Supplemental Digital Content is available in the text.
Keywords: athletes, COVID-19, myocarditis, return to sport, SARS-CoV-2
Background:
Cardiac involvement among hospitalized patients with severe coronavirus disease 2019 (COVID-19) is common and associated with adverse outcomes. This study aimed to determine the prevalence and clinical implications of COVID-19 cardiac involvement in young competitive athletes.
Methods:
In this prospective, multicenter, observational cohort study with data from 42 colleges and universities, we assessed the prevalence, clinical characteristics, and outcomes of COVID-19 cardiac involvement among collegiate athletes in the United States. Data were collected from September 1, 2020, to December 31, 2020. The primary outcome was the prevalence of definite, probable, or possible COVID-19 cardiac involvement based on imaging definitions adapted from the Updated Lake Louise Imaging Criteria. Secondary outcomes included the diagnostic yield of cardiac testing, predictors for cardiac involvement, and adverse cardiovascular events or hospitalizations.
Results:
Among 19 378 athletes tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, 3018 (mean age, 20 years [SD, 1 year]; 32% female) tested positive and underwent cardiac evaluation. A total of 2820 athletes underwent at least 1 element of cardiac triad testing (12-lead ECG, troponin, transthoracic echocardiography) followed by cardiac magnetic resonance imaging (CMR) if clinically indicated. In contrast, primary screening CMR was performed in 198 athletes. Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG (21 of 2999 [0.7%]), cardiac troponin (24 of 2719 [0.9%]), and transthoracic echocardiography (24 of 2556 [0.9%]). Definite, probable, or possible SARS-CoV-2 cardiac involvement was identified in 21 of 3018 (0.7%) athletes, including 15 of 2820 (0.5%) who underwent clinically indicated CMR (n=119) and 6 of 198 (3.0%) who underwent primary screening CMR. Accordingly, the diagnostic yield of CMR for SARS-CoV-2 cardiac involvement was 4.2 times higher for a clinically indicated CMR (15 of 119 [12.6%]) versus a primary screening CMR (6 of 198 [3.0%]). After adjustment for race and sex, predictors of SARS-CoV-2 cardiac involvement included cardiopulmonary symptoms (odds ratio, 3.1 [95% CI, 1.2, 7.7]) or at least 1 abnormal triad test result (odds ratio, 37.4 [95% CI, 13.3, 105.3]). Five (0.2%) athletes required hospitalization for noncardiac complications of COVID-19. During clinical surveillance (median follow-up, 113 days [interquartile range=90 146]), there was 1 (0.03%) adverse cardiac event, likely unrelated to SARS-CoV-2 infection.
Conclusions:
SARS-CoV-2 infection among young competitive athletes is associated with a low prevalence of cardiac involvement and a low risk of clinical events in short-term follow-up.
Clinical Perspective.
What Is New?
The Outcomes Registry for Cardiac Conditions in Athletes was created to investigate the prevalence and clinical outcomes of cardiovascular disease, including cardiac involvement after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, among young competitive athletes.
Our data indicate a low prevalence of cardiac involvement (0.5% to 3.0%) after SARS-CoV-2 infection among young competitive athletes undergoing return-to-play cardiac testing.
No adverse cardiac events related to SARS-CoV-2 infection have been observed among >3000 collegiate athletes during short-term clinical surveillance.
What Are the Clinical Implications?
The findings suggest that asymptomatic or mildly symptomatic athletes who have fully recovered from SARS-CoV-2 infection may return to sport without cardiac testing.
Cardiac evaluation inclusive of 12-lead ECG, troponin, and transthoracic echocardiography should be considered in athletes with moderate or cardiopulmonary symptoms during initial illness or on return to exercise.
Cardiac magnetic resonance imaging is most useful in athletes with high pretest probability for SARS-CoV-2 cardiac involvement as defined by the presence of cardiopulmonary symptoms or abnormalities on cardiac testing (ECG, troponin, transthoracic echocardiography); however, the significance of cardiac magnetic resonance imaging findings in the absence of symptoms remains unknown.
Editorial, see p 267
Cardiac involvement associated with adverse outcomes is common among hospitalized patients with severe coronavirus disease 2019 (COVID-19).1 Limited data exist on the prevalence and clinical relevance of cardiac involvement in nonhospitalized—and otherwise healthy—populations including young competitive athletes. Postviral myocarditis is a well-documented cause of sudden cardiac death during exercise.2–4 Concern for COVID-19 myocarditis contributed to the cancellation of organized sports and led to the development of expert consensus recommendations for the cardiac evaluation of athletes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.5–8
Observational data among middle-aged people recovering from SARS-CoV-2 infection document high rates of potential cardiac involvement (78%) as evidenced by elevated cardiac biomarkers and cardiac magnetic resonance imaging (CMR) abnormalities.9 Single-center observational cohort studies of collegiate athletes undergoing return-to-play (RTP) cardiac testing inclusive of CMR report highly variable rates of SARS-CoV-2 cardiac involvement (1.4% to 56%) using heterogeneous, nonstandardized definitions of disease.10–13 A subsequent study in professional athletes undergoing RTP cardiac testing reported a lower prevalence of cardiac involvement (0.6%) when using a testing strategy of 12-lead ECG, blood cardiac troponin assay, and transthoracic echocardiography (TTE), followed by CMR when clinically indicated.14 The prevalence, clinical correlates, and outcomes of SARS-CoV-2 cardiac involvement in competitive athletes are unclear.
The Outcomes Registry for Cardiac Conditions in Athletes was designed to address these issues. This registry collected data documenting cardiac diagnoses, noncardiac diagnoses, and attendant clinical outcomes among competitive collegiate athletes returning to organized athletics during the SARS-CoV-2 pandemic.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. This was a prospective observational cohort study. The primary aim was to determine the prevalence of cardiac involvement in collegiate athletes diagnosed with SARS-CoV-2 infection. Secondary aims were to assess the diagnostic yield of cardiac testing, identify risk factors for cardiac involvement, and determine the prevalence of adverse cardiac events or hospitalizations after SARS-CoV-2 infection in collegiate athletes. Deidentified data were collected from participating institutions from September 1, 2020, to December 31, 2020, inclusive of all athletes with previous SARS-CoV-2 infection. All aspects of this study were approved by the Massachusetts General Brigham Institutional Review Board (protocol 2020P002667).
