Abstract
Purpose
To evaluate the integrative potential of neural stem cells (NSCs) with the visual system and characterize effects on the survival and axonal regeneration of axotomized retinal ganglion cells (RGCs).
Methods
For in vitro studies, primary, postnatal rat RGCs were directly cocultured with human NSCs or cultured in NSC-conditioned media before their survival and neurite outgrowth were assessed. For in vivo studies, human NSCs were transplanted into the transected rat optic nerve, and immunohistology of the retina and optic nerve was performed to evaluate RGC survival, RGC axon regeneration, and NSC integration with the injured visual system.
Results
Increased neurite outgrowth was observed in RGCs directly cocultured with NSCs. NSC-conditioned media demonstrated a dose-dependent effect on RGC survival and neurite outgrowth in culture. NSCs grafted into the lesioned optic nerve modestly improved RGC survival following an optic nerve transection (593 ± 164 RGCs/mm2 vs. 199 ± 58 RGCs/mm2; P < 0.01). Additionally, RGC axonal regeneration following an optic nerve transection was modestly enhanced by NSCs transplanted at the lesion site (61.6 ± 8.5 axons vs. 40.3 ± 9.1 axons, P < 0.05). Transplanted NSCs also differentiated into neurons, received synaptic inputs from regenerating RGC axons, and extended axons along the transected optic nerve to incorporate with the visual system.
Conclusions
Human NSCs promote the modest survival and axonal regeneration of axotomized RGCs that is partially mediated by diffusible NSC-derived factors. Additionally, NSCs integrate with the injured optic nerve and have the potential to form neuronal relays to restore retinofugal connections.
Keywords: neural stem cells, retinal ganglion cell, optic nerve transection, survival, regeneration, integration
Retinal ganglion cell (RGC) injury occurs in multiple pathologies including glaucoma and neurodegenerative diseases. Unfortunately, damaged RGC axons projecting to central vision-associated targets do not spontaneously regenerate.1 Additionally, axonal damage activates signaling pathways in RGC axons and cell bodies that lead to RGC death after injury.2 Because lost RGCs are not replaced, the ensuing vision loss is permanent. Strategies to restore vision must protect RGCs after injury and regenerate lost retinofugal projections. One such strategy is to use stem cells that have the potential to support both neuronal survival and axon regeneration and to reconstitute damaged neuronal circuits by forming neuronal relays.
Stem cells derived from various sources have been assessed for the potential to enhance neuronal survival. Mesenchymal stem cells derived from bone marrow sources secrete neurotrophic factors and improve the survival of RGCs.3,4 Neural stem cells (NSCs) derived from cerebellar sources also constitutively secrete neurotrophic factors that promote neuronal survival, as well as axonal regeneration.5 Furthermore, induced pluripotent stem cells cotransplanted with RGCs in vivo increase the survival and neurite extension of intravitreally transplanted RGCs.6
In the field of spinal cord injury and regeneration, corticospinal axons are similar to RGC axons in that they also do not spontaneously regenerate and are refractory to attempts to induce regeneration. However, the transplantation of NSCs have challenged this long-held dogma and made significant advances in spinal cord repair. When NSCs are transplanted into the lesion site of a spinal cord injury, corticospinal axons robustly regenerate into the cell graft and form synapses with the grafted NSCs.7 NSCs also differentiate into neurons and extend axons that integrate into existing host neural circuitry.8,9 The resulting “neuronal relays” across the spinal cord lesion site reconstitutes spinal circuits and restores motor function. Whether NSCs could similarly exert a neuroregenerative effect on RGC axons and form neuronal relays to reestablish retinofugal connections is unknown. This study aimed to assess whether human NSCs could promote RGC survival and axon regeneration after injury to the adult optic nerve and to evaluate the integrative potential of NSCs as a neuronal relay in the injured optic nerve.
Material and Methods
Animals and Stem Cells
All animal use adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research Protocols. Protocols were reviewed and approved by the Veterans Affairs San Diego Healthcare System Institutional Animal Care and Use Committee. Transgenic Fischer 344 (F344) Tg(UBC-EGFP) rats ubiquitously expressing green fluorescent protein (GFP) under the control of the human ubiquitin-C promoter were obtained from the Rat Resource and Research Center (University of Missouri, Columbia, MO, USA). T-cell–deficient athymic nude rats (Hsd:RH-Foxn1rnu) seven to eight weeks of age were obtained from Envigo (Placentia, CA, USA). NSCs generated from the H9 human embryonic stem cell line were obtained from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA).
RGC Purification
Primary RGCs were isolated from GFP-expressing F344 rats between postnatal days two to four and purified by magnetic separation methods. Animals were euthanized by decapitation. Retinas were dissected from the eyes and dissociated into a single-cell suspension using a Neural Tissue Dissociation Kit - Postnatal Neurons (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. RGCs were purified from the single-cell suspension using a Retinal Ganglion Cell Isolation Kit - Rat (Miltenyi Biotec) in accordance with the manufacturer's instructions. Briefly, the cell suspension was incubated with Biotinylated Depletion Antibodies and CD90.1 Microbeads specific for RGCs followed by incubation with Anti-Biotin MACSiBeads Particles. The cell suspension was applied to a magnetic MACSiMAG separator to negatively select for microglia and endothelial cells. Positive selection for RGCs was achieved by applying the cell suspension to a magnetic MS column. Purified primary RGCs were eluted from the column with a purity of 95%. All cell culture experiments were maintained at 37°C with 5% CO2.
NSC Cultures
H9 NSCs were transduced with a lentivirus to constitutively express the fluorescent protein tdTomato under a CAG promoter. Transduced, fluorescent NSCs were purified by fluorescence activated cell sorting. NSCs expressing tdTomato were cultured in T-25 tissue culture flasks coated with poly-D-lysine (PDL; 20 µg/mL; MilliporeSigma, Burlington, MA, USA) and laminin (5 µg/mL; MilliporeSigma). NSC growth media composed of ENStem-A Neural Expansion Media (MilliporeSigma) supplemented with GlutaMAX (1X; Thermo Fisher Scientific), penicillin/streptomycin (1X; Thermo Fisher Scientific), and fibroblast growth factor-2 (FGF-2; 20 ng/mL; MilliporeSigma) was changed every other day until cells were at least 90% confluent. Confluent cells were passaged with Accutase (Thermo Fisher Scientific) at 1:2 or 1:3 dilutions while maintaining passage numbers less than 30. The pluripotency of human NSC cultures were verified experimentally by in vitro neuronal differentiation and immunohistochemical labeling.
RGC-NSC/NSC-Conditioned Media Cultures
To prepare NSC-conditioned media, NSCs were grown to 90% confluence with NSC growth media on PDL- and laminin-coated tissue culture flasks. The NSC growth media was removed, and cells were rinsed with PBS and replaced with RGC growth media prepared with Neural Basal Media (Thermo Fisher Scientific) supplemented with B-27 supplement (1X; Thermo Fisher Scientific), GlutaMAX (1X; Thermo Fisher Scientific), penicillin-streptomycin (1X; Thermo Fisher Scientific), sodium pyruvate (1 mM; Thermo Fisher Scientific), 3,3′,5-triiodo-L-thyronine (40 ng/mL; MilliporeSigma), insulin (5 µg/mL; MilliporeSigma), N-acetyl cysteine (5 µg/mL; MilliporeSigma), and Forskolin (10 µM; MilliporeSigma). The conditioned media was collected after 24 hours, filtered through a 0.22 µm filter, and stored at −80°C until use.
