Abstract
Simple Summary
In mammals, imprinted genes play key roles in embryonic growth and postnatal behavior and their misregulation has been associated with several developmental syndrome and cancers. At present, more than 150 imprinted genes have been identified in humans and mice. Cattle is an economically important farm animal, but compared to humans and mice, few genes have been reported as imprinted in this species. MKRN3, MAGEL2 and NDN are three maternally imprinted genes in the human Prader-Willi and Angelman syndromes imprinted locus at 15q11-q13. In this study, we determined that the bovine MKRN3, MAGEL2 and NDN genes are three paternally expressed gene, and their expression is regulated by the DNA methylation. This work could advance the genomic imprinting by increasing the knowledge of imprinted genes in bovine and also facilitate future studies to detect the physiological roles of MKRN3, MAGEL2 and NDN genes.
Abstract
Genomic imprinting is the epigenetic mechanism of transcriptional regulation that involves differential DNA methylation modification. Comparative analysis of imprinted genes between species can help us to investigate the biological significance and regulatory mechanisms of genomic imprinting. MKRN3, MAGEL2 and NDN are three maternally imprinted genes identified in the human PWS/AS imprinted locus. This study aimed to assess the allelic expression of MKRN3, MAGEL2 and NDN and to examine the differentially methylated regions (DMRs) of bovine PWS/AS imprinted domains. An expressed single-nucleotide polymorphism (SNP)-based approach was used to investigate the allelic expression of MKRN3, MAGEL2 and NDN genes in bovine adult tissues and placenta. Consistent with the expression in humans and mice, we found that the MKRN3, MAGEL2 and NDN genes exhibit monoallelic expression in bovine somatic tissues and the paternal allele expressed in the bovine placenta. Three DMRs, PWS-IC, MKRN3 and NDN DMR, were identified in the bovine PWS/AS imprinted region by analysis of the DNA methylation status in bovine tissues using the bisulfite sequencing method and were located in the promoter and exon 1 of the SNRPN gene, NDN promoter and 5’ untranslated region (5’UTR) of MKRN3 gene, respectively. The PWS-IC DMR is a primary DMR inherited from the male or female gamete, but NDN and MKRN3 DMR are secondary DMRs that occurred after fertilization by examining the methylation status in gametes.
Keywords: cow, MKRN3 gene, MAGEL2 gene, NDN gene, imprinting, DNA methylation, DMR
1. Introduction
Genomic imprinting is a phenomenon in which a subset of mammalian genes is completely or preferentially expressed from maternally or paternally inherited alleles. Over 100 imprinted genes cluster in 20–25 chromosomal regions in the human and mouse genomes and include both protein-coding and noncoding RNA genes in each imprinted cluster [1]. Compared with humans and mice, fewer imprinted genes have been identified in cattle. Imprinted genes play key roles in both pre- and postnatal development. In humans, aberrant imprinting is responsible for certain congenital syndromes [2], while perturbed imprinting is involved in cancers [3].
Necdin (NDN), melanoma antigen-like gene 2 (MAGEL2) and makorin ring finger protein 3 (MKRN3) are three paternally expressed genes located at the proximal end of the PWS/AS imprinted region, one of the best-studied imprinted clusters on human chromosome 15q11-q13. This imprinted cluster is associated with two human neurobehavioral disorders, Prader-Willi syndrome (PWS) and Angelman syndrome (AS). PWS is thought to arise from the loss of multiple paternal gene expression within the segment from MKRN3 to the small nucleolar RNA (snoRNA) genes [4,5]. In contrast, loss of the maternally inherited copy of the UBE3A gene (E6-AP) leads to AS [6,7].
The cis-acting element regulating monoallelic gene expression in imprinted clusters is known as imprinting control regions (ICRs). The differential DNA methylation regions (DMRs) at CpG-rich ICRs are inherited from germline DMRs (gDMRs), which typically regulate the monoallelic expression of imprinted genes [8,9]. Apart from gDMRs, additional second DMRs are subsequently acquired after the implantation stages in response to the gDMR, which are also important for the imprinted expression of a certain gene in the cluster [10]. There are three important maternally methylated DMRs in the human PWS/AS imprinted region: a germline DMR of the PWS imprinting center (PWS-IC) [11] and somatic NDN [12] and MKRN3 DMRs [13]. PWS-IC serves as the master regulator of imprinting of the PWS/AS region and hierarchically controls NDN and MKRN3 DMRs in somatic tissues [14,15]. The homologous region of the human PWS/AS domain is on mouse chromosome 7C [10]. The regulatory mechanisms of genomic imprinting and genes hosted in the PWS/AS region are exquisitely maintained between mice and humans. Comparative genomics is an important way to identify functional DNA elements to regulate the expression of imprinted gene in a locus [16]. Here, we were interested in investigating the imprinting status of the cow NDN, MAGEL2 and MKRN3 genes and in identifying the three maternally methylated DMRs in the bovine PWS/AS imprinted region.
2. Materials and Methods
2.1. Tissue and Placental Sample Collection
Tissue samples, including heart, liver, spleen, lung, kidney, muscle, fat and brain, of 34 female Holstein cows were collected from a local abattoir. A total of 30 placental tissues of Holstein cows were collected immediately after spontaneous delivery from local cattle farm. For each placenta, the corresponding maternal whole blood and paternal sperm from five bulls used for artificial insemination were also collected to identify the parental genotype. All samples were immediately frozen in liquid nitrogen after collection for further DNA or RNA extraction. The procedures of using animals were approved by the Agriculture Research Animal Care Committee of Hebei Agriculture University. All animal experiments of this study were approved by the Agriculture Research Animal Care Committee of Hebei Agricultural University (No. 14021).
2.2. DNA Extraction and PCR Amplification for Identification of SNPs
Genomic DNA was extracted from the liver tissues of adult cattle, placentas, and their corresponding mother’s whole blood and father’s sperm using the DNA Extraction Kit (Sangon Biotech, Shanghai, China). MKRN3, MAGEL2 and NDN are three protein-coding genes with a single exon. SNP sites in the MKRN3, MAGEL2 and NDN genes were identified by direct sequencing of the PCR products. Gene-specific primers were designed according to the mRNA sequences of the bovine MKRN3 gene (XM_024982255.1), the MAGEL2 gene (XM_002696471.5) and the NDN gene (NM_001014982.1). The primer sequences, the length of products and AT (annealing temperature) are shown in Table 1.
