Abstract
This work details the use of amber suppression-mediated genetic incorporation of unnatural amino acids (UAAs), specifically p-azido-l-phenylalanine (pAzF) and p-acetyl-l-phenylalanine (pAcF), to develop site-specifically labeled antibody Fab fragments. These antibody fragment conjugates represent a novel class of imaging agents with optimal stability, efficacy, and pharmacological properties, which have demonstrated promising potential for probing and understanding the in vivo bio-distributions of protein targets of interest. This chapter provides general guidelines for preparing these Fab conjugates, and details of follow-up bioassays such as single-agent based positron emission tomography (PET) imaging of immune-checkpoint protein PD-L1, and the use of GCN4-mediated switchable antibody conjugates for near-infrared fluorescent imaging of cancer-related biomarkers.
1. Introduction
Conjugation with small molecules (Hernandez et al., 2016; Lewis Phillips et al., 2008; Wang et al., 2015), oligomers (Sano, Smith, & Cantor, 1992), and proteins (Gill, Forouzandeh, Rahbarizadeh, Ramezani, & Rasaee, 2006), etc. has endowed versatile functions to antibodies, resulting in antibody conjugates capable of being used for broad applications such as immunoassay, imaging-based detection, diagnosis, and therapeutic development (Lyu et al., 2018). In addition to the development of antibody-drug conjugates (ADCs), which have emerged as promising immunotherapeutics (Lewis Phillips et al., 2008; Ricart, 2011; Wang et al., 2015), antibodies conjugated with radioisotopes or fluorophores are increasingly pursued for positron emission tomography (PET) or near-infrared fluorescence (NIRF) imaging, thereby becoming a critical component of modern precision medicine (Adumeau, Sharma, Brent, & Zeglis, 2016a, 2016b; Hernandez et al., 2016). While effective, most conjugates were based on full-length IgG antibodies which have limited tissue and tumor penetration (Chattegee et al., 2016; England et al., 2018; Heskamp et al., 2015; Hettich, Braun, Bartholoma, Schirmbeck, & Niedermann, 2016). On top of this, common conjugates including FDA-approved ADCs have been prepared via random conjugation with naturally occurring lysine and cysteine residues, resulting in heterogeneous constructs with varying efficacies, stabilities, and suboptimal pharmacological properties (Adumeau et al., 2016a, 2016b; Schumacher, Hackenberger, Leonhardt, & Helma, 2016). Early studies, such as THIOMABs, utilized antibodies bearing engineered cysteine residues to prepare site-specific immunoconjugates (Adumeau et al., 2016a), and revealed higher signal-to-noise imaging intensity ratios in vivo in comparison to random conjugates, demonstrating the positive effects of the precise control of conjugation sites and stoichiometry. Nevertheless, most cysteine conjugation methods rely on maleimdyl thioester bonds that possess suboptimal biological stability, thereby limiting the broad applications of the THIOMAB approach (Adumeau et al., 2016a). Other site-specific strategies such as expressed protein ligation (EPL) could result in relatively stable constructs (Muir, 2003). Yet, the ligation site is usually limited to the C-terminal, which may not generate the most stable conjugates, and makes it challenging for multi-site conjugation (Muir, 2003; Wang, Xie, & Schultz, 2006).
Amber suppression-mediated genetic incorporation of unnatural amino acids (UAA), on the other hand, has recently emerged as a promising site-specific protein conjugation strategy, due to the stably bonded constructs and flexibility in site-selections it offers (Adumeau et al., 2016b). Through site-directed mutation, the genetic code of the target site can be changed to the amber stop codon (TAG) (Liu & Schultz, 2010; Wang et al., 2006). During the translation of recombinant proteins, a pair of orthogonal tRNA and aminoacyl tRNA synthetase were employed to charge the amber codon (TAG) with the desired UAA (Wals & Ovaa, 2014) (Fig. 1). This pair has been evolved to recognize UAA specifically, and function orthogonally to intrinsic tRNA/aminoacyl transferases (Liu & Schultz, 2010; Wang et al., 2006). This novel approach allows the generation of antibodies which site-specifically incorporated UAAs such as p-acetyl-l-phenylalanine (pAcF) and p-azido-l-phenylalanine (pAzF) that can be coupled to molecules of interest via a stable linkage (Adumeau et al., 2016b; Cao et al., 2015; Lyu et al., 2018; Wissler et al., 2019). Protein therapeutics based on the UAA conjugates have displayed better in vivo performance than the cysteine conjugates, in terms of efficacy, stability, and toxicology (Jackson et al., 2014; Liu & Schultz, 2010; Tian et al., 2014).