Isolated RGCs were plated at a density of approximately 150 cells/mm2 on PDL and laminin coated 96-well plates in RGC growth media. RGCs were allowed to adhere to the plate for at least one hour before experimental manipulations. For RGCs directly cocultured with NSCs, NSCs were plated with RGCs at a density of 150 cells/mm2 in RGC growth media. For RGCs cultured in NSC-conditioned media, half of the RGC media was removed and replaced with either RGC growth media supplemented with brain-derived neurotrophic factor (BDNF; 50 ng/mL; Peprotech, Cranbury, NJ, USA) or ciliary neurotrophic factor (CNTF; 5 ng/mL; Peprotech, Cranbury, NJ) or thawed NSC-conditioned RGC growth media diluted to achieve final concentrations of 10%, 33%, or 66% while maintaining similar volumes across all conditions. Cells were cultured for two days before proceeding to outgrowth or viability assays.
In Vitro RGC Viability Assays
RGC viability in cultures with NSC-conditioned media was measured with CellTiter-Glo 2.0 (Promega, Madison, WI, USA) according to the manufacturer's instructions. A volume of CellTiter-Glo 2.0 equal to the amount of media was added to the cells. Cells were placed on an orbital shaker for two minutes. After 10 minutes at room temperature, luminescence was measured with a plate reader (Bio-Rad, Hercules, CA, USA). Five replicates were used for each data point.
In Vitro RGC Outgrowth Assays
For RGCs cocultured with NSCs, cultures were fixed with 4% paraformaldehyde (PFA). Fixed cultures were immunolabeled by incubating with primary antibodies to βIII-tubulin (1:1000; Promega), GFP (1:1000; Life Technologies, Carlsbad, CA, USA) and mCherry (1:1000; Sicgen, Cantanhede, Portugal) for one hour at 25°C followed by Alexa-conjugated secondary antibodies (Molecular Probes, Carlsbad, CA, USA) for one hour at 25°C using standard protocols. Fluorescent images were acquired using automated fluorescence microscopy (BZ-X700; Keyence, Itasca, IL). Images were stitched and neurite lengths of βIII-tubulin+/GFP+/tdTomato− cells were measured using the Simple Neurite Tracer plugin in FIJI ImageJ software (National Institutes of Health, Bethesda, MD).
For RGC cultures with NSC-conditioned media, the fluorogenic substrate calcein AM (Life Technologies, Carlsbad, CA) was added to wells to achieve a final concentration of 1 µM. After 30 minutes at 37°C, fluorescent, viable cells were imaged using automated fluorescence microscopy (BZ-X700; Keyence, Itasca, IL, USA). Images were stitched, and neurite lengths from fluorescent cells were measured using the Simple Neurite Tracer plugin in FIJI ImageJ software (National Institutes of Health, Bethesda, MD, USA).
In Vivo Optic Nerve Transection and NSC Transplant
Transection and transplantation of NSCs into the optic nerve was performed as previously described.10 Athymic nude rats were anesthetized using an intraperitoneal injection of ketamine (50 mg/kg), xylazine (2.6 mg/kg), and acepromazine (0.5 mg/kg). Adequate anesthesia depth was confirmed by the absence of reflex with toe pinch. The animal was placed in a stereotaxic frame on top of a heating pad. The left eye was anesthetized with a drop of proparacaine hydrochloride 0.5% and sterilized with a 5% povidone-iodine solution. A 4-0 Vicryl suture was placed in the superior eyelid, and the eyelid was everted. A superior peritomy was performed and the superior rectus muscle was disinserted from the globe. The eye was infraducted and blunt dissection of the posterior aspect of the globe was performed to reveal the optic nerve. The optic nerve was crushed with a pair of jeweler forceps 1.5 to 2.0 mm posterior to the globe. A glass pulled pipette was inserted into the optic nerve at the crush site. PBS or a cell suspension of NSCs (250,000 cells/µL) in PBS was injected into the optic nerve using a Picospritzer at 20 psi to transect the optic nerve while preserving the optic nerve sheath. The conjunctiva was reapposed to the limbus. Polymyxin B (10,000 U/g) antibiotic ointment was applied to the eye. A temporary tarsorrhaphy was placed. Animals received subcutaneous injections of an antibiotic, nonsteroidal anti-inflammatory, and fluids and were housed independently in a heated cage to recover after surgery.
Tissues were harvested two or four weeks after optic nerve transections. Three days before tissue collection, animals received intravitreal injections of cholera toxin subunit B (CTB; 2 µL, 1 µg/µL; List Biological Labs, Campbell, CA, USA) to anterogradely label RGC axons. Animals were perfused transcardially with cold PBS, followed by 4% PFA, and post fixed in 4% PFA overnight. The eyes were dissected and stored in PBS. The optic nerves were dissected and cryoprotected in 30% sucrose.
Retina and Optic Nerve Immunohistochemistry
For retina flat mounts, the retinas were dissected and washed in PBS. Nonspecific binding was blocked and permeabilization was achieved with a solution of 0.25% Triton-X100 and 10% donkey serum in PBS for 1 hour at room temperature. Primary antibody to RBPMS (1:500; PhosphoSolutions, Aurora, CO, USA) was applied and incubated at 4°C for three days. Retinas were then washed with PBS. Alexa-conjugated secondary antibodies (Molecular Probes) were applied and incubated at 4°C for 1 day. Retinas were washed with PBS. Four radial incisions were made. The retinas were flat-mounted onto a microscope slide. Slides were dried overnight and coverslipped with Mowiol.
Optic nerves were embedded in optimal cutting temperature compound (Scigen, Gardena, CA). Optic nerves were sectioned on a cryostat longitudinally (10 µm) and directly mounted onto gelatin-subbed microscope slides. Every fourth section was processed for analysis. Optic nerve sections were blocked and permeabilized with 0.25% Triton-X100 and 10% donkey serum in Tris-buffered saline solution for one hour at room temperature. Primary antibodies to CTB (1:1000; List Biological Labs), mCherry (1:1000; Sicgen), glial fibrillary acidic protein (GFAP) (1:1000; EnCor Biotechnology, Gainesville, FL, USA), βIII-tubulin (1:1000; BioLegend, San Diego, CA, USA), postsynaptic density protein 95 (PSD-95) (1:1000; Abcam, Cambridge, MA, USA), vesicular glutamate transporter-2 (VGLUT2) (1:1000; MilliporeSigma), or NeuN (1:1000; EnCor Biotechnology) were applied and incubated at 4°C for one day. Alexa-conjugated secondary antibodies (Molecular Probes) were applied and incubated at room temperature for one hour. Slides were dried overnight and coverslipped with Mowiol.
Retina and Optic Nerve Imaging and Quantification
Images of flat mounted retinas and optic nerves were acquired using an Axio Scan (Zeiss, White Plains, NY, USA). Fluorescent images of the entire retina or optic nerve sections were acquired using an objective ×10 with a Z-stack. Shading correction was applied to each image and Z-stacks were flattened using maximum projections. Shading corrected and flattened images were stitched to generate images of the entire retina for quantification of RGC densities or of the longitudinal optic nerve sections for quantification of RGC axon regeneration and GFAP immunoreactivity.
RGC survival in retina flat mounts was quantified using ImageJ. In each quadrant of the retina, four fields (each measuring 500 µm × 500 µm with two fields 2 mm from the optic disc and two fields 1 mm from the optic disc) were specified. A total of 16 fields per eye were sampled. The densities of immuno-positive cells (cells per mm2) were averaged over all the fields in each retina and then averaged over all subjects in each experimental group.
RBPMS intensity from retina flat mounts was quantified using ImageJ. The mean pixel intensity from the 16 fields used to quantify RGC survival were averaged for each eye and then averaged over all subjects in each experimental group.
RGC axon regeneration in the optic nerve was quantified using ImageJ. GFAP immunoreactivity was used to delineate the proximal border of the optic nerve transection in each optic nerve section. Axon growth was quantified by counting the number of CTB-labeled axons that extended past the proximal lesion border. The total number of axons in each optic nerve was estimated using the following equation: total axon number = πr2 × (average number of axons/mm width)/t where r is the radius of the nerve width and t is the thickness of the section.