Table 1.
Primers used in SNP identification, allelic expression and DNA methylation analysis.
| Primer | Sequence (5′-3′) | AT (°C) |
Product Size (bp) |
Application |
|---|---|---|---|---|
| MKRN3-F MKRN3-R |
CGCCCGTCACGTCTGA TACAGCTCTGCCCACGAAAG |
55 | 568 | Identification of SNP rs42331804 and allelic expression analysis ofMKRN3 |
| MAGEL2-F1 MAGEL2-R1 | GAAAAACTTGCCTACCACATC CCACATCCCTGAGCAAGAGTA |
56 | 696 | Identification of SNP rs211249225 and allelic expression analysis ofMAGEL2in tissues |
| MAGEL2-F2 MAGEL2-R2 |
CGTAGGCATTCTCTTCTCTC AACCTGTGACTGGATCTGC |
56 | 1045 | Identification of SNP rs110762305 and allelic expression analysis ofMAGEL2in placenta |
| NDN-F NDN-R |
AGAAACACTCCACCTTCG CTACCCCAATACACAGCC |
55 | 644 | Identification of SNP rs468002089 and allelic expression analysis of NDN |
| PWS-IC-F1 PWS-IC-R1 |
AAGGAAATTGATAGTAAGTATATTAGAGT AACCCAAATCCCCAATAAA |
59 | 791 | PWS-IC DMR identification and analysis |
| PWS-IC-F2 PWS-IC-R2 |
GTTATTAGTGGAAAGTTTGAGGAAA ACCACACGACTAACCTAACCC |
59 | 472 | |
| MKRN3 DMR-F1 MKRN3 DMR-R1 |
TGTAAGAATTATTAGAAAATAAAGAGTAGA CCCAATCCCTACTTCCTATACCTA |
54 | 541 | MKRN3 DMR identification and analysis |
| MKRN3 DMR-F2 MKRN3 DMR-R2 |
TATATAGATATAAAATGAAGTGAATAAAG CCTACTTCCTTCTCTAAACAAA |
52 | 406 | |
| NDN DMR-F1 NDN DMR-R1 |
AGTTTTAATAGGACGTTTGGGGAGG AATCTCCCTCTTCGCCTAAAACCTA |
59 | 526 | NDN DMR identification and analysis |
| NDN DMR-F2 NDN DMR-R2 |
GGGAGTGATTATTGAGGTTTA ACAAAATTAAAATTACACACATCCTTACT |
59 | 331 |
PCR was performed in a 25-μL volume containing 1 μL of forward and reverse primers (10 μmol/L), 1 μL of genomic DNA template (100 ng), 12.5 μL of ES Tap Master Mix (CWBIO, Beijing, China) and 9.5 μL of ddH2O. The PCR conditions were as follows: 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, AT for 30 s, 72 °C for 30 s and a final extension at 72 °C for 10 min. The amplified products were recovered and purified with a UNIQ-10 column DNA gel extraction kit (Sangon, Shanghai, China) and were sequenced directly using an ABI PRISM 3730 automated sequencer (Applied Biosystems, Massachusetts, MA, USA). An overlapping doublet peak in the sequencing chromatogram reveals the existence of a SNP. Heterozygous animals with identified SNPs were employed to analyze allelic expression.
2.3. Allelic Expression Analysis by RT-PCR
Total RNA was extracted from the tissues (including heart, liver, spleen, lung, kidney, muscle, fat, and brain) of heterozygous cows and heterozygous placentas using a TRIzol RNA extraction kit (Invitrogen, Massachusetts, MA, USA), and DNase-Ι treatment was performed to remove possible contamination of genomic DNA. The OD value was measured using an ND-1000 spectrophotometer (NanoDrop, Carlsbad, CA, USA), and a final concentration of 1000 ng/μL was prepared for reverse transcription (RT) experiments. The RT reaction was performed using oligo (dT) primers with a reverse transcription (RT) kit (Promega, Beijing, China) according to the manufacturer’s guidelines. The reaction volume and RT-PCR conditions were the same as those of PCR for SNP identification, except the template of genomic DNA was replaced with cDNA. The MKRN3, MAGEL2 and NDN genes have single exons, and gene-specific primers for the amplification of genomic DNA were also used to amplify cDNA.
2.4. DNA Methylation Analysis by Bisulfite Sequencing PCR (BSP)
According to the locations of the PWS-IC DMR, the MKRN3 DMR and the NDN DMR in humans, the CpG islands were predicted around the promoters of the SNRPN, MKRN3 and NDN genes, and two methylation-specific primers for each CpG island were designed using the online website Methprimer 2.0 (http://www.urogene.org/methprimer2/, accessed on 1 November 2018). Genomic DNA (approximately 500 ng) extracted from tissues of heterozygous cattle, heterozygous placentas, sperm, and oocytes was sodium bisulfate treated with the EZ DNA Methylation-GoldTM Kit (Zymo, Beijing, China). Nested PCR was performed to amplify the target products using FastPfu Fly DNA Polymerase with the treated DNA as template. The second-round PCR was performed using 1 μL of a tenfold dilution of the first-round PCR product as template, then the amplicons were purified and cloned into pMD19-T vectors (Takara, Beijing, China) for sequencing. For each product, at least 20 clones were sequenced. For each detected tissue, the percentage of mCpG(mCpG/CpG) in both parental strands was calculated. The sequences of the methylation-specific primers and AT are shown in Table 1.
3. Results
3.1. Monoallelic Expression of MKRN3, MAGEL2 and NDN in Bovine Tissues
To investigate the allelic expression of MKRN3, MAGEL2 and NDN genes in bovine tissues, three SNPs, the c.1271T>G SNP (rs42331804) in the MKRN3 sequence (XM_024982255.1), the c.457A>G SNP (rs211249225) in the MAGEL2 sequence (XM_002696471.5) and the c.1336A>C SNP (rs468002089) in the NDN sequence (NM_001014982.1), were first detected by direct sequencing of PCR products. Heterozygous animals with these SNPs were used for allelic expression analysis of the MKRN3, MAGEL2 and NDN genes (Figure 1). RT-PCR was performed on total RNA isolated from eight tissues (heart, liver, spleen, lung, kidney, muscle, fat and brain) of heterozygous animals. The amplified products of RT-PCR were sequenced directly. The sequencing chromatograms at the polymorphism sites indicated monoallelic expression of the MKRN3, MAGEL2 and NDN genes in bovine heart, liver, spleen, lung, kidney, muscle, fat and brain tissues (Figure 1).