Fig. 1.
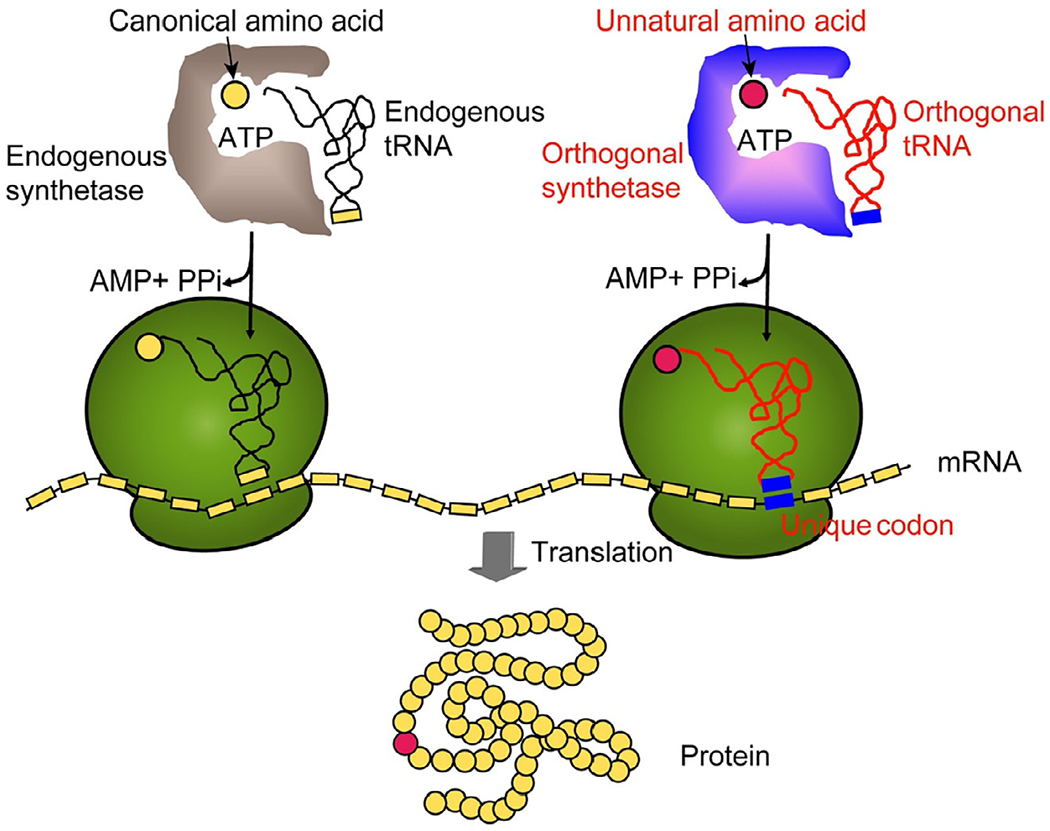
General scheme illustrating the amber suppression-mediated genetic incorporation of unnatural amino acid. Adapted with permission from Wang, Q., Parrish, A. R., & Wang, L. (2009). Expanding the genetic code for biological studies. Chemical Biology, 16(3), 323–336. Copyright (2009) Elsevier Ltd.