GFAP immunoreactivity in optic nerves was quantified using ImageJ. The proximal border of the optic nerve transection was demarcated based on GFAP immunoreactivity and an area within 100 µm proximal to the lesion border was defined in every optic nerve section. The mean pixel intensity was measured for each optic nerve and averaged for each experimental group.
The number of tdTomato-expressing NSC axons along the optic nerve was quantified using ImageJ. Lines spaced 500 µm apart starting from the distal border of the host/graft interface were superimposed on optic nerve images. The number of tdTomato positive fibers crossing each line were counted manually. The total number of tdTomato positive axons for each optic nerve was estimated using the formula: total axon number extending distance d = πr2 × (average number of axons/mm width)/t where d is the distance from the distal host/graft interface, r is the radius of the nerve width, and t is the thickness of the optic nerve section.
Statistical Analyses
Student's t-test was used to compare RGCs cocultured with NSCs in vitro to identify differences in neurite length and neurite extension between groups and to compare RGC axon growth following optic nerve transection in vivo. For experiments in which more than two conditions were compared, ANOVA with post-hoc Tukey test was performed. P < 0.05 was considered significant.
Results
Human NSCs Enhance RGC Survival and Outgrowth
The potential for human NSCs to increase the regenerative capacity of postnatal RGCs was assayed with RGCs directly cocultured with NSCs expressing tdTomato in vitro (Figs. 1A, 1B). RGCs cultured with NSCs extended neurites associated with clusters of NSCs. After two days in culture, RGCs directly cocultured with NSCs extended neurites that were 70% longer (P < 0.05) than RGCs cultured in isolation (Fig. 1C). The addition of NSCs to RGC cultures did not influence the percentage of RGCs with neurites (Fig. 1D). Thus NSCs exert a growth-promoting effect on RGC neurite outgrowth.
Figure 1.
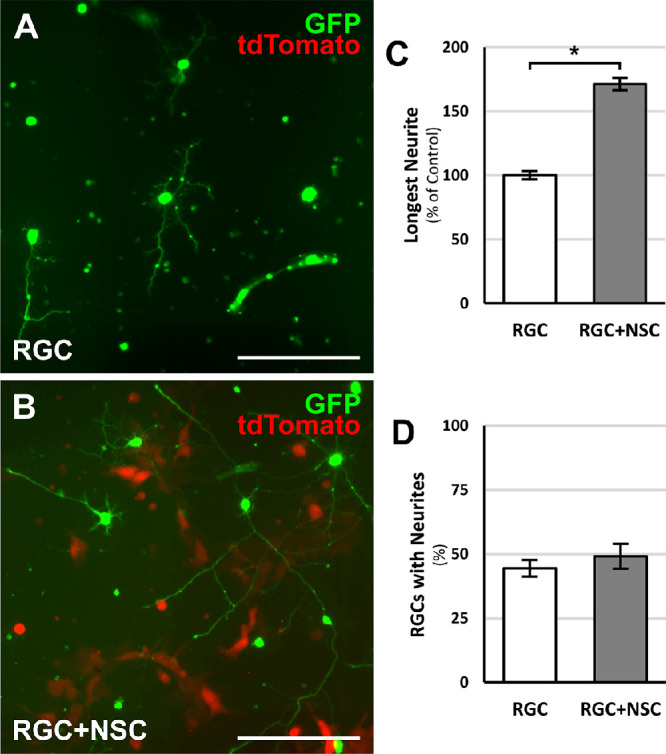
RGC-NSC direct cocultures promote RGC process outgrowth. Representative images of (A) GFP-positive RGCs cultured in isolation under standard conditions or (B) GFP-positive RGCs directly cocultured with tdTomato-expressing NSCs. (C) After two days in culture, an increase in the longest RGC process length was observed in RGCs directly cocultured with NSCs. (D) RGC-NSC direct cocultures did not change the percentage of RGCs with processes. *P < 0.05; Student's t-test. N = 4 experiments; n > 1000 cells/experiment; error bars represent SEM. Scale bars: 100 µm.
To examine whether NSC-derived, diffusible factors contributed to the RGC neurite growth promoting qualities of NSCs, RGCs were cultured in NSC-conditioned media (Figs. 2A, 2B). RGCs cultured with NSC-conditioned media comprising 10% of the growth media also extended neurites 70% longer (P < 0.05) than RGCs cultured in standard media (Fig. 2C). Furthermore, the neurite lengths of RGCs cultured in 10% NSC-conditioned media were comparable to RGCs cultured with BDNF and CNTF. With increasing concentrations of NSC-conditioned media, RGCs continued to demonstrate increased neurite outgrowth but at reduced magnitudes.
Figure 2.
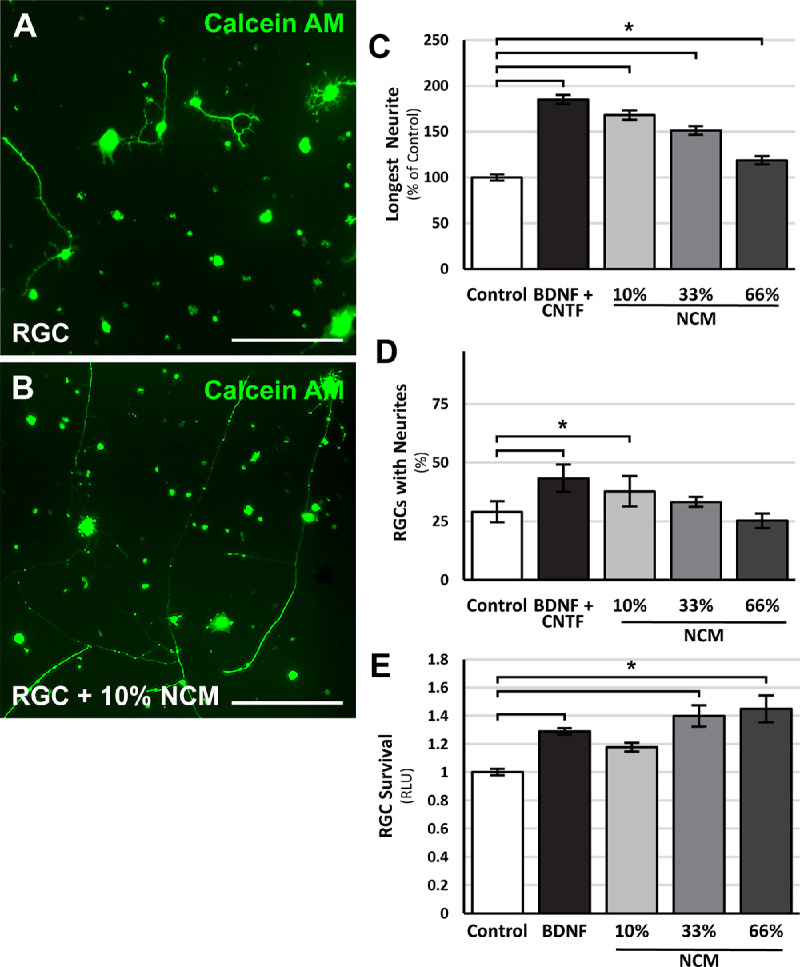
NSC-conditioned media promotes RGC survival and neurite outgrowth. Representative images of calcein AM-stained RGCs cultured in (A) standard RGC media or (B) 10% NSC-conditioned RGC media. RGCs cultured with BDNF and CNTF or increasing concentrations of NSC-conditioned media (NCM) demonstrated dose-dependent (C) increases in neurite outgrowth and (D) increases in the percentage of RGCs with neurite. (E) RGCs cultured with BDNF or increasing concentrations of NCM showed increase rates of survival. *P < 0.05; one-way ANOVA with post-hoc Tukey test. N = 4 experiments; n > 1000 cells/experiment; error bars represent SEM. Scale bars: 100 µm.