Figure 1.
Monoallelic expression of MKRN3, MAGEL2 and NDN in bovine tissues detected by RT-PCR. (A) Monoallelic expression of MKRN3 in eight adult somatic tissues, including heart, liver, spleen, lung, kidney, muscle, fat and brain. The arrows indicate the SNP of rs42331804. (B) The SNP of rs211249225 in the MAGEL2 gene and monoallelic expression of the bovine MAGEL2 gene in eight detected tissues. (C) The SNP of rs468002089 in the NDN gene and monoallelic expression of the NDN gene.
3.2. Paternally Expressed MKRN3, MAGEL2 and NDN in Bovine Placenta
The SNPs c.1271T>G (rs42331804) and c.1336A>C (rs468002089) were also evaluated to investigate the imprinted status of MKRN3 and NDN in bovine placentas, respectively. A new SNP, c.2640A>G (rs110762305), was identified in the MAGEL2 sequence(XM_002696471.5) and was used to analyze the imprinted expression of MAGEL2 in placentas because no heterozygous placenta with the c.457A>G (rs211249225) SNP was found.
Based on SNP of c.1271T>G (rs42331804) in MKRN3, five heterozygous individuals were identified from 30 placentas. RT-PCR was also performed on total RNA isolated from heterozygous placentas. The amplified products of RT-PCR were the sequenced, and the results indicated that monoallelic (G) expression occurred in all five placentas. To determine which parental allele was expressed, the corresponding genomic DNA isolated from the maternal blood and paternal sperm samples was amplified. In three placentas (3–5, 1 = 18 and 1 = 12), the paternal and maternal genotypes were homozygous GG and heterozygous TG, respectively; thus, MKRN3 exhibits paternal expression (allele G). For placentas 3501 and 3–16, both of the paternal and maternal genotypes were heterozygous TGs; therefore, we could not determine which parental allele was expressed (Figure 2A).
Figure 2.
Paternal expression of MKRN3, MAGEL2 and NDN in the bovine placenta. (A) Sequencing results of SNP (rs42331804) in the MAGEL2 gene. Paternal expression was deduced by comparing genotypes of gDNA, cDNA and parental gDNA in five heterozygous placentas. (B) Paternal expression of the MAGEL2 gene based on the sequencing results of the SNP rs110762305 in four heterozygous placentas. (C) Paternal expression of NDN deduced by sequencing results of SNP rs468002089 in three heterozygous placentas.
Four heterozygous placentas were found based on the c.2640A>G (rs110762305) SNP in the MAGEL2 gene. Monoallelic (G) expression occurred in all four heterozygous placentas. Analysis of the parental genotypes revealed that the allele (G) was derived from the paternal allele (Figure 2B). In the three heterozygous placentas with the c.1336A>C (rs468002089) SNP of the NDN gene, the expressed allele (C) also came from the paternal allele (Figure 2C).
3.3. Identification of Bovine PWS-IC, MKRN3 and NDN DMR
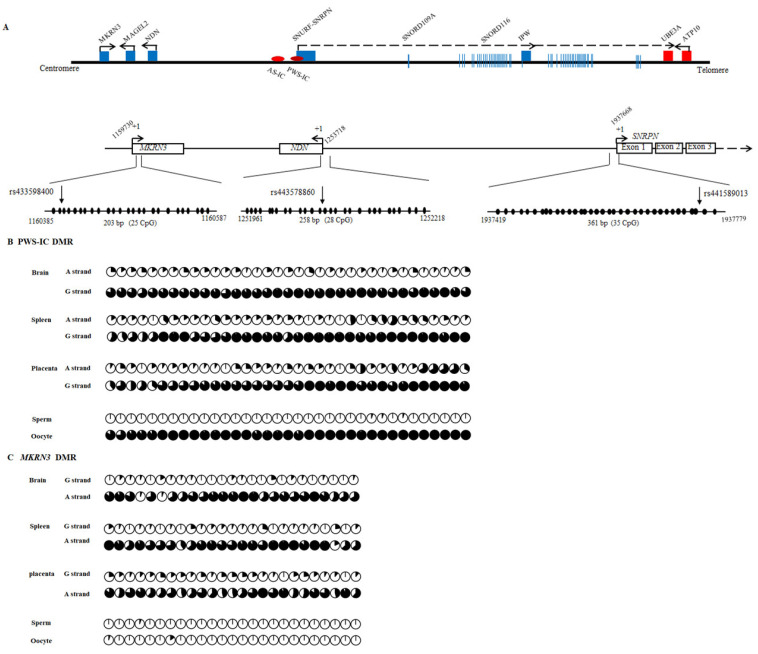
In humans, three DMRs, PWS-IC, MKRN3 DMR and NDN DMR, are located around the promoters of the SNRPN, MKRN3 and NDN genes, respectively. To evaluate the role of DNA methylation in the regulation of monoallelic or imprinted expression of the bovine MKRN3, MAGEL2 and NDN genes, the locations of these three bovine DMRs were first identified. Similar to what has been observed in humans, three CpG islands (CGIs) were identified in the promoter and exon 1 of the bovine SNRPN gene, the 5’UTR (5’ untranslated region) of the MKRN3 gene and the promoter region of the NDN gene using CpG Plot analysis (http://www.ebi.ac.uk/Tools/emboss/cpgplot/, accessed on 1 November 2018). The locations of these three CGIs were of potential DMRs of PWS-IC, MKRN3 and NDN. Methylation-specific primers were designed for bisulfite sequencing analysis of a 472-bp fragment containing 35 CpG dinucleotides in the PWS-IC CGI, a 406-bp fragment containing 25 CpGs in the MKRN3 CGI and a 331-bp fragment containing 28 CpGs in the NDN CGI (Figure 3A). Because SNRPN, MKRN3 and NDN genes exhibit similar paternal or monoallelic expression in bovine tissues, placental and two somatic tissues were selected to study their methylation.
Figure 3.