Recruitment, Eligibility Criteria, and Data Collection
All National Collegiate Athletic Association institutions that implemented SARS-CoV-2 infection testing and performed cardiac preparticipation evaluations for athletes infected with SARS-CoV-2 were eligible. Athletes with confirmed SARS-CoV-2 infection by laboratory testing (polymerase chain reaction, antigen, or antibody) and who underwent local cardiovascular evaluation were included. Athletes were often tested for SARS-CoV-2 infection multiple times by participating institutions, and were included in this study if they had any positive SARS-CoV-2 test. Each athlete is only represented once in the registry based on the initial positive SARS-CoV-2 test. Evaluations included a clinical assessment and a range of cardiac testing as determined by the institution including 1 or more of the following: 12-lead ECG, cardiac troponin assay, TTE, and CMR. Triad testing refers to the combination of ECG, troponin, and TTE. Athletes were excluded if they had only a clinical assessment with no additional cardiac testing or if they had not yet started their cardiac evaluation.
The Massachusetts General Hospital served as the central data collection center. Each participating institution submitted data using a standardized data capture tool and deidentified original clinical reports of the cardiovascular testing performed. Raw imaging data for both TTE and CMR were not available to the study investigators and diagnoses were therefore adjudicated using original clinical reports. Submitted data collection spreadsheets were reviewed for completeness and accuracy before being transcribed into a central REDCap database. Further details on recruitment and data collection methods are provided in the Methods in the Data Supplement.
Key Definitions
Symptom severity was categorized using reported symptoms from the initial infection and the presence of any recurring symptoms with return to exercise as initially proposed for collegiate athletes.5 Prespecified symptom severity categories included asymptomatic, mild, moderate, and cardiopulmonary.5 Mild symptoms were defined as cough, fatigue, gastrointestinal symptoms (nausea, vomiting, or diarrhea), headache, anosmia, ageusia, rhinorrhea, sore throat, or nasopharyngeal congestion. Moderate symptoms were defined as the presence of COVID-19 toes or fingers, chills, fever, or myalgias. Cardiopulmonary symptoms were defined as chest pain, shortness of breath, palpitations, or exercise intolerance. If an athlete had symptoms in multiple categories, he or she was assigned the most severe category on the basis of the symptoms. ECGs were deemed normal or abnormal using international criteria for ECG interpretation in athletes.15 Abnormal ECG, TTE, and CMR reports were adjudicated as related to SARS-CoV-2 infection or unrelated to SARS-CoV-2 infection using prespecified definitions (Table I in the Data Supplement). A troponin measurement was considered abnormal if it was >99% upper limit of normal per the local laboratory commercial assay standards. Borderline abnormal triad testing was defined as any cardiac test that was considered abnormal by the local institution and led to CMR testing but did not meet prespecified study definitions after adjudication (Table I in the Data Supplement). These included detectable troponin level but not >99th percentile, nonspecific ECG abnormalities not fulfilling the international criteria, and TTE findings including abnormalities of unclear significance or those at the upper or lower limit of the normal range and considered to potentially represent pathology (ie, left ventricular ejection fraction 50%).
Study definitions were derived for both myocardial and pericardial involvement with imaging components adapted from the Updated Lake Louise Imaging Criteria (Table I in the Data Supplement).16 Definite myocardial involvement was defined as CMR T1 abnormality or presence of late gadolinium enhancement + T2 abnormality or CMR T2 abnormality + 1 or more supportive findings (left ventricular ejection fraction <45%, small or greater pericardial effusion, pericardial enhancement, or troponin >99% upper limit of normal). Probable myocardial involvement was defined as CMR T1 abnormality or presence of late gadolinium enhancement + 1 or more supportive findings (same as definite myocardial involvement). Possible myocardial involvement was defined as isolated CMR T1 abnormality or presence of late gadolinium enhancement. SARS-CoV-2 pericardial involvement was defined as a small or greater pericardial effusion or pericardial enhancement on CMR. Any athlete meeting criteria for myocardial involvement of SARS-CoV-2 infection who also had pericardial involvement was labeled as definite, probable, or possible myopericardial involvement based on the definitions for myocardial involvement.
Outcomes for this study included adverse cardiovascular events (new clinically significant arrhythmias, clinical heart failure, or sudden cardiac arrest or death) or hospitalizations related to SARS-CoV-2. Follow-up time was defined as the date of symptom onset or date of positive SARS-CoV-2 test if asymptomatic (or if symptoms were not reported) to the date of last cardiovascular outcomes update from each institution.
Statistical Analysis
Patient characteristics, SARS-CoV-2 symptoms, and cardiac testing results were described using basic descriptive methods including frequency distributions, means and SDs, and medians and interquartile ranges; 95% CIs for all prevalence estimates were calculated by exact binomial distribution. Because of the small number of observed SARS-CoV-2 cardiac cases and the known small sample bias with maximum likelihood estimation, the Firth penalized logistic regression method was used to assess unadjusted associations with definite, probable, and possible SARS-CoV-2–related cardiac involvement. A multivariable Firth logistic regression model using backwards variable selection was built to identify independent predictors of SARS-CoV-2 myocardial/pericardial involvement. The final model was determined on the basis of goodness of fit using Akaike information criteria. Odds ratios (ORs) and 95% CIs are reported. Secondary analyses modeling only definite and probable SARS-CoV-2–related cardiac involvement were also conducted using the same approach. Statistical analyses were performed using SAS (version 9.4; SAS Institutes) and R: A Language and Environment for Statistical Computing (R Core Team; https://www.R-project.org/).