NSC-conditioned media also increased the percentage of RGCs with neurites at low concentrations, similar to the effects of BDNF and CNTF (Fig. 2D). At higher concentrations, the effect of NSC-conditioned media on the percentage of RGCs with neurites was reduced.
The effect of NSC-derived diffusible factors on RGC survival was also examined using a luminescence assay that is comparable to cell viability staining assays.2 RGCs cultured with NSC-conditioned media exhibited a modest but significant, dose-dependent increase in RGC survival compared to control RGCs (Fig. 2E). The effect of NSC-conditioned media on RGC survival was comparable to the effects of BDNF. These findings suggest that NSC-derived diffusible factors are partially responsible for NSC effects on RGC survival and neurite outgrowth.
Human NSCs Transplanted into the Transected Optic Nerve Promote RGC Survival and Axonal Growth into Grafted NSCs
To examine the effect of human NSCs on adult RGC survival and axon regeneration, we turned to the rat optic nerve transection model. Athymic, nude rats were used to avoid host rejection of a human NSC xenograft. Animals were subjected to a unilateral optic nerve transection and human NSCs were transplanted into the lesion site at the time of injury, providing NSCs access to the axonal compartment of injured RGCs.
The effect of optic nerve transplanted NSCs on RGC survival following axonal injury was evaluated by quantifying the number of RGC cell bodies in retina flat mounts from animals that underwent an optic nerve exposure only or optic nerve transection with or without an NSC graft. Optic nerve transection significantly reduced the density of surviving RGCs two weeks following injury (Figs. 3A–3D); only 10% of RGCs survived optic nerve transection in the absence of an NSC graft (1926.7 ± 345.6 RBPMS+ cells/mm2 vs. 199.2 ± 58.2 RBPMS+ cells/mm2, P < 0.05; Fig 3G). In the presence of an optic nerve NSC graft (Figs. 3E, 3F), RGC survival improved 2.5-fold to 25% (593.0 ± 164.7 RBPMS+ cells/mm2, P < 0.05; Fig 3G). Quantification of RBPMS staining intensity in RGCs as an indicator of cell viability did not demonstrate a significant difference among groups (P = 0.08; Fig. 3H). Thus optic nerve transplanted NSCs promote modest survival of adult RGCs in vivo after an acute optic nerve injury.
Figure 3.
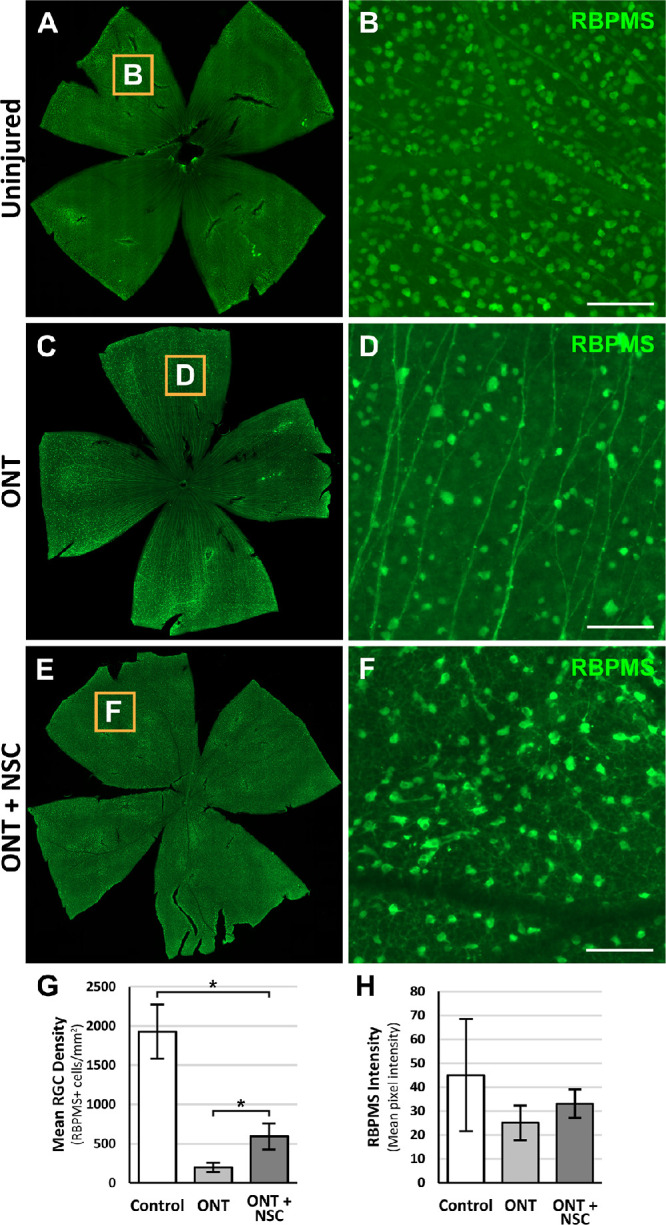
Optic nerve transplanted NSCs following ONT promote modest RGC survival. Representative retinal flat mounts stained for RBPMS and magnified insets from animals two weeks after receiving (A, B) no injury, (C, D) ONT, or (E, F) ONT with NSC graft. (G) Quantification of RGC survival demonstrates increased RGC densities with an optic nerve NSC graft compared to a ONT alone. (H) Quantification of RGC RBPMS intensity was similar among groups, suggestive of comparable cell viabilities. *P < 0.05; one-way ANOVA with post-hoc Tukey test. n = 6 animals per group; error bars represent SD. Scale bars: 100 µm. ONT, optic nerve transection.
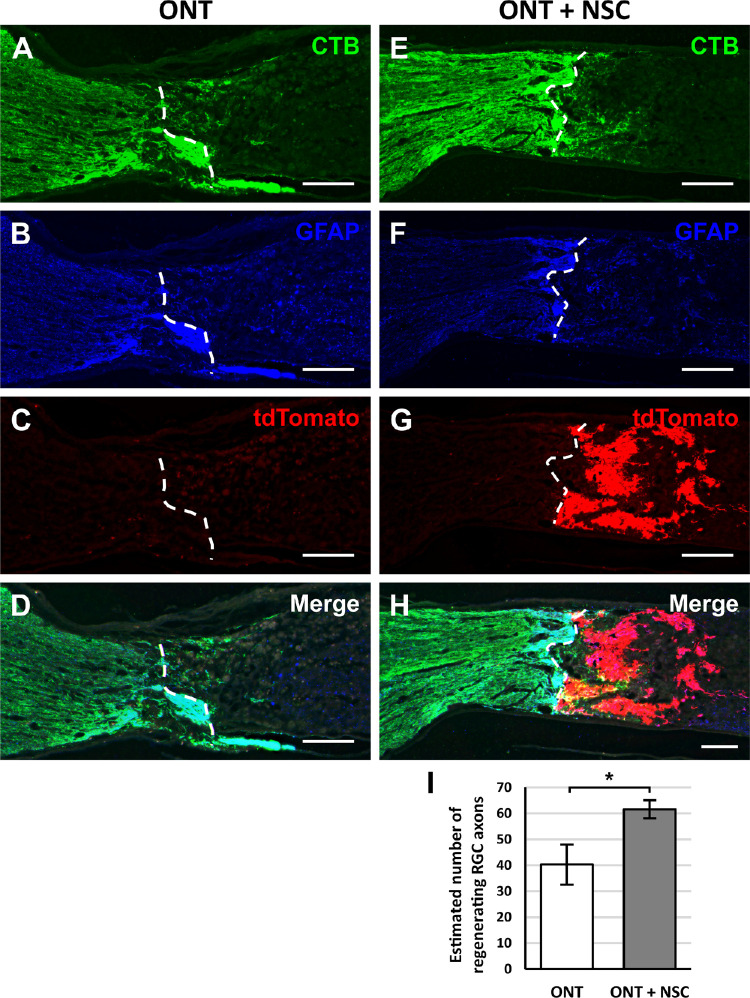
To determine whether optic nerve transplanted NSCs promote RGC axon regeneration in vivo, athymic, nude rats received an optic nerve transection with or without an NSC graft. RGC axons were labeled with intravitreal CTB injections two weeks after the optic nerve transection. The number of CTB-labeled RGC axons regenerating beyond the proximal transection border demarcated by GFAP immunolabeling were quantified (Figs. 4A–4H). The presence of an NSC graft significantly but modestly increased the number RGC axons regenerating beyond the proximal lesion border and into the lesion site by 1.5-fold compared to animals lacking NSC grafts (61.6 ± 8.5 axons vs. 40.3 ± 9.1 axons, P < 0.05, Fig. 4I). CTB-labeled RGC axons were not observed extending beyond the transection/graft site. Immunolabeling for GFAP immunoreactivity at the interface between the proximal optic nerve and the transection/graft site (Figs. 5A–5C) revealed a significant increase in GFAP immunoreactivity at the host-lesion junction in the presence of an NSC graft compared to an optic nerve transection without a cell graft (Fig. 5D). These findings suggest that NSCs are capable of promoting RGC axon growth into a lesion to a modest extent and that NSCs can modify the host tissue response to injury.