The structure of bovine PWS/AS imprinted region and the locations and methylation profiles of the PWS-IC, MKRN3 and NDN DMRs in this region. (A) The structure of the PWS/AS imprinted region and the relative location of the PWS-IC, MKRN3 and NDN DMRs in this locus. Maternally and paternally expressed genes are represented by red and blue squares, respectively. The transcription orientation is denoted with an arrow. The SNPs used to determine allele-specific methylation are shown with vertical arrows. (B) DNA methylation profiles of PWS-IC DMR in the brain, spleen, placenta, sperm and oocytes. (C) DNA methylation profiles of MKRN3 DMR in the brain, spleen, placenta, sperm and oocytes. (D) DNA methylation profiles of NDN DMRs in the brain, spleen, placenta, sperm and oocytes. The allele origin of each clone was determined using SNPs in the heterozygous individuals. Methylation of each CpG site is presented with pie charts. The percent of methylated cytosines is represented in black, and the percent of unmethylated cytosines is represented in white at each site. The complete bisulfite maps of PWS-IC, MKRN3 and NDN DMRs may be found in Figure S1.
The methylation status of PWS-IC CGI was tested in the brains, spleens and placentas (Figure 3B and Figure S1). A G/A polymorphism (rs441589013) was identified and used to discriminate the two parental alleles. As shown in Figure S1, there was a methylation level difference between the two parental alleles in the detected brains, spleens and placentas. The average methylation levels of the parental alleles were 88.0% (G allele) and 13.75% (A allele) in brains, 88.5% (G allele) and 19.2% (A allele) in spleens, and 82.4% (G allele) and 22.3% (A allele) in placentas. The difference in methylation level between parental alleles was more than 50% and exhibited a DMR-like methylation pattern; thus, it was named PWS-IC DMR. When examining the sperm and oocyte methylation levels (Figure 3B and Figure S1), unmethylation was observed in sperm across this region, and high hypermethylation was observed in the oocyte. These results suggest that the methylation pattern of PWS-IC DMR is established at the gamete stage and belongs to the primary regulatory DMR.
The methylation status of MKRN3 CGI was analyzed in the brains, spleens and placentas (Figure 3C and Figure S1). A G/A SNP (rs433598400) distinguishes parental alleles into G and A alleles. The methylation pattern showed a DMR-like methylation pattern in all the detected tissues, and the methylation level of allele G was obviously lower than that of allele A (Figure S1). In NDN CGI, a G/T SNP (rs443578860) was used to distinguish parental alleles, and a methylation status similar to that of MKRN3 CGI was observed in the detected brains, spleens and placentas (Figure 3D and Figure S1). The methylation level of the maternal allele of T was higher than that of the paternal allele of G (>50%). Therefore, the MKRN3 CGI and the NDN CGI were named MKRN3 DMR and NDN DMR, respectively. For MKRN3 DMR and NDN DMR, hypomethylation was observed in both sperm and oocytes, indicating that the methylation patterns of MKRN3 DMR and NDN DMR are established post fertilization and belong to the second regulatory DMR.
4. Discussion
Parental genomes are not functionally equivalent in embryonic development and body composition in mammalian species [17]. Genomic imprinting is a phenomenon of parent-of-origin effects(POE), which causes gene monoallelically expressed in paternal- or maternal-specific manner [18]. the parent-of-origin effects on quantitative trait loci (QTL) has been reported in livestock, such as cattle [19,20], pigs [21,22] and sheep [23,24]. Cattle, an economically important domestic farm animal species, serve as a potential model species in research on human preimplantation embryo development [25] and in determining the genetic etiology of sporadic human disorders [26]. To date, about 50 genes are experimentally identified to be imprinted in cattle (www.geneimprint.com). Most of these imprinted genes are validated by SNP-based method [27,28,29]. The high throughput sequencing technology provide new method for the discovery of new imprinted genes at the transcriptome level and has been performed in characterization of genomic imprinting in mice and humans [30,31,32,33]. Chen et al. assessed the imprinted gene expression in the bovine conceptus by the next generation sequencing technology and identified eight novel bovine imprinted genes and demonstrated that cis-eQTL effects can lead to the monoallelic expression of genes [34]. Imumorin et al. reported that quantitative trait loci with POE could affect seven growth and carcass traits in genome of Angus×Brahman cattle cross breds, and indicated that POE on quantitative traits is common in mammals, and the effects of imprinted gene on quantitative traits should be investigated [35].
MKRN3, MAGEL2 and NDN are three protein-coding genes located in the human 15q11-13 imprinted region, and the absence of the paternal contribution of this chromosomal region could lead to human Prader-Willi syndrome, characterized by neurogenetic disorders [12,36,37]. The MKRN3 gene, also known as ZNF127, is a protein called makorin ring finger protein 3. MKRN3 serves as a repressor regulating puberty in humans [38,39]. The genetic alterations of MKRN3 have been confirmed as the cause of central precocious puberty (CPP), including deleterious mutations in the coding region [38,39,40] and the promoter and 5′-UTR regulatory regions of the MKRN3 gene [41,42]. NDN and MAGEL2, two members of the NDN/MAGEL2 gene family, encode necdin protein, which is concurrently inactivated in patients with PWS [43,44]. MKRN3, MAGEL2 and NDN are candidate genes of PWS, but a deficiency of paternal deletion MKRN3, MAGEL2 and NDN is not sufficient to result in PWS [45]. NDN gene could serve as a phylogenetic marker for studying the evolution and conservation in Bovidae species for its sufficient variation in gene sequence [46].
NDN/Ndn [12,44,47], MAGEL2/Magel2 [36] and MKRN3/Mkrn3 [13,48] were first identified to be paternally expressed in the central nervous system (CNS) and then confirmed to be imprinted in the E15.5 whole brains of mice [49]. NDN and MAGEL2 are also confirmed to be imprinted in adult human tissues [33]. In this study, we showed that bovine MKRN3, MAGEL2 and NDN genes are monoallelically expressed in adult somatic tissues, similar to their orthologs in humans and mice, although the tissue distribution of expression differs. In addition, MKRN3 also exhibits paternally expressed genes in the rabbit brain [50].