Results
Study Population
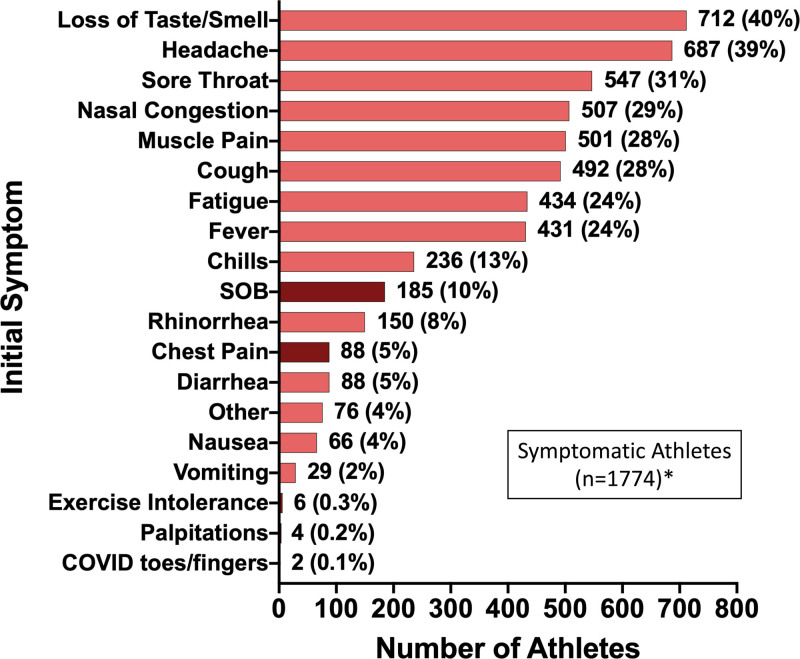
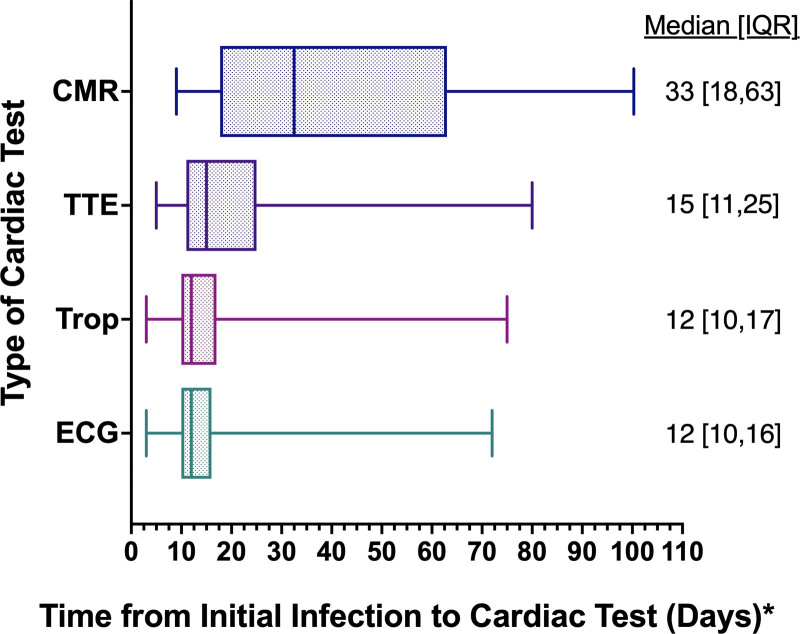
Among 19 378 athletes enrolled during the study period, 3384 (17.5%) tested positive for SARS-CoV-2 infection and 3018 (15.6%) met study inclusion criteria (Figure I in the Data Supplement). Baseline characteristics for the cohort and for the subgroup with SARS-CoV-2 cardiac involvement are presented in Table 1. The cohort, comprising athletes from 42 colleges or universities, includes representation from 26 distinct sporting disciplines including American-style football (36%), baseball (9%), cross country/track and field (8%), lacrosse (6%), and basketball (6%) (Table II in the Data Supplement). The majority of athletes were asymptomatic (33%) or had mild symptom burden (29%), most commonly accounted for by loss of taste or smell (40%), headache (39%), and sore throat (31%) during the acute phase of SARS-CoV-2 infection (Figure 1). Cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, or exercise intolerance) during the acute illness or on return to exercise were reported by 13% of athletes. The most commonly used (74%) cardiovascular testing protocol was triad testing (Table 1). A total of 198 athletes underwent CMR performed as part of the primary cardiovascular screening protocol irrespective of symptom burden or the results of other testing and 119 underwent CMR for clinical indications (Table III in the Data Supplement). The time from initial diagnosis of SARS-CoV-2 infection to the completion of each component of cardiac triad testing and CMR is shown in Figure 2. Abnormal triad testing possibly related to SARS-CoV-2 cardiac involvement was detected by TTE (24 of 2556 [0.9%]), 12-lead ECG (21 of 2999 [0.7%]), and cardiac troponin testing (24 of 2719 [0.9%]), including 5 athletes tested with high-sensitivity troponin I and the other 19 athletes tested with conventional troponin I or troponin T assays. Of athletes with abnormal triad testing possibly related to SARS-CoV-2, 65 had a single abnormal triad test, 2 athletes had 2 abnormal triad tests (12-lead ECG and TTE), and no athlete had abnormalities on all 3 triad tests.
Figure 1.
Prevalence of initial symptoms in athletes with symptomatic SARS-CoV-2 infection. Maroon bars indicate cardiopulmonary symptoms. *Athletes with known initial symptoms. COVID indicates coronavirus disease; SARS-COV-2, severe acute respiratory syndrome coronavirus 2; and SOB, shortness of breath.