Figure 4.
Optic nerve transplanted NSCs following ONT promote modest, short-distance RGC axon regeneration. Representative images from longitudinal optic nerve sections that received an (A–D) optic nerve transection (ONT) or (E–H) an ONT with NSC graft. Dashed white lines demarcate the areas in which CTB labeling was quantified. (A, E) Intravitreal CTB injections label regenerated RGCs axons at the transection site. (B, F) Immunostaining for GFAP identifies the host tissue and was used to demarcate the proximal border of the transection (white line dotted). (C, D) NSCs constitutively express tdTomato to identify the location and extent of the cell graft. (D, H) Merged images demonstrate the localization of CTB-labeled axons, tdTomato NSCs, and GFAP borders. (I) Quantification of CTB-positive RGC axons beyond the proximal lesion border (dashed line) showed a modest increase in regenerating RGC axons when a NSC graft was present. *P < 0.05; Student's t-test. n = 6 animals per group; error bars represent SD. Scale bars: 200 µm. ONT, optic nerve transection.
Figure 5.
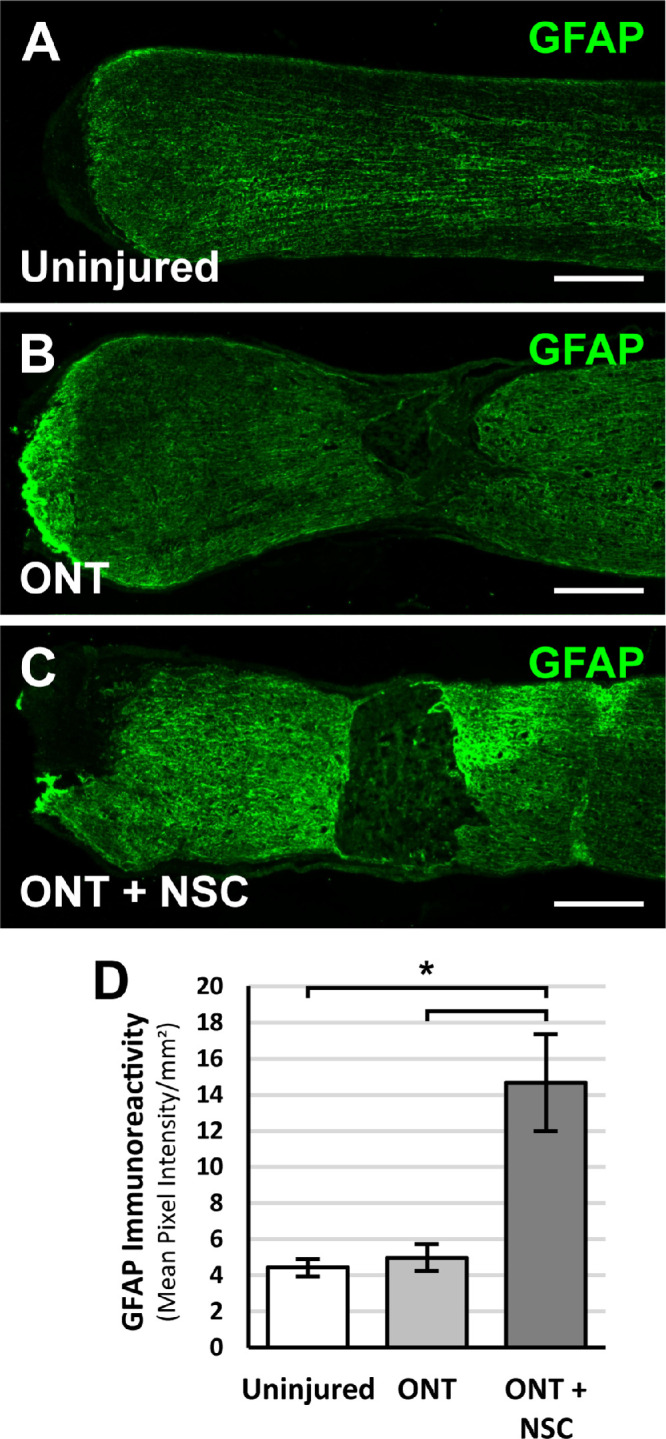
Optic nerve transplanted NSCs following ONT modify the host response following injury. Representative images from longitudinal optic nerve sections stained for GFAP 2 weeks after (A) unlesioned control, (B) ONT, or (C) ONT with NSC graft. (D) Quantification of GFAP immunoreactivity demonstrates increased GFAP immunoreactivity at the proximal transected optic nerve end with an NSC graft. P < 0.05; one-way ANOVA with post-hoc Tukey test. n = 6 animals per group; error bars represent SD. Scale bars: 200 µm. ONT, optic nerve transection.
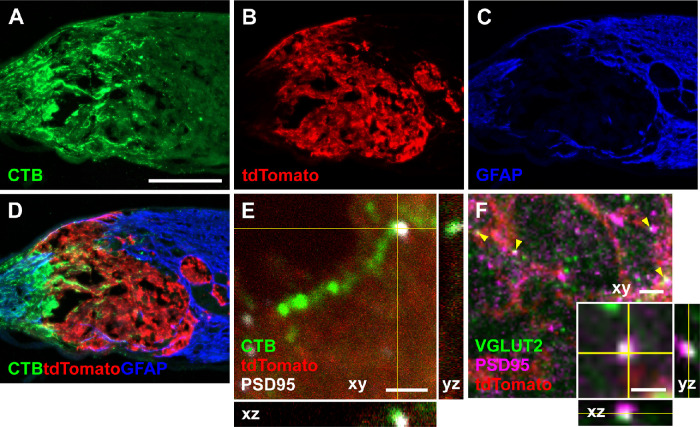
As part of an optic nerve neuronal relay, regenerating RGC axons must form synapses with optic nerve transplanted NSCs. To evaluate whether regenerating host RGC axons were capable of forming synapses with optic nerve transplanted NSCs, athymic, nude rats received an optic nerve transection and an optic nerve NSC graft. Two weeks after injury and grafting, regenerating RGC axons were labeled with CTB. Optic nerve sections were immunolabeled for CTB-positive axons (Fig. 6A) within the tdTomato-expressing NSC graft (Fig. 6B) at the optic nerve transection site (Figs. 6C, 6D) and the postsynaptic marker PSD-95 (Fig. 6E). Regenerating RGC axons could be observed in proximity to PSD-95, suggestive of synapses between host RGC axons and NSCs. Immunolabeling for VGLUT2 and PSD-95 within the NSC graft also demonstrated overlap of the presynaptic and postsynaptic markers (Fig. 6F), supporting the possibility of functional synapses. Thus one component of an optic nerve relay, synapses between RGCs and NSCs, are structurally possible.