Most well-known imprinted genes are highly expressed in pregnancy and term placental tissue and play critical roles in regulating human placental function and fetal development [51,52,53,54]. Some investigations of imprinted genes focus on placental tissue because placental tissue is not included in the Genotype-Tissue Expression (GTEx) project. The number of identified imprinted genes in the human placenta is consistent with the data on the mouse placenta [55]. MKRN3 has been reported to be imprinted in the human placenta [56]. In this study, we showed that MKRN3, MAGEL2 and NDN are paternally expressed in bovine placentas.
The parent-of-origin monoallelic expression of genes in an imprinted cluster is typically governed by DMRs inherited from the germline in the imprinting control region (ICR) [57,58]. In contrast to germline DMRs (gDMRs), differential DNA methylation in secondary DMRs (sDMRs) is acquired during embryonic development. The majority of sDMRs are usually regulated by a gDMR [59]. There are 24 identified imprinted gDMRs in the mouse genome: 21 maternal gDMRs are acquired during oogenesis, and three paternal gDMRs are acquired during spermatogenesis [58,60].
The ICR controlling genomic imprinting across the PWS/AS domain is composed of two parts: PWS-IC and AS-IC. In both mice and humans, PWS-IC is a maternal gDMR and is associated with the 5’ region of the Snrpn/SNRPN gene [61,62,63]. PWS-IC controls the paternal epigenotype on the paternally inherited chromosome [11,64]. The deletion of paternally inherited PWS-IC results in epigenetically silenced paternally expressed genes, such as NDN, MAGEL2 and MKRN3 [14,62,64,65,66,67,68]. These paternally expressed genes are also associated with a secondary differentially methylated region, which is acquired during embryonic development and exhibits paternally unmethylated and maternally hypermethylated regions [68,69,70].
In this study, we identified that the bovine primary DMR of PWS-IC was located in the promoter and the first exon of the bovine SNRPN gene, with hypomethylation in sperm and high hypermethylation in the oocyte. These results are consistent with the findings of previous studies by O’Doherty et al. [71] and Lucifero et al. [72]. The methylation of PWS-IC was consistent with the paternal or monoallelic expression of bovine MKRN3, MAGEL2 and NDN, indicating that their expression was controlled by PWS-IC. For MKRN3 and NDN gene, two somatic DMRs also identified to locate in the 5’UTR of the MKRN3 gene and the promoter region of the NDN gene, respectively. Their locations and methylation patterns are similar to those in humans and mice; therefore, the imprinting regulation of this region appears to be conserved in humans, mice and cows.
5. Conclusions
The MKRN3, MAGEL2 and NDN genes exhibit monoallelic or maternal imprinted expression in bovine somatic and placenta tissues, which was controlled by PWS-DMR located in the promoter and the first exon of the bovine SNRPN gene. The expression of MKRN3 and NDN gene was also controlled by two somatic DMRs, MKRN3 DMR and NDN DMR, respectively.
Acknowledgments
We thank farmers for assisting with collecting tissue and placental samples.
Abbreviations
| AT | annealing temperature |
| DMRs | differentially methylated regions |
| SNP | single-nucleotide polymorphism |
| 5’UTR | 5’ untranslated region |
| NDN | necdin |
| MAGEL2 | melanoma antigen-like gene 2 |
| MKRN3 | makorin ring finger protein 3 |
| PWS | Prader-Willi syndrome |
| AS | Angelman syndrome |
| snoRNA | small nucleolar RNA |
| ICRs | imprinting control regions |
| gDMRs | germline DMRs |
| sDMRs | secondary DMRs |
| PWS-IC | PWS imprinting center |
| CGIs | CpG islands |
| GTEx | Genotype-Tissue Expression |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11071985/s1, Figure S1. Methylation profiles of PWS-IC, MKRN3 DMR and NDN DMR in bovine brain, spleen, placenta, sperms and oocytes. A: Methylation profiles of PWS-IC DMR. B: Methylation profiles of MKRN3 DMR. C. Methylation profiles of NDN DMR Methylated and unmethylated CpG dinucleotides are represented by filled and open circles, respectively.
Author Contributions
Conceptualization, S.L. and C.Z.; methodology, J.L., W.C., D.L., L.J., S.G. and Y.D.; investigation, X.L., Y.D. and L.J.; resources, S.L.; writing—original draft preparation, J.L. and S.L.; writing—review and editing, J.L., S.L., W.C. and C.Z.; visualization, J.L. and S.L.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. and C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Hebei Province Natural Science Foundation of China (C2020204004) and The National Natural Science Foundation of China (31372312).
Institutional Review Board Statement
The study was conducted according to the University of Hebei Agricultural of China guide for the care and use of animals in research and teaching.