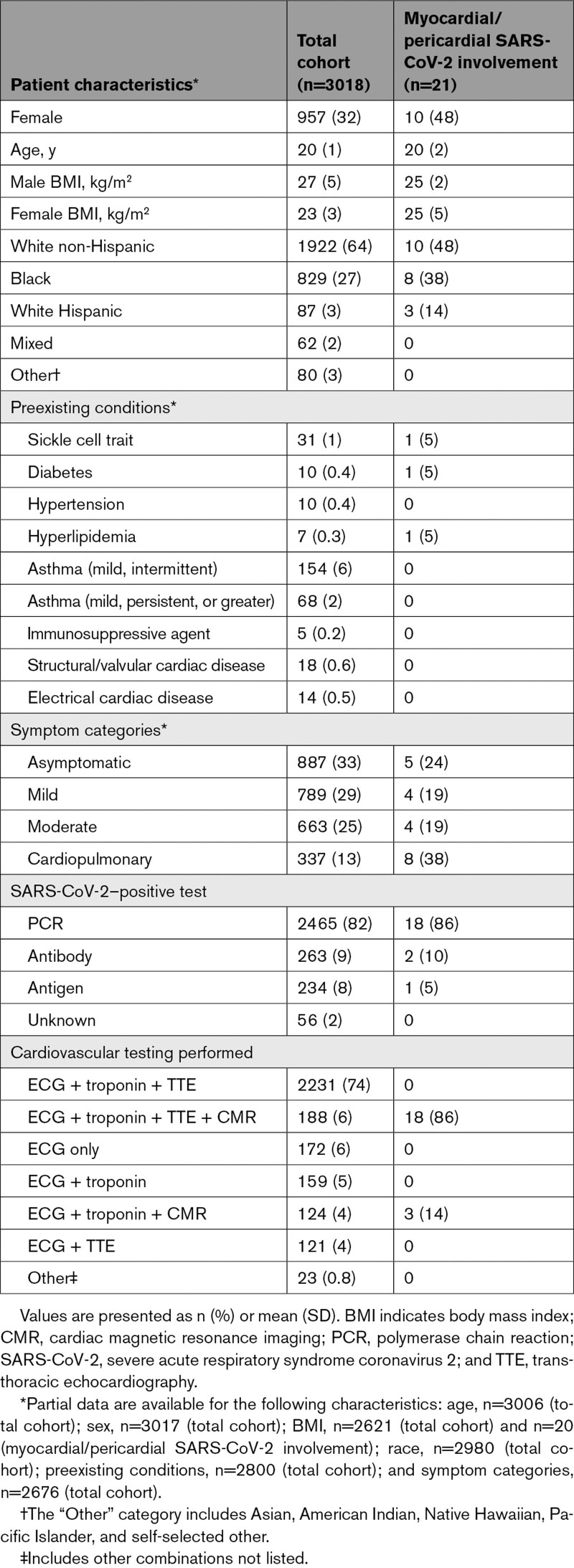
Table 1.
Patient Characteristics for the Total Cohort and for Athletes With Definite, Probable, or Possible Myocardial or Pericardial SARS-CoV-2 Involvement
Figure 2.
Time from initial infection to cardiac testing. *Time from initial infection calculated as the longest time from date of initial symptom onset or date of positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to date of cardiac test. The total athletes with known time frame were as follows: cardiac magnetic resonance imaging (CMR), n=302; ECG, n=2765; troponin (Trop), n=2537; and transthoracic echocardiography (TTE), n=2406. Midline is the median; box, interquartile range (IQR); whiskers, 95% CI.
Cardiac Involvement After SARS-CoV-2 Infection
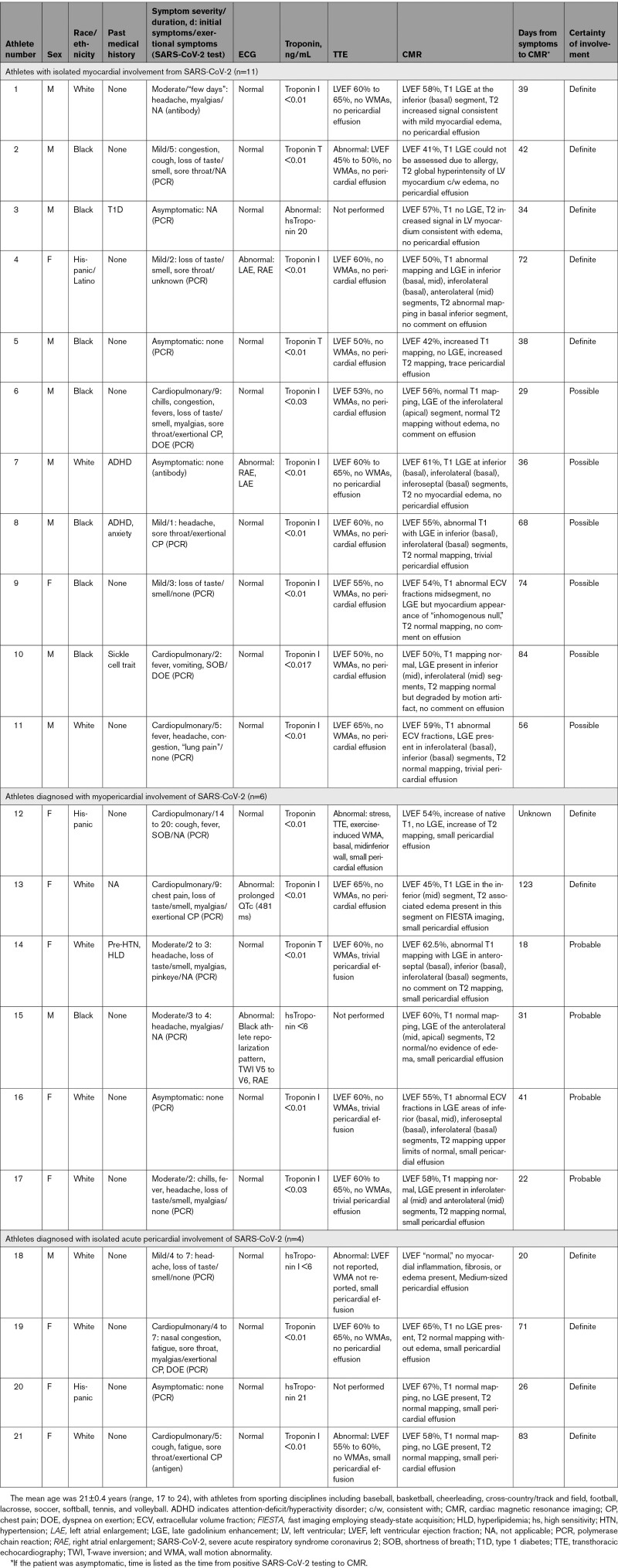
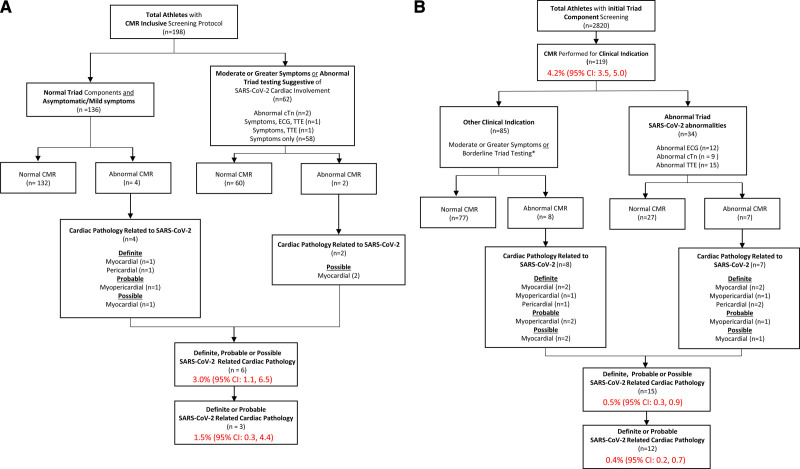
A total of 21 athletes (0.7% [95% CI, 0.4–1.1]) had definite, probable, or possible SARS-CoV-2 cardiac involvement (Table 2). This included 6 of 198 (3.0% [95% CI, 1.1–6.5]) athletes identified by primary screening CMR, of whom 3 (1.5% [95% CI, 0.3–4.4]) had definite or probable cardiac involvement (Figure 3A), and 15 of 2820 (0.5% [95% CI, 0.3–0.9]) athletes identified by 1 or more elements of cardiac triad testing or moderate or greater symptoms followed by clinically indicated CMR, of whom 12 (0.4% [95% CI, 0.2–0.7]) had definite or probable cardiac involvement (Figure 3B). For athletes in the clinically indicated CMR cohort with at least 1 abnormal triad test (n=34), 6 (17.7% [95% CI, 6.8–34.5]) had definite or probable cardiac involvement.
Table 2.
Clinical Characteristics and Cardiac Evaluations for Athletes With Myocardial or Pericardial Involvement From SARS-CoV-2 as Adjudicated by CMR
Figure 3.
Results of primary CMR versus clinically indicated CMR screening protocols. A, Athletes undergoing primary CMR screening protocol. B, Athletes undergoing clinically indicated CMR screening protocol. *Borderline triad testing as indication for CMR included detectable troponin level but not >99th percentile, nonspecific ECG abnormalities not fulfilling international criteria, and transthoracic echocardiogram (TTE) findings including abnormalities of unclear significance or those at the upper or lower limit of the normal range and considered to potentially represent pathology (ie, left ventricular ejection fraction 50%). CMR indicates cardiac magnetic resonance imaging; cTn, cardiac troponin; and SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
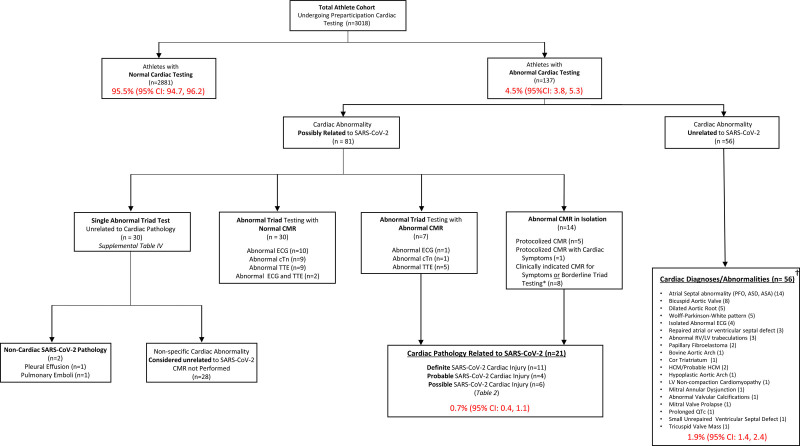