Figure 6.
Optic nerve transplanted NSCs form synapses with transected RGC axons. (A–D) Longitudinal optic nerve section 2 weeks after optic nerve transection and grafting of tdTomato-expressing NSCs in the injury site. (A) Cholera toxin B (CTB) labeled RGC axons regenerate into the injury site. (B) NSCs expressing tdTomato transplanted into the transected optic nerve survive at the transection site. (C) GFAP labeling demarcates the glial response to injury and the boundaries of the optic nerve transection. (D) Merged images demonstrate CTB labeled RGC axons regenerating into the NSC graft. (E) Confocal image of a CTB-labeled RGC axon within the tdTomato-expressing NSC graft in proximity to the postsynaptic marker PSD-95, suggestive of synapses within the NSC graft. (F) Confocal image of VGLUT2 and PSD95 immunostaining within the tdTomato-expressing NSC graft demonstrating overlap of presynaptic and postsynaptic markers (yellow arrowheads); (F inset) high magnification of a puncta of VGLUT2 and PSD95 overlap. Scale bar: 200 µm (A–D); 2 µm (E, F); 1 µm (F inset).
Human NSCs Differentiate into Neurons and Integrate with the Transected Optic Nerve
Another component necessary for an optic nerve relay is the incorporation of grafted NSCs with the host visual system. To evaluate whether NSCs have the potential to integrate with the injured visual system as in spinal cord injury models, human NSCs grafted into the transected optic nerve were evaluated for phenotypic markers. Four weeks after transplantation, NSCs extended tdTomato-positive processes along the distal optic nerve (Figs. 7A–7D) and could be observed approaching the optic chiasm (Figs. 7E, 7F). The number of NSC-derived axons along the optic nerve continued to increase over the course of four weeks (Fig. 7G). NSC-derived projections were not observed extending toward the globe.
Figure 7.
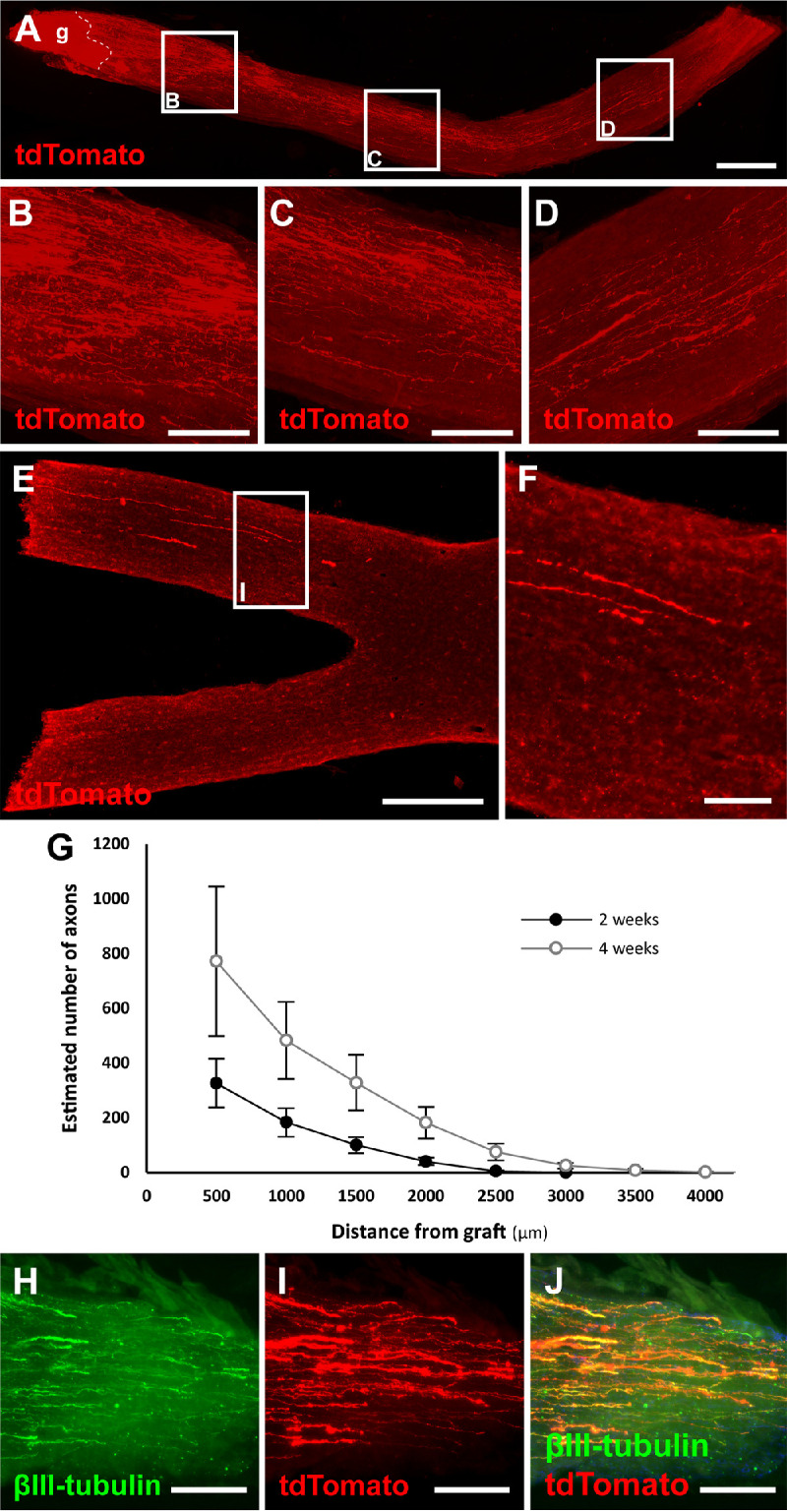
NSCs transplanted into an optic nerve transection survive, differentiate, and extend axons toward the optic chiasm after 4 weeks. (A) Longitudinal optic nerve section 4 weeks after transection (dashed line) and tdTomato expressing NSC graft (g) extending axons along the distal optic nerve. (B–D) Magnifications of insets from (A) demonstrate robust axonal extension along the length of the optic nerve. (E, F) After only four weeks, NSC-derived axons can be seen approaching the optic chiasm. (G) Quantification of the number of NSC graft-derived axons counted at specific distances from the graft site. n = 6 animals per group; error bars represent SEM. (H–J) NSC-derived processes colocalize with the neuronal marker βIII-tubulin. Scale bar: 500 µm (A); 100 µm (B–G); 500 µm (H); 100 µm (I).
NSCs have the potential to differentiate into glial or neuronal cells. Immunolabeling optic nerve grafted NSCs for the immature neuronal marker βIII-tubulin demonstrated colocalization with tdTomato-expressing NSCs processes (Figs. 7H–7J). Immunolabeling the NSC graft for NeuN to mark mature neurons did not demonstrate immunopositivity at this early time point (data not shown). Thus optic nerve–transplanted NSCs differentiate into neurons, extend axons into the distal optic nerve, and have the potential to integrate with the injured visual system.
Discussion
The present study demonstrates that human NSCs have the capacity to promote RGC survival and axon regeneration. NSC-derived, diffusible factors are partially responsible for these neuroprotective and neuroregenerative effects on RGCs. Additionally, NSCs have the potential to integrate with the injured visual system, receiving synaptic inputs from RGCs axons and providing proof of concept for a “neuronal relay” to restore retinofugal projections to downstream vision-associated targets. NSC-derived neuronal relays (Fig. 8) could be transformative for optic nerve regeneration by shifting the burden of long-distance axonal regeneration from mature RGC to neuronal stem cells with greater potential for axon growth and potentially enabling novel vision restorative therapies such as whole eye transplantation.