More recently, imaging studies using probes comprised of UAA-mediated conjugation have been increasingly reported (Lyu et al., 2018; Wissler et al., 2019; Wu, Zhu, et al., 2016), including our lab’s development of UAA-based site-specific antibody Fab fragment conjugates. Along our efforts to develop a class of immunoconjugates based novel imaging agents, we were particularly interested in Fab fragments, which retain the binding of the antibody but are much smaller in size, with the Fc effector domain removed (Lyu et al., 2018; Wissler et al., 2019). The small size of Fab fragments led to shorter in vivo circulation half-lives, which incur less background signals for imaging (Badescu et al., 2014). Compared to other antibody fragments such as ScFv, Fab still possess constant regions that are ideal for modifications and conjugations without compromising Fab’s binding with antigens (Lyu et al., 2018). We have since developed a site-specific αPD-L1 Fab conjugate with the isotope chelator 1,4,7-triazacyclononane-N,N′,N″-triacetic acid (NOTA), based on the incorporation of pAzF (Wissler et al., 2019) (Fig. 2). This immuno-PET tracer was used to capture the significant in vivo expression of PD-L1 in mouse tissues such as brown adipose and spleen (Wissler et al., 2019). Given the complications in antibody conjugation regarding yields, time, and labor (Young & Schultz, 2010), we have also developed a switchable antibody conjugate strategy (Lyu et al., 2018) (Fig. 2), which is focused on synthesizing and optimizing only one site-specific conjugate (αGCN4-Fab conjugate with Cy7 dye based on UAA pAcF) that is capable of recognizing GCN4 as a switch, thereby allowing for variability in the GCN4-fused IgG warhead. This GCN4-mediated switchable conjugate has been demonstrated to allow for NIRF imaging of different tumor types in vivo (Lyu et al., 2018). Here we describe the detailed protocols for the synthesis of UAA-based site-specific Fab conjugates with NOTA and Cy7, as well as a multitude of in vitro and in vivo assays to confirm the utility of this site-specific conjugation platform for molecular imaging.
Fig. 2.
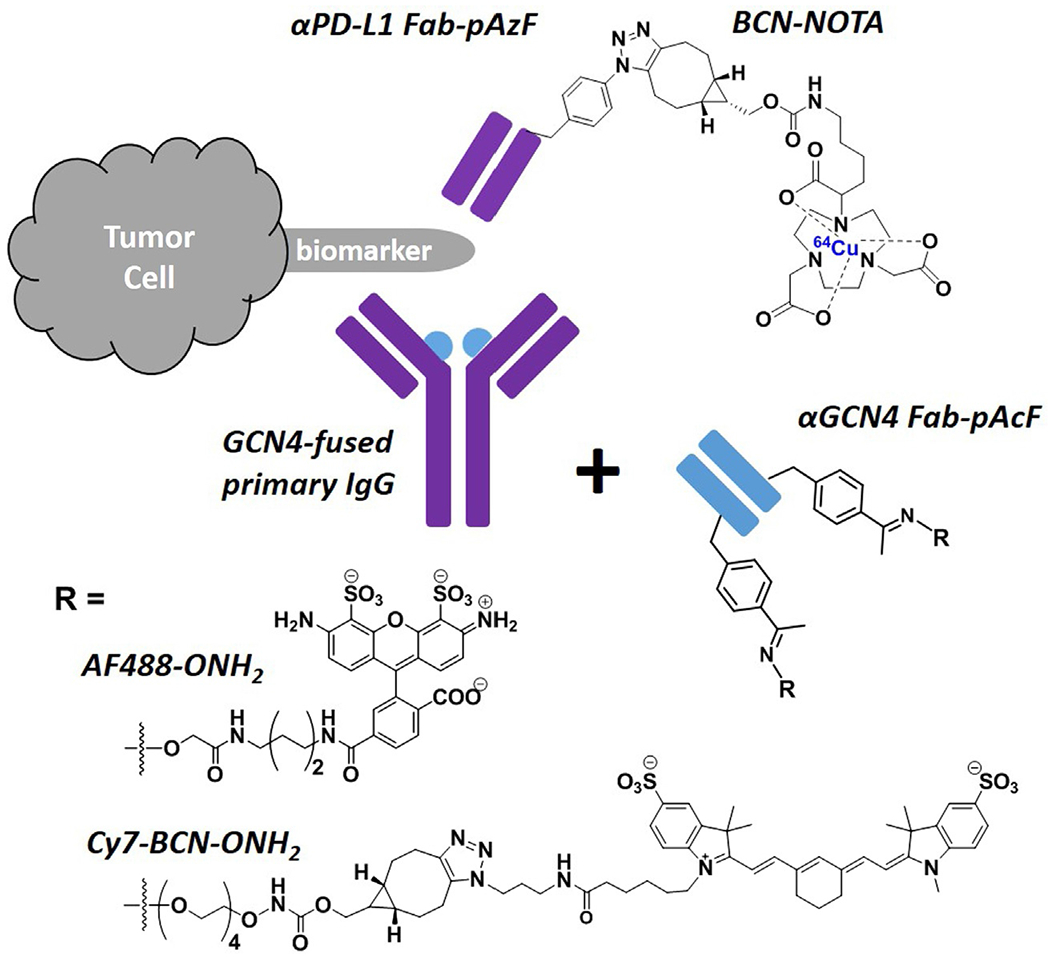
Summary of developed site-specific Fab conjugates based on the incorporation of UAA (pAzF or pAcF). Blue dot: GCN4 peptide fused onto primary antibodies.