In the overall cohort, 137 of 3018 athletes (4.5% [95% CI, 3.8–5.3]) who underwent any form of cardiac screening had abnormal testing (Figure 4). Eighty-one (2.7% [95% CI, 2.1–3.3]) had abnormal cardiac testing possibly related to SARS-CoV-2 infection and 56 (1.9% [95% CI, 1.4–2.4]) had cardiac abnormalities unrelated to SARS-CoV-2 infection (a combination of known and new cardiac findings). Among athletes with 1 or more abnormal triad test results potentially related to SARS-CoV-2 (n=67), 37 underwent CMR, whereas the remaining 30 were ultimately deemed to have isolated testing abnormalities unrelated to SARS-CoV-2 (Table IV in the Data Supplement). Of the 37 athletes who underwent CMR for an abnormal triad test, only 2 (5.4%) had multiple abnormal tests (Figure 4). Fourteen athletes had normal triad testing but were found to have CMR abnormalities potentially related to SARS-CoV-2.
Figure 4.
Cardiovascular testing results among NCAA athletes after SARS-CoV-2 infection. *Borderline triad testing as indication for CMR included detectable troponin level but not >99th percentile, nonspecific ECG abnormalities not fulfilling international criteria, and TTE findings including abnormalities of unclear significance or those at the upper or lower limit of the normal range and considered to potentially represent pathology (ie, left ventricular ejection fraction 50%). †New and known diagnoses. ASA indicates atrial septal aneurysm; ASD, atrial septal defect; CMR, cardiac magnetic resonance imaging; cTn, cardiac troponin; HCM, hypertrophic cardiomyopathy; LV, left ventricular; NCAA, National Collegiate Athletic Association; PFO, patent foramen ovale; RV, right ventricular; SARS-COV-2, severe acute respiratory syndrome coronavirus 2; and TTE, transthoracic echocardiogram.
Predictors of SARS-CoV-2 Cardiac Involvement
Significant univariable predictors of definite, probable, or possible cardiac involvement included White Hispanic race (OR, 7.6 [95% CI, 2.2–26.1]), basketball participation (OR, 5.1 [95% CI, 1.8–14.5]), cardiopulmonary symptoms during acute infection or on resumption of exercise (OR, 4.2 [95% CI, 1.4–12.4]), and 1 or more abnormal triad test results (OR, 48.2 [95% CI, 18.5–125.6]) potentially related to SARS-CoV-2 infection (Table V in the Data Supplement). On multivariable analysis, after adjusting for sex and race, cardiopulmonary symptoms (OR, 3.1 [95% CI, 1.2–7.8]) and any abnormal triad test result (OR, 37.4 [95% CI, 13.3–105.3]) were predictive of cardiac involvement (Table VI in the Data Supplement).