Figure 8.
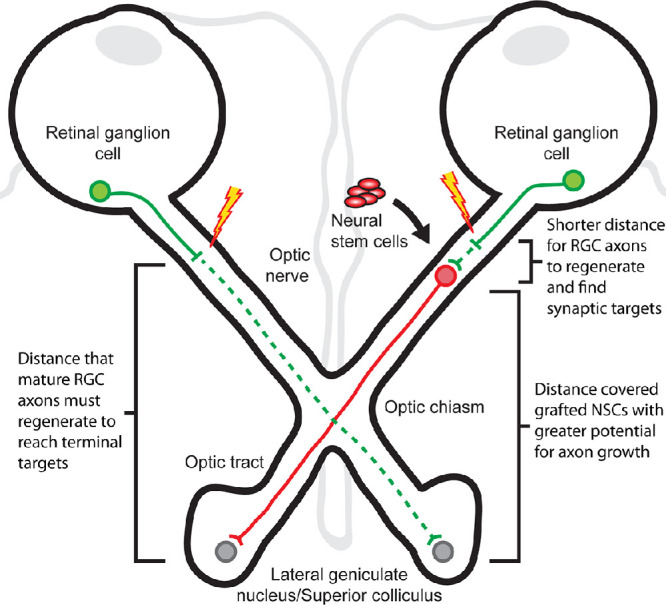
Schematic diagram of a NSC-derived optic nerve relay. (Left) Mature RGCs have a limited potential for axonal regeneration and, if axotomized, must regenerate a considerable distance (green dashed line) to reach targets in the lateral geniculate nucleus or superior colliculus. (Right) NSCs have a greater intrinsic potential for axon growth. When transplanted into the optic nerve to form an optic nerve relay, NSCs shorten the distance mature RGCs need to regenerate to reform synapses (green dashed line) and address the need for long-distance growth to reach terminal targets (red solid line).
This study contributes to existing knowledge of interactions between stem cells and neurons in the central nervous system. Marrow stromal cells have demonstrated neuroprotective effects on RGC survival in experimental glaucoma models.11 Induced pluripotent stem cells (iPSCs) confer neuroprotection on RGCs in vitro and enhance survival of intravitreally cotransplanted RGCs in vivo.6 In the injured spinal cord, NSCs grafted into the lesion site promote regeneration of corticospinal axons that are normally refractory to regenerative strategies.7,12 This study found that NSCs also confer modest neuroprotection and neuroregeneration effects to RGCs and RGC axons. Whether these effects are comparable among iPSCs, marrow stromal cells, and other cell types could be compared to identify the responsible factors and determine an ideal cell type for integration.
RGC survival following injury is critical for restorative therapies. The exact mechanism by which axotomized RGCs die remains unanswered. It has been proposed that axotomy deprives RGCs of retrograde neurotrophic signals from synaptic targets.13,14 Our study demonstrates that NSCs modestly improve the survival of RGCs in vitro and that the engraftment of NSCs at the injured axonal compartment also decreases, or at least delays, RGC death in vivo. This suggests that intervening on the axonal compartment is sufficient to provide a degree of RGC neuroprotection, likely by directly or indirectly providing neurotrophic support. Although the degree of RGC survival imparted by an NSC graft was significant, more than 70% of RGCs were still lost two weeks after injury. Therefore additional neuroprotective interventions are necessary to enhance RGC protection, possibly by combinatorial therapies that intervene on the cell soma through intravitreal approaches and along the retinofugal pathways to RGC axons.
Soluble NSC-derived factors were found to demonstrate dose-dependent neuroprotective and neuroregenerative effects on RGCs in vitro. RGCs cultured in low concentrations of NSC-conditioned media demonstrated enhanced neurite outgrowth. At higher concentrations of NSC-conditioned media, RGCs exhibited increased survival at the expense of neurite outgrowth. This finding is consistent with studies demonstrating that the cellular pathways regulating cell survival and axonal regeneration are distinct and, in some cases, antagonistic. Overexpressing Bcl-2 in RGCs specifically enhances RGC survival but does not promote axon regenerations.1 The deletion of dual leucine zipper kinase (DLK) significantly improves RGC survival after optic nerve injury but inhibits RGC axon regeneration in response to otherwise robust regenerative manipulations.15 Interestingly, inhibition of the germinal cell kinase four kinases that act upstream of DLK can improve neuronal survival without detrimentally affecting RGC axon regeneration, dissociating survival from regeneration.16 Therefore, because the degree of RGC axon regeneration promoted by optic nerve transplanted NSCs is modest and unlikely to be of functional significance without further augmentation, future studies that seek to form restorative neuronal relays should combine RGC intrinsic approaches that optimize both RGC survival and axon regeneration with optic nerve grafted NSCs. Examples of RGC intrinsic interventions with the potential to synergize with our proposed optic nerve relay strategy include combinatorial approaches that inhibit germinal cell kinase four kinases to enhance RGC survival while promoting RGC axon regeneration through the inhibition of phosphatase and tensin homology or suppressor of cytokine signaling 3 along with growth promoting factors such as CNTF and oncomodulin.17–20
Astrocytic scars have long been regarded as barriers to axon regrowth. In this study, the transplantation of NSCs into the optic nerve lesion site increased the levels of GFAP immunoreactivity at the proximal host-graft junction. Despite the increase in GFAP reactivity, NSC grafts increased the degree of RGC axonal growth into the lesion site beyond the area of glial activation. This raises the issue of whether a “glial scar,” or more accurately glial activation, really does prevent regeneration.21 Possible explanations for our findings are that the neuroregenerative effects exerted by the NSCs are sufficient to overcome the inhibitory effects of glial activation, either through the release of secreted factors or direct cellular interactions between NSCs and host tissue. Alternatively, NSCs may favor the induction of A2 astrocytes that are protective and upregulate neurotrophic factors instead of A1 astrocytes that are neurotoxic and cause neuronal death.22 The nature of the changes that NSCs induce on host tissue and methods to enhance NSC regenerative effects, possibly by supplementing transplants with neurotrophic factors, are additional items to be studied.
In spinal cord injury research, novel NSC approaches have achieved significant advancements in the field. NSCs directed toward spinal cord phenotypes dramatically promote the regeneration of corticospinal axons.7,23 Transplanted NSCs receive synaptic inputs from regenerating corticospinal axons and extend axons into host tissue to synapse with denervated neural circuitry.9,12,24,25 These NSC-derived neuronal relays between corticospinal axons and spinal neurons improve the recovery of motor function after spinal cord injury.8,26 We demonstrate in this study that NSCs transplanted into the lesioned optic nerve similarly extend axons at a maximal rate of up to 2 mm/week into host tissue, approaching the optic chiasm by four weeks. Additionally, NSCs promote the growth of RGC axons into the lesion and can receive synaptic inputs from RGC axons. These findings raise the possibility of establishing neuronal relays in the visual system. Given sufficient time for axonal extension and neuronal maturation, NSCs may form functional neuronal relays between RGC axons and retinofugal targets. The ability to reestablish retinofugal connections with a neuronal relay would be transformative, addressing the need for long-distance axon regeneration by injured adult RGC and potentially enabling vision restorative therapies such as whole eye transplants.