Data Availability Statement
The data reported in this study is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edwards C.A., Ferguson-Smith A.C. Mechanisms regulating imprinted genes in clusters. Curr. Opin. Cell. Biol. 2007;19:281–289. doi: 10.1016/j.ceb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Soellner L., Begemann M., Mackay D.J., Grønskov K., Tümer Z., Maher E.R., Temple I.K., Monk D., Riccio A., Linglart A., et al. Recent advances in imprinting disorders. Clin. Genet. 2017;91:3–13. doi: 10.1111/cge.12827. [DOI] [PubMed] [Google Scholar]
- 3.Uribe-Lewis S., Woodfine K., Stojic L., Murrell A. Molecular mechanisms of genomic imprinting and clinical implications for cancer. Expert. Rev. Mol. Med. 2011;13:e2. doi: 10.1017/S1462399410001717. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls R.D., Saitoh S., Horsthemke B. Imprinting in Prader-Willi and Angelman syndromes. Trends Genet. 1998;14:194–200. doi: 10.1016/S0168-9525(98)01432-2. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy S.B., Schwartz S., Miller J.L., Driscoll D.J. Prader-Willi syndrome. Genet. Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain S.J. RNAs of the human chromosome 15q11-q13 imprinted region. Wiley Interdiscip. Rev. RNA. 2013;4:155–166. doi: 10.1002/wrna.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rougeulle C., Glatt H., Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat. Genet. 1997;17:14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- 8.Barlow D.P., Bartolomei M.S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 2014;6:a018382. doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pólvora-Brandão D., Joaquim M., Godinho I., Aprile D., Álvaro A.R., Onofre I., Raposo A.C., de Almeida L.P., Duarte S.T., da Rocha S.T. Loss of hierarchical imprinting regulation at the Prader-Willi/Angelman syndrome locus in human iPSCs. Hum. Mol. Genet. 2018;27:3999–4011. doi: 10.1093/hmg/ddy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie W., Barr C.L., Kim A., Yue F., Lee A.Y., Eubanks J., Dempster E.L., Ren B. Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell. 2012;148:816–831. doi: 10.1016/j.cell.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith E.Y., Futtner C.R., Chamberlain S.J., Johnstone K.A., Resnick J.L. Transcription is required to establish maternal imprinting at the Prader–Willi syndrome and Angelman syndrome locus. PLoS Genet. 2011;7:e1002422. doi: 10.1371/journal.pgen.1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jay P., Rougeulle C., Massacrier A., Moncla A., Mattei M.G., Malzac P., Roëckel N., Taviaux S., Lefranc J.L., Cau P., et al. The human necdin gene, NDN, is maternally imprintedand located in the Prader-Willi syndrome chromosomal region. Nat. Genet. 1997;17:357–361. doi: 10.1038/ng1197-357. [DOI] [PubMed] [Google Scholar]
- 13.Jong M.T.C., Gray T.A., Ji Y., Glenn C.C., Saitoh S., Driscoll D.J., Nicholls R.D. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum. Mol. Genet. 1999;8:783–793. doi: 10.1093/hmg/8.5.783. [DOI] [PubMed] [Google Scholar]
- 14.Bielinska B., Blaydes S.M., Buiting K., Yang T., Krajewska-Walasek M., Horsthemke B., Brannan C.I. De novo deletions of SNRPN exon 1 in early human and mouse embryos result in a paternal to maternal imprint switch. Nat. Genet. 2000;25:74–78. doi: 10.1038/75629. [DOI] [PubMed] [Google Scholar]
- 15.Ohta T., Gray T., Rogan P., Buiting K., Gabriel J., Saitoh S., Muralidhar B., Bilienska B., Krajewska-Walasek M., Driscoll D., et al. Imprinting-mutation mechanisms in Prader–Willi syndrome. Am. J. Hum. Genet. 1999;64:397–413. doi: 10.1086/302233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Donato M., Hussain T., Rodulfo H., Peters S.O., Imumorin I.G., Thomas B.N. Conservation of Repeats at the Mammalian KCNQ1OT1-CDKN1C Region Suggests a Role in Genomic Imprinting. Evol. Bioinform. Online. 2017;13:1176934317715238. doi: 10.1177/1176934317715238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surani M.A., Barton S.C., Norris M.L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- 18.Barlow D.P. Gametic imprinting in mammals. Science. 1995;270:1610–1613. doi: 10.1126/science.270.5242.1610. [DOI] [PubMed] [Google Scholar]
- 19.Kuehn C., Edel C., Weikard R., Thaller G. Dominance and parent-of-origin effects of coding and non-coding alleles at the acylCoA-diacylglycerol-acyltransferase (DGAT1) gene on milk production traits in German Holstein cows. BMC Genet. 2007;8:62. doi: 10.1186/1471-2156-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pausch H., Flisikowski K., Jung S., Emmerling R., Edel C., Götz K.U., Fries R. Genome-wide association study identifies two major loci affecting calving ease and growth-related traits in cattle. Genetics. 2011;187:289–297. doi: 10.1534/genetics.110.124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Laere A.-S., Nguyen M., Braunschweig M., Nezer C., Collette C., Moreau L., Archibald A.L., Haley C., Buys N., Tally M., et al. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature. 2003;425:832–836. doi: 10.1038/nature02064. [DOI] [PubMed] [Google Scholar]
- 22.Boysen T.J., Tetens J., Thaller G. Detection of a quantitative trait locus for ham weight with polar over dominance near the ortholog of the callipyge locus in an experimental pig F2 population. J. Anim. Sci. 2010;88:3167–3172. doi: 10.2527/jas.2009-2565. [DOI] [PubMed] [Google Scholar]
- 23.Cockett N.E., Jackson S.P., Shay T.L., Farnir F., Berghmans S., Snowder G.D., Nielsen D.M., Georges M. Polar over dominance at the ovine callipyge locus. Science. 1996;273:236–238. doi: 10.1126/science.273.5272.236. [DOI] [PubMed] [Google Scholar]
- 24.Matika O., Sechi S., Pong-Wong R., Houston R.D., Clop A., Woolliams J.A., Bishop S.C. Characterization of OAR1 and OAR18 QTL associated with muscle depth in British commercial terminal sire sheep. Anim. Genet. 2010;42:172–180. doi: 10.1111/j.1365-2052.2010.02121.x. [DOI] [PubMed] [Google Scholar]
- 25.Hansen P.J. Current and future assisted reproductive technologies for mammalian farm animals. In: Cliff lamb G., DiLorenzo N., editors. Current and Future Reproductive Technologies and World Food Production. Volume 752. Springer; New York, NY, USA: 2014. pp. 1–22. Advances in Experimental Medicine and Biology. [DOI] [PubMed] [Google Scholar]