Clinical Outcomes
No adverse cardiac events (median follow-up period, 130 days [interquartile range, 97–160]) were reported in athletes with definite, probable, or possible SARS-CoV-2 cardiac involvement (n=21). Among the entire cohort of athletes positive for SARS-CoV-2 (n=3018; median follow-up period, 113 days [interquartile range, 90–146]), we observed 1 adverse cardiac event (successfully resuscitated sudden cardiac arrest). This athlete underwent CMR 17 days after SARS-CoV-2 infection symptom onset without findings suggestive of acute cardiac involvement, rendering the cause of this event uncertain and likely unrelated to SARS-CoV-2. Ten athletes were either hospitalized (n=5) or treated in the emergency department (n=5) for noncardiac complications of SARS-CoV-2 infection. Noncardiac SARS-CoV-2 complications included submassive pulmonary embolism (n=1), symptomatic large pleural effusion (n=1), and palmar desquamation secondary to mild multisystem inflammatory syndrome (n=1).
Discussion
We report data characterizing collegiate athletes undergoing cardiovascular evaluation after SARS-CoV-2 infection with the following key findings. First, cardiac evaluation conducted in accordance with current recommendations,5,7 which suggest risk stratification based on SARS-CoV-2 symptom burden to determine the role of triad testing and CMR, had a diagnostic yield for definite, probable, or possible cardiac involvement of 0.5% (95% CI, 0.3–0.9). Second, the prevalence of definite, probable, or possible SARS-CoV-2 cardiac involvement, derived from the first multicenter cohort of competitive athletes undergoing screening CMR, was 3.0% (95% CI, 1.1–6.5). Our data suggest these represent the lower and upper estimates (0.5% to 3.0%) of cardiac involvement after SARS-CoV-2 infection in young otherwise healthy individuals. Third, clinical predictors of SARS-CoV-2 cardiac involvement include cardiopulmonary symptoms during acute infection or on return to exercise and any abnormality on triad testing suggestive of SARS-CoV-2 cardiac involvement. In this cohort, a stepwise approach that used the presence of moderate severity or cardiopulmonary symptoms or any abnormal triad test to trigger a diagnostic CMR would have identified 9 of 11 (81.8%) athletes diagnosed with definite or probable myocardial or myopericardial involvement. Last, no athlete diagnosed with definite, probable, or possible cardiac involvement had an adverse cardiac event through the follow-up period.
The prevalence and clinical implications of cardiac sequalae after SARS-CoV-2 infection among people not requiring hospitalization have yet to be determined. Defining disease prevalence is of paramount importance to understand diagnostic testing performance and inform recommendations for population-based screening. Among a German cohort recovering from SARS-CoV-2 infection (mean age, 49 years; 33% hospitalized for symptoms of SARS-CoV-2 infection), 78% were reported to have cardiac involvement as defined by abnormalities on CMR.9 Recent single-center cross-sectional studies of collegiate athletes undergoing screening CMR after SARS-CoV-2 infection have documented highly variable prevalence estimates of cardiac involvement, ranging from 1.4% to 56%.10–13 The basis for this heterogeneity is multifactorial with contributions from variable study designs, limited sample sizes, and inconsistent definitions of cardiac involvement. Findings from the present study, leveraging multicenter CMR data, and the use of strict and conservative definitions that adhere to contemporary clinical imaging criteria suggest that SARS-CoV-2 cardiac involvement among collegiate athletes is less common than previously reported. This finding is similar to the prevalence rate of cardiac involvement recently reported among professional athletes (0.6%) who were assessed using triad testing followed by clinically indicated CMR.14
CMR has emerged as the noninvasive reference standard for confirming myocarditis among patients with disease-specific symptoms or other cardiac testing suggestive of this diagnosis. Numerous factors including the paucity of normative CMR data in athletes, interreader interpretation variability, financial cost, and access to testing underlie uncertainties about the use of CMR as a primary screening tool. Our data highlight important tradeoffs pertaining to use of CMR as a screening modality versus its use to confirm cardiac involvement. Among athletes undergoing clinically indicated CMR based on symptom burden or an abnormal triad test (n=119), 12.6% (95% CI, 7.2–17.9) were diagnosed with definite, probable, or possible cardiac involvement. This diagnostic yield was 4-fold higher than that observed when CMR was used as part of a primary screening protocol (6 of 198 [3.0%; 95% CI, 1.1–6.5]). However, 3 of 6 of athletes with definite, probable, or possible cardiac involvement undergoing screening CMR were asymptomatic or had mild symptoms and normal triad testing. CMR detected isolated late gadolinium enhancement or abnormal T1 data, age indeterminant markers of myocardial fibrosis, or abnormal tissue architecture in a small number of athletes with no other features suggestive of acute SARS-CoV-2 cardiac involvement. Although we strongly suspect that these findings were unrelated to recent SARS-CoV-2 infection, we categorized these findings as “possible cardiac involvement,” underscoring the need to better understand their prevalence and clinical significance among young competitive athletes. In aggregate, these issues illustrate the inherent tradeoff between a CMR-inclusive screening protocol that may enhance sensitivity and a clinically driven approach that maximizes specificity.
Autopsy series have established myocarditis as an important cause of sudden death during exercise.2–4 Before the SARS-CoV-2 pandemic, myocarditis was routinely diagnosed among patients presenting with postviral cardiovascular symptoms, malignant arrhythmias, or myocardial dysfunction. Diagnostic CMR criteria and sport eligibility recommendations were developed on the basis of considerable experience with this clinical presentation.16–18 It remains unclear whether isolated cardiac abnormalities detected through CMR-based screening harbor a risk similar to typical clinically diagnosed myocarditis and how often these findings occur in other viral infections. Our SARS-CoV-2 cardiac involvement prevalence estimate is similar to documented prevalence rates of myocarditis after influenza infection.19 Although many collegiate athletes in this cohort did not undergo CMR as part of their RTP screening algorithm, we observed no adverse cardiac events in this group. Whereas longer follow-up is of critical importance, our data suggest that the potential for subclinical cardiac involvement characterized by isolated CMR abnormalities is of low short-term clinical risk.