It is likely that the retinotopic organization and connectivity of axons from various RGC subtypes to terminal central targets must be correctly restored to perceive an image. Currently, robust survival and axon regeneration are only within the purview of alpha and intrinsically photosensitive RGCs, two of at least 40 different subtypes of RGCs conveying different aspects of vision in the mouse retina.27 Whether the visual information for other RGC subtypes can be preserved remains an area of ongoing investigation. Additionally, maintaining retinal topography is an obstacle that must be overcome in any strategy seeking to regenerate retinofugal pathways, be it by enhancing RGC intrinsic regenerative properties, directly replacing RGCs, or establishing neuronal relays.19,28 Whether spontaneous and accurate topographical axon guidance is retained, there is sufficient postchiasmal plasticity to make sense of disorganized visual inputs, or images can be digitally remapped to corresponding retinal locations to enable meaningful vision is an important question that must be addressed in future experiments.12,29 In the absence of techniques that preserve retinotopic connectivity, whole eye transplantation could still be beneficial to patients unable to perceive light by restoring the detection of luminance and synchronizing biological functions with circadian rhythms. However, as strategies are developed that further enhance survival of multiple RGC subtypes, promote axon regeneration, and accurately guide axon growth and synapse formation, whole eye transplants that enable functional vision may be a possibility.
This is the first study to address RGC survival and regeneration by applying stem cells specifically to the injured axonal compartment and to assess the integrative potential of NSCs transplanted in the optic nerve. RGC survival and axonal regeneration after injury is limited by the loss of target-derived neurotrophic factors, reduced cell-intrinsic regenerative potentials, and post-traumatic changes in the extracellular environment. Our study demonstrates that NSCs at the site of axonal injury are capable of supporting a modest degree of RGC survival and axon growth and altering the extracellular environment. A number of questions remain to be addressed. The exact mechanism by which NSCs exert neuroprotective and neuroregenerative effects are unclear and a potential area for further investigation and augmentation. Stem cell–derived neurotrophic factors and extracellular vesicles containing miRNA acting in a paracrine fashion have demonstrated effects on RGCs in rodent glaucoma models when introduced into the vitreous cavity.30 It is possible that NSCs transplanted in the optic nerve release similar diffusible factors that are able to act on the injured axon as opposed to the RGC soma. In addition, extended time courses are needed to determine whether the survival and regenerative effects of NSCs on RGCs are sustained or enhanced beyond four weeks. Future studies must also determine which RGC subtypes are capable of forming synapses with grafted NSCs and whether there is a preference for specific RGC subtypes. Furthermore, NSCs continue to undergo differentiation many months following transplantation and longer-term studies could elucidate the extent to which NSCs mature and integrate with synaptic targets in the lateral geniculate nucleus or superior colliculus. Another variable to consider is whether more terminally differentiated NSCs or NSCs derived from iPSCs possess a greater capacity to influence RGC survival and axon regeneration and to integrate with existing neural circuitry. Further refinement of this neuronal relay strategy could enable studies that seek to explore whether the injured visual system can accommodate new neuronal inputs, how to direct and target the growth of new axons in the visual system, and, ultimately, whether optic nerve relays can be used to make whole eye transplantations possible.
Acknowledgments
Supported in part by a K12 Career Development grant (5K12EY024225-04, National Eye Institute); a P30 core grant (P30EY022589, National Eye Institute); a Mentoring for the Advancement of Physician Scientists (American Glaucoma Society); Academic Senate Health Sciences Research Award (UCSD); Physician Scientist Award (Research to Prevent Blindness); Catalyst for a Cure Award (Glaucoma Research Foundation); and an unrestricted grant from Research to Prevent Blindness (New York, NY).
Disclosure: J.L. Do, None; S. Allahwerdy, None; R.C.C. David, None; R.N. Weinreb, Aerie Pharmaceuticals (C), Allergan (C), Bausch & Lomb (C), Eyenovia (C), Novartis (C); Heidelberg Engineering (F), Carl Zeiss Meditec (F), Konan (F), Optovue (F), Centervue (F); M.H. Tuszynski, None; D.S. Welsbie, Oriole Therapeutics (I), Oriole Therapeutics (C), Oriole Therapeutics (P)
References
- 1. Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002; 33: 689–702. [DOI] [PubMed] [Google Scholar]
- 2. Welsbie DS, Mitchell KL, Jaskula-Ranga V, et al.. Enhanced functional genomic screening identifies novel mediators of dual leucine zipper kinase-dependent injury signaling in neurons. Neuron. 2017; 94: 1142–1154.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bull ND, Johnson TV, Welsapar G, DeKorver NW, Tomarev SI, Martin KR.. Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies. Investig Ophthalmol Vis Sci. 2011; 52: 3309–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson T V., Dekorver NW, Levasseur VA, et al.. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014; 137: 503–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu P, Jones LL, Snyder EY, Tuszynski MH.. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003; 181: 115–129. [DOI] [PubMed] [Google Scholar]
- 6. Wu S, Chang K-C, Nahmou M, Goldberg JL.. Induced pluripotent stem cells promote retinal ganglion cell survival after transplant. Investig Opthalmology Vis Sci. 2018; 59: 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kadoya K, Lu P, Nguyen K, et al.. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016; 22: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu P, Wang Y, Graham L, et al.. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012; 150: 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu P, Woodruff G, Wang Y, et al.. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014; 83: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Do JL, Allahwerdy S, David RC, Weinreb RN, Welsbie DS.. Sheath-preserving optic nerve transection in rats to assess axon regeneration and interventions targeting the retinal ganglion cell axon. J Vis Exp. 2020; 163: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR.. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010; 51(4): 2051–2059, doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumamaru H, Lu P, Rosenzweig ES, Kadoya K, Tuszynski MH.. Regenerating Corticospinal Axons Innervate Phenotypically Appropriate Neurons within Neural Stem Cell Grafts. Cell Rep. 2019; 26: 2329–2339.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pearson HE, Stoffler DJ.. Retinal ganglion cell degeneration following loss of postsynaptic target neurons in the dorsal lateral geniculate nucleus of the adult cat. Exp Neurol. 1992; 116: 163–171. [DOI] [PubMed] [Google Scholar]
- 14. Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ.. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Investig Ophthalmol Vis Sci. 2000; 41: 764–774. [PubMed] [Google Scholar]
- 15. Watkins TA, Wang B, Huntwork-Rodriguez S, et al.. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci USA. 2013; 110: 4039–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel AK, Broyer RM, Lee CD, et al.. Inhibition of GCK-IV kinases dissociates cell death and axon regeneration in CNS neurons. Proc Natl Acad Sci. 2020; 117: 33597–33607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin Y, Henzl MT, Lorber B, et al.. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006; 9: 843–852. [DOI] [PubMed] [Google Scholar]
- 18. Smith PD, Sun F, Park KK, et al.. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009; 64: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park KK, Liu K, Hu Y, et al.. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008; 322(5903): 963–966, doi: 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D.. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009; 29: 14334–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson MA, Burda JE, Ren Y, et al.. Astrocyte scar formation aids CNS axon regeneration. Nature. 2016; 532(7598): 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liddelow SA, Guttenplan KA, Clarke LE, et al.. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017; 541(7638): 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumamaru H, Kadoya K, Adler AF, et al.. Generation and post-injury integration of human spinal cord neural stem cells. Nat Methods. 2018; 15: 723–731. [DOI] [PubMed] [Google Scholar]
- 24. Lu P, Ceto S, Wang Y, et al.. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J Clin Invest. 2017; 127: 3287–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adler AF, Lee-Kubli C, Kumamaru H, Kadoya K, Tuszynski MH.. Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem Cell Reports. 2017; 8: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenzweig ES, Brock JH, Lu P, et al.. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018; 24: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rheaume BA, Jereen A, Bolisetty M, et al.. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun. 2018; 9: 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL.. Transplanted neurons integrate into adult retinas and respond to light. Nat Commun. 2016; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dulin JN, Adler AF, Kumamaru H, et al.. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun. 2018; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mead B, Amaral J, Tomarev S.. Mesenchymal stem cell–derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Invest Opthalmol Vis Sci. 2018; 59: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]