2. Genetic incorporation of UAA to fab fragments
2.1. Construction of plasmids for wild type and mutant Fab’s
The sequence information for the variable and constant regions of αPD-L1 (avelumab) and αGCN4 antibodies can be obtained through literature (Zahnd et al., 2004) or patents (Nastri et al., 2014), which is the key for antibody plasmid construction. Plasmids for the expression of antibody Fab fragments can be feasibly synthesized via molecular cloning on the commercially available pBAD plasmid system that adopts a StII signaling peptide. The coding sequences can be directly synthesized from IDT, or quickly constructed by overlapping PCR of the fragments, followed by sub-cloning into the pBAD vector using a pair of restriction enzymes (NheI, ApaI) and T4-ligase-mediated ligation. Site-specific mutation to the amber codon can then be efficiently introduced via directed mutagenesis using the kit from New England Biolabs or Agilent Technologies. For UAA incorporation, the tyrosyl aminoacyl-tRNA synthetase pair from the archaea Methanococcus jannaschii (Mj) was introduced to the E. coli expression machinery, which is orthogonal to other intrinsic tRNA synthetase/tRNA pairs (Wang, Brock, Herberich, & Schultz, 2001). The mutant tRNA synthetases have been evolved to selectively aminoacylate their cognate tRNAs with pAcF (Kim et al., 2013), or pAzF (Young et al., 2011). Thus, a separate pUltra plasmid that encodes the Mj-TyrRS/tRNA pair for pAcF (Kim et al., 2013) or pAzF (Young et al., 2011) was directly used, along with the pBAD plasmid that encodes the Fab fragment during protein expression.
2.1.1. Equipment and materials
PCR Thermocycler (Bio-Rad)
Sub-Cell GT Electrophoresis Cell (Bio-Rad)
NanoDrop Lite Spectrometer (Thermo Scientific)
Gene Pulser Xcell Electroporator (Bio-Rad)
MaxQ 8000 Orbital Shaker (Thermo Scientific)
Heratherm Microbiological Incubator (Thermo Scientific)
Allegra Centrifuge (X-14R) (Beckman Coulter)
pBAD plasmid, pULTRA plasmid (Thermo Scientific)
Restriction enzymes, CutSmarter Buffer (New England BioLabs)
T4 ligase, ligase buffer (New England BioLabs)
DNA Clean & Concentrator (Zymo Research)
Zymoclean Gel DNA Recovery Kit (Zymo Research)
Quick-DNA Miniprep Kit (Zymo Research)
Q5 Site-Directed Mutagenesis Kit (New England BioLabs)
SOC Medium (Fisher Scientific)
Luria-Bertani Broth and agar (Sigma Aldrich)
Glycerol (Fisher Scientific)
Antibiotics: ampicillin, spectinomycin (Sigma Aldrich)
Escherichia coli strain TOP10 (Sigma Aldrich)
2.1.2. Protocol
Mix the pBAD template plasmid (~5 μg) with 2 μL ApaI in the 100 μL reaction solution containing the CutSmarter buffer. Incubate the mixture at room temperature for 2 h. Add approximately 2 μL of the other restriction enzyme, NheI, and incubate the mixture at 37 °C overnight.
Assemble the DNA strands corresponding to the coding sequences of the target proteins (αPD-L1 or αGCN4) in the order of “Light Chain Variable Domain (VL)—Light Chain Constant Domain (CL)—Heavy Chain Variable Domain (VH)—Heavy Chain Constant Domain 1(CH1)” by overlapping PCR, and enzyme digest the strands following the same procedure as step 1.
Purify products from steps 1–2 with DNA agarose gel electrophoresis, and extract the DNA via the Zymoclean Gel DNA recovery kit. Measure the concentrations accordingly on NanoDrop.
Mix approximately 100 ng each of the enzyme digested pBAD template and the antibody coding strand. To the final ligation solution (20 μL), add about 1 μL of T4 ligase and 2 μL of the 10× ligation buffer. After ligation at room temperature for 1 h, extract the DNA mixture containing possible products by the DNA Clean & Concentrator kit.