There are several limitations of this study. First, screening protocol heterogeneity across participating institutions may have affected our estimates of prevalence and diagnostic yield. Nonetheless, this study examines the largest number of athletes who have undergone some form of cardiac testing after SARS-CoV-2 infection (n=3018) and the largest multicenter CMR-inclusive screening cohort (n=198) of competitive athletes to date. Second, diagnostic testing results reported for both TTE and CMR were extracted from the primary clinical reports provided by participating institutions. Whereas this approach captures the actual clinical experience of our cohort, the unblinded clinical interpretation of the testing may have resulted in a degree of observer bias on the part of the interpreting physician. Unbiased imaging adjudication by a centralized core facility represents an important area of future work. Third, there is no uniformly accepted definition of cardiac involvement attributable to SARS-CoV-2 infection. We therefore used fundamental components of the Updated Lake Louise Imaging Criteria16 to generate de novo definitions of possible, probable, and definite cardiac involvement and presented full clinical profiles of athletes meeting these criteria. Future work is warranted to validate this approach. Last, we acknowledge the relatively short duration of our clinical follow-up and underscore the need for ongoing clinical surveillance of this cohort. However, the median of 113 days (interquartile range, 90–146) follow-up captured coincides with the period of highest risk of adverse events during exercise after acute myocardial injury.
Implications for RTP SARS-CoV-2 Cardiac Testing
Findings from this study provide an opportunity to assess and refine consensus recommendations for preparticipation cardiac screening of athletes after SARS-CoV-2 infection.5,7 Given the low prevalence of cardiac involvement and the absence of adverse events during short-term clinical surveillance related to SARS-CoV-2 infection among both collegiate and professional athletes,14 we propose the following considerations regarding future RTP cardiac testing after SARS-CoV-2 infection. First, our data support that athletes with asymptomatic or mild symptom burden from acute SARS-CoV-2 infection do not require additional cardiac testing before resumption of organized athletics. Second, triad testing in the form of a 12-lead ECG, cardiac troponin, and TTE should be considered among athletes after acute SARS-CoV-2 infection who experience moderate systemic or cardiopulmonary symptoms, with the presence of cardiopulmonary symptoms representing a particularly important marker of risk. Last, our data suggest that the diagnostic yield of CMR will be optimized by confining its use to athletes presenting with increased clinical pretest probability of acquired myocardial pathology as defined by the presence of SARS-CoV-2–related cardiopulmonary symptoms or abnormalities on triad testing. These recommendations are generally consistent with the most recent RTP cardiac testing guidelines.
Conclusion
In a large prospective registry of collegiate athletes, SARS-CoV-2 infection occurred in 17.5% of athletes. Clinical evaluation revealed a low prevalence (0.5% to 3.0%) of definite, probable, or possible SARS-CoV-2 cardiac involvement and a low risk of adverse cardiac events during short-term follow-up. The clinical relevance of cardiac testing abnormalities after SARS-CoV-2 infection among athletes without other clinical features of myocardial involvement requires additional study. Future studies with extended clinical follow-up and appropriate control populations including athletes without SARS-CoV-2 infection are needed to better inform risk and the refinement of evidence-based screening strategies.
Acknowledgments
The authors thank the collaborators for the Outcomes Registry for Cardiac Conditions in Athletes registry.
Sources of Funding
This work was funded in part by a grant from the American Medical Society for Sports Medicine Foundation and American Medical Society for Sports Medicine Foundation Collaborative Research Network. Dr Moulson is supported by the University of British Columbia Clinician Investigator Program.
Disclosures
Dr Patel reports advisory board participation from Amgen, Bayer, Janssen, Heartflow, and Medscape; grant funding from National Heart, Lung, and Blood Institute, Bayer, Janssen, Heartflow, and Idorsia; and support from the Joel Cournette Foundation for research on athlete’s hearts. Dr. Baggish has received funding from the National Institute of Health/National Heart, Lung, and Blood Institute, the National Football Players Association, and the American Heart Association and receives compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University.
Supplemental Materials
Methods
Data Supplement Figure I
Data Supplement Tables I–VI
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CMR
- cardiac magnetic resonance imaging
- COVID-19
- coronavirus disease 2019
- OR
- odds ratio
- RTP
- return to play
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- TTE
- transthoracic echocardiography
N. Moulson and B.J. Petek contributed equally.
A complete list of the members of the Outcomes Registry for Cardiac Conditions in Athletes Study Group is provided in the Data Supplement.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
This work was presented as an abstract at the American Medical Society for Sports Medicine annual meeting, April 13–18, 2021.
The Data Supplement, podcast, and transcript are available with this article at www.ahajournals.org/doi/suppl/10.1161/circulationaha.121.054824.
For Sources of Funding and Disclosures, see page 265.
Contributor Information
Nathaniel Moulson, Email: NMOULSON@mgh.harvard.edu.
Bradley J. Petek, Email: bpetek@partners.org.
Jonathan A. Drezner, Email: jdrezner@uw.edu.
Kimberly G. Harmon, Email: kharmon@u.washington.edu.
Stephanie A. Kliethermes, Email: Kliethermes@ortho.wisc.edu.
Manesh R. Patel, Email: manesh.patel@duke.edu.