- 26.Bourneuf E., Otz P., Pausch H., Jagannathan V., Michot P., Grohs C., Piton G., Ammermüller S., Deloche M.C., Fritz S., et al. Rapid Discovery of De Novo Deleterious Mutations in Cattle Enhances the Value of Livestock as Model Species. Sci. Rep. 2017;7:11466. doi: 10.1038/s41598-017-11523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaitoun I., Khatib H. Comparative genomic imprinting and expression analysis of six cattle genes. J. Anim. Sci. 2008;86:25–32. doi: 10.2527/jas.2007-0150. [DOI] [PubMed] [Google Scholar]
- 28.Zaitoun I., Khatib H. Assessment of genomic imprinting of SLC38A4, NNAT, NAP1L5, and H19 in cattle. BMC Genet. 2006;7:49. doi: 10.1186/1471-2156-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneda M., Takahashi M., Yamanaka K.I., Saito K., Taniguchi M., Akagi S., Watanabe S., Nagai T. Epigenetic analysis of bovine parthenogenetic embryonic fibroblasts. J. Reprod. Dev. 2017;63:365–375. doi: 10.1262/jrd.2017-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Clark A.G. Using next-generation RNA sequencing to identify imprinted genes. Heredity. 2014;113:156–166. doi: 10.1038/hdy.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babak T., Deveale B., Armour C., Raymond C., Cleary M.A., van der Kooy D., Johnson J.M., Lim L.P. Global survey of genomic imprinting by transcriptome sequencing. Curr. Biol. 2008;18:1735–1741. doi: 10.1016/j.cub.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 32.DeVeale B., van der Kooy D., Babak T. Critical evaluation of imprinted gene expression by RNA-Seq: A new perspective. PLoS Genet. 2012;8:e1002600. doi: 10.1371/journal.pgen.1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baran Y., Subramaniam M., Biton A., Tukiainen T., Tsang E.K., Rivas M.A., Pirinen M., Gutierrez-Arcelus M., Smith K.S., Kukurba K.R., et al. The landscape of genomic imprinting across diverse adult human tissues. Genome Res. 2015;25:927–936. doi: 10.1101/gr.192278.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z., Hagen D.E., Wang J., Elsik C.G., Ji T., Siqueira L.G., Hansen P.J., Rivera R.M. Global assessment of imprinted gene expression in the bovine conceptus by next generation sequencing. Epigenetics. 2016;11:501–516. doi: 10.1080/15592294.2016.1184805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imumorin I.G., Kim E.H., Lee Y.M., De Koning D.J., van Arendonk J.A., De Donato M., Taylor J.F., Kim J.J. Genome Scan for Parent-of-Origin QTL Effects on Bovine Growth and Carcass Traits. Front. Genet. 2011;2:44. doi: 10.3389/fgene.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boccaccio I., Glatt-Deeley H., Watrin F., Roeckel N., Lalande M., Muscatelli F. The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader-Willi region. Hum. Mol. Genet. 1999;8:2497–2505. doi: 10.1093/hmg/8.13.2497. [DOI] [PubMed] [Google Scholar]
- 37.Sutcliffe J.S., Han M., Christian S.L., Ledbetter D.H. Neuronally-expressed necdin gene: An imprinted candidate gene in Prader-Willi syndrome. Lancet. 1997;350:1520–1521. doi: 10.1016/S0140-6736(05)63943-8. [DOI] [PubMed] [Google Scholar]
- 38.Abreu A.P., Dauber A., Macedo D.B., Noel S.D., Brito V.N., Gill J.C., Cukier P., Thompson I.R., Navarro V.M., Gagliardi P.C., et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 2013;368:2467–2475. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abreu A.P., Macedo D.B., Brito V.N., Kaiser U.B., Latronico A.C. A new pathway in the control of the initiation of puberty: The MKRN3 gene. J. Mol. Endocrinol. 2015;54:R131–R139. doi: 10.1530/JME-14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simsek E., Demiral M., Ceylaner S., Kırel B. Two Frameshift Mutations in MKRN3 in Turkish Patients with Familial Central Precocious Puberty. Horm. Res. Paediatr. 2017;87:405–411. doi: 10.1159/000450923. [DOI] [PubMed] [Google Scholar]
- 41.Fanis P., Skordis N., Toumba M., Papaioannou N., Makris A., Kyriakou A., Neocleous V., Phylactou L.A. Central Precocious Puberty Caused by Novel Mutations in the Promoter and 5′-UTR Region of the Imprinted MKRN3 Gene. Front. Endocrinol. 2019;10:677. doi: 10.3389/fendo.2019.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macedo D.B., França M.M., Montenegro L.R., Cunha-Silva M., Bessa D.S., Abreu A.P., Kaiser U.B., Mendonca B., Jorge A.A., Brito V.N., et al. Central Precocious Puberty Caused by a Heterozygous Deletion in the MKRN3 Promoter Region. Neuroendocrinology. 2018;107:127–132. doi: 10.1159/000490059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S., Kozlov S., Hernandez L., Chamberlain S.J., Brannan C.I., Stewart C.L., Waverick R. Expression and imprinting of MAGEL2 suggesta role in Prader-Willi syndrome and the homologous murine imprinting phenotype. Hum. Mol. Genet. 2000;9:1813–1819. doi: 10.1093/hmg/9.12.1813. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald H.R., Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum. Mol. Genet. 1997;6:1873–1878. doi: 10.1093/hmg/6.11.1873. [DOI] [PubMed] [Google Scholar]
- 45.Kanber D., Giltay J., Wieczorek D., Zogel C., Hochstenbach R., Caliebe A., Kuechler A., Horsthemke B., Buiting K. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader–Willi syndrome. Eur. J. Hum. Genet. 2009;17:582–590. doi: 10.1038/ejhg.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters S.O., De Donato M., Hussain T., Rodulfo H., Babar M.E., Imumorin I.G. Sequence variation of necdin gene in Bovidae. J. Anim. Sci. Technol. 2018;60:32. doi: 10.1186/s40781-018-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watrin F., Roëckel N., Lacroix L., Mignon C., Mattei M.-G., Disteche C., Muscatelli F. The mouse Necdin gene is expressed from the paternal allele only and lies in the 7C region of the mouse chromosome 7, a region of conserved synteny to the human Prader–Willi syndrome region. Eur. J. Hum. Genet. 1997;5:324–332. doi: 10.1159/000484784. [DOI] [PubMed] [Google Scholar]
- 48.Hershko A., Razin A., Shemer R. Imprinted methylation and its effect on expression of the mouse Zfp127 gene. Gene. 1999;234:323–327. doi: 10.1016/S0378-1119(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 49.Buettner V.L., Walker A.M., Singer-Sam J. Novel paternally expressed intergenic transcripts at the mouse Prader-Willi/Angelman Syndrome locus. Mamm. Genome. 2005;16:219–227. doi: 10.1007/s00335-004-2452-7. [DOI] [PubMed] [Google Scholar]