Elute the ligated plasmid out from the DNA concentrator column with 6 μL water, and transform 1 μL of the elutes into competent cells made from TOP 10 E. coli cells (Warren, 2011) via gene pulser electroporator. Recover the cells in 500 μL SOC medium with continuous shaking at 250 rpm and 37 °C for 1 h, and plate 50 μL of the medium on an Ampicillin containing LB agar plate.
After overnight incubation at 37 °C, pick the colonies from the plate and amplify each of them in 10 mL of Ampicillin containing LB media. Mix 0.5 mL of each saturate culture with 0.5 mL of 50% glycerol, and store them at −80 °C as stock solutions. Extract the plasmids for the rest of the culture with the Quick-DNA Miniprep Kit and validate the sequences by Sanger sequencing.
For site-directed mutation to amber codon, design the primers containing the TAG codon via the online software (NEBaseChanger). Mix ~20 ng of the vector that has been correctly inserted the Fab protein sequence in the PCR tube with forward and reverse primers (final concentration 0.5 μM) in the Q5 hot start high-fidelity 2× Master Mix. Perform 25 cycles of PCR amplification, followed by the KLD reaction.
Transform 5 μL of the KLD reaction mixture to competent cells, and perform the recovery, plating, and colony picking as described above. Validate the incorporation of TAG codon at the desired site by sanger sequencing.
If needed, steps 7–8 can be repeated to introduce more than one amber codon in the antibody sequence. With the incorporation of more than one equivalent of UAA, a Fab fragment can be conjugated with multiequivalents of payloads, which is the case for the αGCN4 switchable Fab conjugate (Lyu et al., 2018).
For future expression of mutant Fabs, transform both the correct pBAD plasmid (with amber codon mutation) and the pULTRA plasmid coding the Mj-TyrRS/tRNA pair for UAA incorporation into the E. coli competent cells (e.g., BL21). Plate the cells onto the agar plate that contains antibiotics for both plasmids. The next day, pick the colonies and amply them in the LB media with both antibiotics added. Extract the plasmids from part of the saturated culture by miniprep, and validate the two plasmids through DNA agarose gel electrophoresis. Mix the rest of the culture with 50% glycerol and store at −80 °C as the stock.
2.2. Fab expression and purification
All the engineered Fab fragments are expected to be readily expressible in E. coli. Compared to wild type, some modifications in the expression procedures are necessary in order to incorporate UAAs by amber suppression. Briefly, the nutritious cell culture media should be supplemented with UAAs. Antibiotic spectinomycin corresponding to the pULTRA plasmid should be added to the media, in addition to ampicillin that corresponds to the pBAD plasmid. Both antibiotics are required to selectively maintain the viability of cells that carry both pBAD and pUltra plasmids. During the induction of protein expression, IPTG is required to induce the T7 promoter in the pULTRA plasmid, while l-arabinose is needed to initiate the protein expression from the pBAD vector. The following protocol is focused on the expression of mutant Fab fragments. In practice, the UAA-based site-specific labeling also facilitates our exploration of various sites on the antibody that could lead to different stability, thereby resulting in varied expression yields (Lyu et al., 2018; Wissler et al., 2019). In that sense, experimental screening (Lyu et al., 2018; Wissler et al., 2019), along with in silico prediction by the RosettaBackrub algorithm (Wissler et al., 2019) should lead to the optimal mutation site for a given UAA.
2.2.1. Equipment and materials
MaxQ 8000 Orbital Shaker (Thermo Scientific)
Biowave CO 8000 Cell Density Meter (WPA)
Allegra Centrifuge (X-14R) (Beckman Coulter)
NanoDrop Lite Spectrometer (Thermo Scientific)
Nalgene Rapid-Flow Sterile Filter Units with 0.2 μm PES Membrane (Fisher Scientific)
CaptureSelect IgG-CH1 Affinity Matrix (Thermo Scientific)
Amicon Ultra Centrifugal Filters, 10 K MWCO (EMD Millipore)
2× YT medium (Sigma Aldrich)
Antibiotics: ampicillin, spectinomycin (Sigma Aldrich)
l-(+)-Arabinose (Alfa Aesar)
Isopropyl β-d-1-thiogalactopyranoside (IPTG) (VWR)
Lysozyme (VWR)
Dulbecco’s phosphate-buffered saline (DPBS), without calcium and magnesium (Corning)
2.2.2. Protocol
To minimize the possibility of contamination, steps 1–4 should be performed in the area sterilized by 70% ethanol and close to the flame of natural gas. Protein extraction and purification (after step 5) should be performed in a cold room (4 °C) or on ice.