Collaborators: Irfan M. Asif, James Borchers, Katherine M. Edenfield, Michael S. Emery, Kyle Goerl, Brian Hainline, Jonathan H. Kim, William E. Kraus, Rachel Lampert, Matthew Leiszler, Benjamin D. Levine, Matthew W. Martinez, Francis G. O’Connor, Dermot Phelan, Lawrence D. Rink, Herman A. Taylor, Carl Ade, Aryan Aiyer, Jarrah Alfadhli, Chloe Amaradio, Scott Anderson, Stephanie Arlis-Mayor, Jonathan S. Aubry, Andrea Austin, Timothy Beaver, Nicolas Benitez, Brant Berkstresser, Thomas M. Best, Tiffany Bohon, Jonathan P. Bonnet, Elizabeth Boyington, James Bray, Jenna Bryant, Sean Carnahan, Rachel Chamberlain, Samantha Charters, Timothy W. Churchill, Douglas Comeau, Laura E. Cook, Deanna Corey, Amy Costa, Marshall Crowther, Tarun Dalia, Craig Davidson, Kaitlin Davitt, Annabelle De St Maurice, Peter N. Dean, Katelyn DeZenzo, Courtney Dimitris, Jeanne Doperak, Calvin Duffaut, Craig Fafara, Katherine Fahy, Jason Ferderber, Megan Finn, Angelo Galante, Todd Gerlt, Amy Gest, Carla Gilson, Jeffrey Goldberger, Joshua Goldman, Erich Groezinger, Jonathan R. Guin, Heather Halseth, Joshua Hare, Beth Harness, Nicolas Hatamiya, Julie Haylett, Neal Hazen, Yeun Hiroi, Amy Hockenbrock, Amanda Honsvall, Jennifer Hopp, Julia Howard, Samantha Huba, Mustafa Husaini, Lindsay Huston, Calvin Hwang, Laura Irvin, Val Gene Iven, Robert Jones, Donald Joyce, Kristine Karlson, Christian Klein, Chris Klenck, Michele Kirk, Jordan Knight, Laura Knippa, Madeleine Knutson, Louis E. Kovacs, Yumi Kuscher, Andrea Kussman, Chrissy Landreth, Amy Leu, Dylan Lothian, Maureen Lowery, Andrew Lukjanczuk, John M. MacKnight, Lawrence M. Magee, Marja-Liisa Magnuson, Aaron V. Mares, Anne Marquez, Grant McKinley, Megan Meier, Christopher Miles, Emily Miller, Hannah Miller, Raul Mitrani, Robert J. Myerburg, Greg Mytyk, Andrew Narver, Aurelia Nattiv, Laika Nur, Brooke E. Organ, Meredith Pendergast, Frank A. Pettrone, Sourav K. Poddar, Diana Priestman, Ian Quinn, Fred Reifsteck, Morgan Restivo, James B. Robinson, Ryan Roe, Thomas Rosamond, Carrie Rubertino Shearer, Miguel Rueda, Takamasa Sakamoto, Brock Schnebel, Ankit B. Shah, Alan Shahtaji, Kevin Shannon, Polly Sheridan-Young, Siobhan M. Statuta, Mark Stovak, Andrei Tarsici, Kenneth S. Taylor, Kim Terrell, Matt Thomason, Jason Tso, Daniel Vigil, Francis Wang, Jennifer Winningham, and Susanna T. Zorn
References
- 1.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation. 2020; 141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 2.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004; 141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005 [DOI] [PubMed] [Google Scholar]
- 3.Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, Zigman ML, Ellenbogen R, Rao AL, Ackerman MJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. 2015; 132:10–19. doi: 10.1161/CIRCULATIONAHA.115.015431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson DF, Kucera K, Thomas LC, Maleszewski J, Siebert D, Lopez-Anderson M, Zigman M, Schattenkerk J, Harmon KG, Drezner JA. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: a 4-year prospective study [published online March 30, 2020]. Br J Sports Med. doi: 10.1136/bjsports-2020-102666. https://bjsm.bmj.com/content/early/2021/05/09/bjsports-2020-102666.info [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Medical Society for Sports Medicine and the American College of Cardiology. Cardiac Considerations for College Student-Athletes During the COVID-19 Pandemic. Published 2020. https://www.amssm.org/Content/pdf-files/COVID19/NCAA-COVID19/NCAA-COVID-19-Algorithm-12-AUG-2020.pdf
- 6.Baggish A, Drezner JA, Kim J, Martinez M, Prutkin JM. Resurgence of sport in the wake of COVID-19: cardiac considerations in competitive athletes. Br J Sports Med. 2020; 54:1130–1131. doi: 10.1136/bjsports-2020-102516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, Thompson PD, Baggish AL. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021; 6:219–227. doi: 10.1001/jamacardio.2020.5890 [DOI] [PubMed] [Google Scholar]
- 8.Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020; 5:1085–1086. doi: 10.1001/jamacardio.2020.2136 [DOI] [PubMed] [Google Scholar]
- 9.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5:1265–1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, Seetharam K, Riveros D, Beto RJ, II, Balla S, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021; 14:541–555. doi: 10.1016/j.jcmg.2020.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, Slaughter JC, Fitch W, Hughes SG, Soslow JH. COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR). Circulation. 2021; 143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021; 6:116–118. doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, Purtell CS, Schiebler ML, Reeder SB. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging [published online January 14, 2021]. JAMA Cardiol. doi: 10.1001/jamacardio.2020.7444. https://jamanetwork.com/journals/jamacardiology/fullarticle/2775372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening [published online March 4, 2021]. JAMA Cardiol. doi: 10.1001/jamacardio.2021.0565. https://jamanetwork.com/journals/jamacardiology/fullarticle/2777308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drezner JA, Sharma S, Baggish A, Papadakis M, Wilson MG, Prutkin JM, Gerche A, Ackerman MJ, Borjesson M, Salerno JC, et al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med. 2017; 51:704–731. doi: 10.1136/bjsports-2016-097331 [DOI] [PubMed] [Google Scholar]
- 16.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018; 72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 17.Maron BJ, Zipes DP, Kovacs RJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: preamble, principles, and general considerations: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015; 66:2343–2349. doi: 10.1016/j.jacc.2015.09.032 [DOI] [PubMed] [Google Scholar]
- 18.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, et al. ; ESC Scientific Document Group. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021; 42:17–96. doi: 10.1093/eurheartj/ehaa605 [DOI] [PubMed] [Google Scholar]
- 19.Datta R, Helou E, Tucker M, John B, Martinello RA, Malinis M. Detection of influenza myocarditis using national healthcare safety network surveillance definitions accounting for fever in older adults. Infect Control Hosp Epidemiol. 2018; 39:1145–1147. doi: 10.1017/ice.2018.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.