BDNF and GDNF. J Neurobiol. 1999; 41(2): 295–315. [PubMed] [Google Scholar]
- 26. von Bartheld CS, Williams R, Lefcort F, Clary DO, Reichardt LF, Bothwell M.. Retrograde transport of neurotrophins from the eye to the brain in chick embryos: roles of the p75NTR and trkB receptors. J Neurosci. 1996; 16(9): 2995–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson JE, Liu L, Kardami E.. Distinctive patterns of basic fibroblast growth factor (bFGF) distribution in degenerating and regenerating areas of dystrophic (mdx) striated muscles. Dev Biol. 1991; 147: 96–109. [DOI] [PubMed] [Google Scholar]
- 28. Anderson JE, Mitchell CM, McGeachie JK, Grounds MD.. The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res. 1995; 216: 325–334. [DOI] [PubMed] [Google Scholar]
- 29. Do MKQ, Suzuki T, Gerelt B, et al.. Time-coordinated prevalence of extracellular HGF, FGF2 and TGF-β3 in crush injured skeletal muscle. Anim Sci J. 2012; 83: 712–717. [DOI] [PubMed] [Google Scholar]
- 30. Rink S, Chatziparaskeva C, Elles L, et al.. Neutralizing BDNF and FGF2 injection into denervated skeletal muscle improve recovery after nerve repair. Muscle Nerve. 2020; 62(3): 404–412. [DOI] [PubMed] [Google Scholar]
- 31. Allen RE, Dodson MV, Luiten LS.. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp Cell Res. 1984; 152: 154–160. [DOI] [PubMed] [Google Scholar]
- 32. Sheehan SM, Allen RE.. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cellul Physiol. 1999; 181: 499–506. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Schneider MF.. FGF2 activates TRPC and Ca2+ signaling leading to satellite cell activation. Front Physiol. 2014; 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yousef H, Conboy MJ, Mamiya H, et al.. Mechanisms of action of hESC-secreted proteins that enhance human and mouse myogenesis. Aging. 2014; 6(8): 602–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pawlikowski B, Vogler TO, Gadek K, Olwin BB.. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev Dyn. 2017; 246: 359–367. [DOI] [PubMed] [Google Scholar]
- 36. De Micheli AJ, Laurilliard EJ, Heinke CL, et al.. Single-cell analysis of the muscle stem cell hierarchy identifies heterotopic communication signals involved in skeletal muscle regeneration. Cell Rep. 2020; 30: 3583–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saera-Vila A, Kish PE, Louie KW, Grzegorski SJ, Klionsky DJ, Kahana A.. FGF regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell Signal. 2016; 28: 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaneko M, Tsuji T, Kishimoto Y, Sugiyama T, Hirano S.. Regenerative effects of basic fibroblast growth factor on restoration of thyroarytenoid muscle atrophy caused by recurrent laryngeal nerve transection. J Voice. 2017; 32(6): 645–651. [DOI] [PubMed] [Google Scholar]
- 39. Goto T, Ueha R, Sato T, Fujimaki Y, Nito T, Yamasoba T.. Single, high-dose local injection of bFGF improves thyroarytenoid muscle atrophy after paralysis. Laryngoscope. 2020; 130: 159–165. [DOI] [PubMed] [Google Scholar]
- 40. Willoughby CL, Christiansen SP, Mustari MJ, McLoon LK.. Effects of the sustained release of IGF-1 on extraocular muscle of the infant non-human primate: adaptations at the effector organ level. Invest Ophthalmol Vis Sci. 2012; 53: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willoughby CL, Fleuriet J, Walton MM, Mustari MJ, McLoon LK.. Adaptability of the immature ocular motor control system: unilateral IGF-I medial rectus treatment. Invest Ophthalmol Vis Sci. 2015; 56: 3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verma M, Fitzpatrick KR, McLoon LK.. Extraocular muscle repair and regeneration. Curr Ophthalmol Rep. 2017; 5: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ornitz DM, Itoh N.. The fibroblast growth factor signaling pathway. WIREs Dev Biol. 2015; 4: 215–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DiMario J, Buffinger N, Yamada S, Strohman RC.. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989; 244: 6880690. [DOI] [PubMed] [Google Scholar]
- 45. Mitchell P, Steenstrup T, Hannon K.. Expression of fibroblast growth factor family during postnatal skeletal muscle hypertrophy. J Appl Physiol. 1999; 86: 313–319. [DOI] [PubMed] [Google Scholar]
- 46. Flanagan-Steet H, Hannon K, McAvoy MJ, Hullinger R, Olwin BB.. Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev Biol. 2000; 218: 21–37. [DOI] [PubMed] [Google Scholar]
- 47. Iwata Y, Ozaki N, Hirata H, et al.. Fibroblast growth factor-2 enhances functional recovery of reinnervated muscle. Muscle Nerve. 2006; 34: 623–630. [DOI] [PubMed] [Google Scholar]