- 50.Yuan L., Lai L., Duan F., Chen M., Deng J., Li Z. Conservation of imprinting of MKRN3 and NAP1L5 in rabbits. Anim. Gen. 2016;47:507–509. doi: 10.1111/age.12444. [DOI] [PubMed] [Google Scholar]
- 51.Hanna C.W. Placental imprinting: Emerging mechanisms and functions. PLoS Genet. 2020;16:e1008709. doi: 10.1371/journal.pgen.1008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.John R.M. Imprinted genes and the regulation of placental endocrine function: Pregnancy and beyond. Placenta. 2017;56:86–90. doi: 10.1016/j.placenta.2017.01.099. [DOI] [PubMed] [Google Scholar]
- 53.Monk D. Genomic imprinting in the human placenta. Am. J. Obstet. Gynecol. 2015;213:152–162. doi: 10.1016/j.ajog.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 54.Noguer-Dance M., Abu-Amero S., Al-Khtib M., Lefèvre A., Coullin P., Moore G.E., Cavaillé J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum. Mol. Genet. 2010;19:3566–3582. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
- 55.Okae H., Hiura H., Nishida Y., Funayama R., Tanaka S., Chiba H., Yaegashi N., Nakayama K., Sasaki H., Arima T. Re-investigation and RNA sequencing-based identification of genes with placenta-specific imprinted expression. Hum. Mol. Genet. 2012;21:548–558. doi: 10.1093/hmg/ddr488. [DOI] [PubMed] [Google Scholar]
- 56.Pilvar D., Reiman M., Pilvar A., Laan M. Parent-of-origin-specific allelic expression in the human placenta is limited to established imprinted loci and it is stably maintained across pregnancy. Clin. Epigenetics. 2019;11:94. doi: 10.1186/s13148-019-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barlow D.P. Genomic imprinting: A mammalian epigenetic discovery model. Annu. Rev. Genet. 2011;45:379–403. doi: 10.1146/annurev-genet-110410-132459. [DOI] [PubMed] [Google Scholar]
- 58.White C.R., MacDonald W.A., Mann M.R. Conservation of DNA Methylation Programming Between Mouse and Human Gametes and Preimplantation Embryos. Biol. Reprod. 2016;95:61. doi: 10.1095/biolreprod.116.140319. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson-Smith A.C. Genomic imprinting: The emergence of an epigenetic paradigm. Nat. Rev. Genet. 2011;12:565–575. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- 60.Macdonald W.A., Mann M.R.W. Epigenetic regulation of genomic imprinting from germ line to preimplantation. Mol. Reprod. Dev. 2014;81:126–140. doi: 10.1002/mrd.22220. [DOI] [PubMed] [Google Scholar]
- 61.Geuns E., De Rycke M., Van Steirteghem A., Liebaers I. Methylation imprints of the imprint control region of the SNRPN-gene in human gametes and preimplantation embryos. Hum. Mol. Genet. 2003;12:2873–2879. doi: 10.1093/hmg/ddg315. [DOI] [PubMed] [Google Scholar]
- 62.El-Maarri O., Buiting K., Peery E.G., Kroisel P.M., Balaban B., Wagner K., Urman B., Heyd J., Lich C., Brannan C.I., et al. Maternal methylation imprints on human chromosome 15 are established during or after fertilization. Nat. Genet. 2001;27:341–344. doi: 10.1038/85927. [DOI] [PubMed] [Google Scholar]
- 63.El-Maarri O., Seoud M., Coullin P., Herbiniaux U., Oldenburg J., Rouleau G., Slim R. Maternal alleles acquiring paternal methylation patterns in biparental complete hydatidiform moles. Hum. Mol. Genet. 2003;12:1405–1413. doi: 10.1093/hmg/ddg152. [DOI] [PubMed] [Google Scholar]
- 64.Dubose A.J., Smith E.Y., Yang T.P., Johnstone K.A., Resnick J.L. A new deletion refines the boundaries of the murine Prader-Willi syndrome imprinting center. Hum. Mol. Genet. 2011;20:3461–3466. doi: 10.1093/hmg/ddr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horsthemke B., Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am. J. Med. Genet. A. 2008;146:2041–2052. doi: 10.1002/ajmg.a.32364. [DOI] [PubMed] [Google Scholar]
- 66.Rabinovitz S., Kaufman Y., Ludwig G., Razin A., Shemer R. Mechanisms of activation of the paternally expressed genes by the Prader-Willi imprinting center in the Prader-Willi/Angelman syndromes domains. Proc. Natl. Acad. Sci. USA. 2012;109:7403–7408. doi: 10.1073/pnas.1116661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bressler J., Tsai T.-F., Wu M.-Y., Tsai S.-F., Ramirez M.A., Armstrong D., Beaudet A.L. The SNRPN promoter is not required for genomic imprinting of the Prader-Willi/Angelman domain in mice. Nat. Genet. 2001;28:232–240. doi: 10.1038/90067. [DOI] [PubMed] [Google Scholar]
- 68.Sharp A.J., Migliavacca E., Dupré Y., Stathaki E., Sailani M.R., Baumer A., Schinzel A., Mackay D.J., Robinson D.O., Cobellis G., et al. Methylation profiling in individuals with uniparental disomy identifies novel differentially methylated regions on chromosome 15. Genome Res. 2010;20:1271–1278. doi: 10.1101/gr.108597.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chai J.H., Locke D.P., Ohta T., Greally J.M., Nicholls R.D. Retrotransposed genes such as Frat3 in the mouse Chromosome 7C Prader-Willi syndrome region acquire the imprinted status of their insertion site. Mamm. Genome. 2001;12:813–821. doi: 10.1007/s00335-001-2083-1. [DOI] [PubMed] [Google Scholar]
- 70.Robinson W., Knoblauch H., Buiting K., Schmidt K., Gillessen-Kaesbach G., Horsthemke B. Molecular diagnosis of the Prader-Willi and Angelman syndromes by detection of parent-of-origin specific DNA methylation in 15q11-13. Hum. Genet. 1992;90:313–315. doi: 10.1007/BF00220089. [DOI] [PubMed] [Google Scholar]
- 71.O’Doherty A.M., O’Shea L.C., Fair T. Bovine DNA methylation imprints are established in an oocyte size-specific manner, which are coordinated with the expression of the DNMT3 family proteins. Biol. Reprod. 2012;86:67. doi: 10.1095/biolreprod.111.094946. [DOI] [PubMed] [Google Scholar]
- 72.Lucifero D., Suzuki J., Bordignon V., Martel J., Vigneault C., Therrien J., Filion F., Smith L.C., Trasler J.M. Bovine SNRPN methylation imprint in oocytes and day 17 in vitro-produced and somatic cell nuclear transfer embryos. Biol. Reprod. 2006;75:531–558. doi: 10.1095/biolreprod.106.051722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this study is available on request from the corresponding author.