- 48. Syverud BC, VanDusen KW, Larkin LM.. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs. 2016; 202: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olwin BB, Hauschka SD.. Identification of the fibroblast growth factor receptor of Swiss 3T3 cells and mouse skeletal muscle myoblasts. Biochemistry. 1986; 25: 3487–3492. [DOI] [PubMed] [Google Scholar]
- 50. McLoon LK, Rowe J, Wirtschafter J, McCormick KM.. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004; 29(5): 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Christiansen SP, Soulsby ME, Seifen EE.. Effect of antagonist weakening on developed tension in cat extraocular muscle. Invest Ophthalmol Vis Sci. 1995; 36: 2547–2550. [PubMed] [Google Scholar]
- 52. Christiansen SP, Antunes-Foschini RS, McLoon LK.. Effect of recession versus tenotomy surgery without recession in adult rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2010; 51: 5646–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin SY, Paik DJ.. Expression of four growth factors in recessed extraocular muscles of rabbits. Ophthalmic Surg Lasers Imaging. 2006; 37(2): 129–37 [PubMed] [Google Scholar]
- 54. Khan SH, Britto JA, Evans RD, Nischal KK.. Expression of FGFR-2 and FGFR-3 in the normal human fetal orbit. Br J Ophthalmol. 2005; 89: 1643–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosenberg JB, Tepper OM, Medow NB.. Strabismus in craniosynostosis. J Pediatr Ophthalmol Strabismus. 2013; 50(3): 140–148. [DOI] [PubMed] [Google Scholar]
- 56. Dalmas F, Pech-Gourg G, Gallucci A, Denis D, Scavarda D.. Craniosynostosis and oculomotor disorders. Neurochirurgie. 2020; 66: 91–96. [DOI] [PubMed] [Google Scholar]
- 57. De Moerlooze L, Dickson C.. Skeletal disorders associated with fibroblast growth factor receptor mutations. Curr Opin Genet Develop. 1997; 7: 378–385. [DOI] [PubMed] [Google Scholar]
- 58. Margolis S, Pachter BR, Breinin GM.. Structural alterations of extraocular muscle associated with Apert's syndrome. Br J Ophthalmol. 1977; 61: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rudell JC, Stager D Jr., Felius J, McLoon LK.. Morphological differences in the inferior oblique muscles from subjects with over-elevation in adduction. Invest Ophthalmol Vis Sci. 2020; 61(6): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cohen MM Jr., Kreiborg S, Lammer EJ, et al.. Birth prevalence study of Apert syndrome. Am J Med Genet. 1992; 42: 655–659. [DOI] [PubMed] [Google Scholar]
- 61. Kosty J, Vogel TW.. Insights into the development of molecular therapies for craniosynostosis. Neurosurg Focus. 2015; 38: E2. [DOI] [PubMed] [Google Scholar]
- 62. Wilkie AO. Craniosynostosis: genes and mechanisms. Hum Mol Genet. 1997; 6: 1647–1656. [DOI] [PubMed] [Google Scholar]
- 63. Gray TL, Casey T, Selva D, Anderson PJ, David DJ.. Ophthalmic sequelae of Crouzon syndrome. Ophthalmology. 2005; 112: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 64. Kreiborg S, Cohen MM Jr.. Ocular manifestations of Apert and Crouzon syndromes: qualitative and quantitative findings. J Craniofac Surg. 2010; 21: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 65. Rai R, Iwanaga J, Dupont G, et al.. Pfeiffer type 2 syndrome: review with updates on its genetics and molecular biology. Childs Nerv Syst. 2019; 35: 1451–1455. [DOI] [PubMed] [Google Scholar]
- 66. Sawh-Martinez R, Steinbacher DM.. Syndromic craniosynostosis. Clin Plast Surg. 2019; 46: 141–155. [DOI] [PubMed] [Google Scholar]
- 67. Sharma N, Greenwell T, Hammerton M, David DJ, Selva D, Anderson PJ.. The ophthalmic sequelae of Pfeiffer syndrome and the long term visual outcomes after craniofacial surgery. J AAPOS. 2016; 20: 315–319. [DOI] [PubMed] [Google Scholar]
- 68. Khan SH, Nischal KK, Dean F, Hayward RD, Walker J.. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: a review of 141 cases. Br J Ophthalmol. 2003; 87: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ganesh A, Edmond J, Forbes B, et al.. An update of ophthalmic management in craniosynostosis . J AAPOS. 2019; 23: 66–76. [DOI] [PubMed] [Google Scholar]
- 70. Fraysee B, Guillet C, Huchet-Cadiou C, Camerino DC, Gascan H, Léoty C.. Ciliary neurotrophic factor prevents unweighting-induced functional changes in rat soleus muscle. J Appl Physiol. 2000; 88: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 71. Li TA, Feng CY, von Bartheld CS. How to make rapid eye movements “rapid”: the role of growth factors for muscle contractile properties. Plug Arch. 2011; 461: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fitzpatrick KR, Cucak A, McLoon LK.. Changing muscle function with sustained glial derived neurotrophic factor treatment of rabbit extraocular muscle. PLoS One. 2018; 13(8): e0202861. [DOI] [PMC free article] [PubMed] [Google Scholar]