Inoculate the E. coli stock containing the desired plasmids in 8 mL 2× YT media with 100 μg/mL ampicillin and 100 μg/mL spectinomycin. Incubate the mixture by shaking at 37 °C, 250 rpm for overnight.
Dilute the start culture in 800 mL 2× YT media with 100 μg/mL ampicillin, 100 μg/mL spectinomycin, and 1 mM UAA (pAzF or pAcF). Continue to shake the cell culture at 37 °C, 250 rpm.
Monitor the cell growth phase by measuring OD600 using the Cell Density Meter, until the value reaches 0.8–1.0.
Induce the protein expression by adding 0.2% l-arabinose and 0.5 mM IPTG (all are final concentrations). Continue the expression at 30 °C, 250 rpm, for 24 h.
Harvest cells by centrifuging at 4 °C, 500 rpm, for 30 min. Discard the supernatant and freeze the cell pellets at −80 °C for at least 3 h.
Thaw the cell pellets and re-suspend them in 80 mL of the periplasmic lysis buffer (20% sucrose, 30 mM Tris (pH 8.0), 1 mM EDTA) that has been freshly dissolved with 0.2 mg/mL lysozyme. Mix thoroughly by pipetting up and down. Incubate the cell suspension at 37 °C, 250 rpm, for 20 min.
Extract the periplasmic lysates by centrifuging the suspension at 4 °C, 9000 rpm for 30 min.
Filter the supernatant through 0.2 μm membrane to remove debris.
Prepare the CH1 affinity column by equilibrating 1.5 mL IgG-CH1 Affinity Matrix beads slurry in PBS buffer. Allow the filtered periplasmic lysates to flow through the CH1 affinity column twice under gravity to capture Fab fragments.
Wash the CH1 affinity column twice with PBS buffer. Elute Fab fragments with 6 mL of 100 mM aqueous glycine solution (pH 2.8), and collect the eluent in a centrifuge tube with 600 μL 1 M Tris buffer (pH 8.0) pre-added into the bottom of the tube for neutralization.
Concentrate the protein containing eluent using an Amicon Ultra centrifugal filter and exchange the buffer into PBS. Determine the Fab concentration on NanoDrop.
Aliquot and snap-freeze the Fab proteins by liquid nitrogen. Store the aliquots at −80 °C for long-term storage.
3. Linker compound synthesis
A variety of payloads can be conjugated site-specifically to the mutant Fab fragments that possess the UAA as a handle. Depending on the chemical reactivity of the incorporated UAA, different bioorthogonal conjugation chemistries can be employed. For example, pAzF bears electron-deficient aryl azides, and is ideal for the strain-promoted azide-alkyne cycloaddition (SPAAC) conjugation with strained alkyne derivatized linker compound at neutral pH (Dommerholt et al., 2014; Wissler et al., 2019). In the process of developing a site-specific immuno-PET tracer, we have designed and synthesized a NOTA derivative that is capable of chelating 64Cu, a metallic radionuclide for PET, and was tethered to the strained bicyclo[6.1.0]non-4-yne (BCN) linker for SPAAC conjugation with the pAzF containing αPD-L1 Fab mutant (Wissler et al., 2019). The NOTA derivative design was inspired by the enhanced chelation capabilities of hexadentate NOTA with branched substitution (3p-C-NOTA) (Wu, Kang, et al., 2016). On the other hand, pAcF is a typical UAA tag for coupling with molecules of interest via a stable oxime linkage (Axup et al., 2012; Hutchins et al., 2011; Kazane et al., 2012; Kularatne et al., 2014). To develop the switchable antibody conjugate, we have conjugated the pAcF containing αGCN4 Fab with alkoxy-amine derivatized Alexa Fluor 488 (AF488), or the alkoxy-amine modified PEGylated BCN linker in tandem with the sulfo-cyanine 7 (Cy7) azide (Lyu et al., 2018). Given that all alkoxy-amine related dyes and linkers are commercially available, this section will focus on the preparation of the NOTA-BCN payload (Fig. 3) for PET imaging.
Fig. 3.
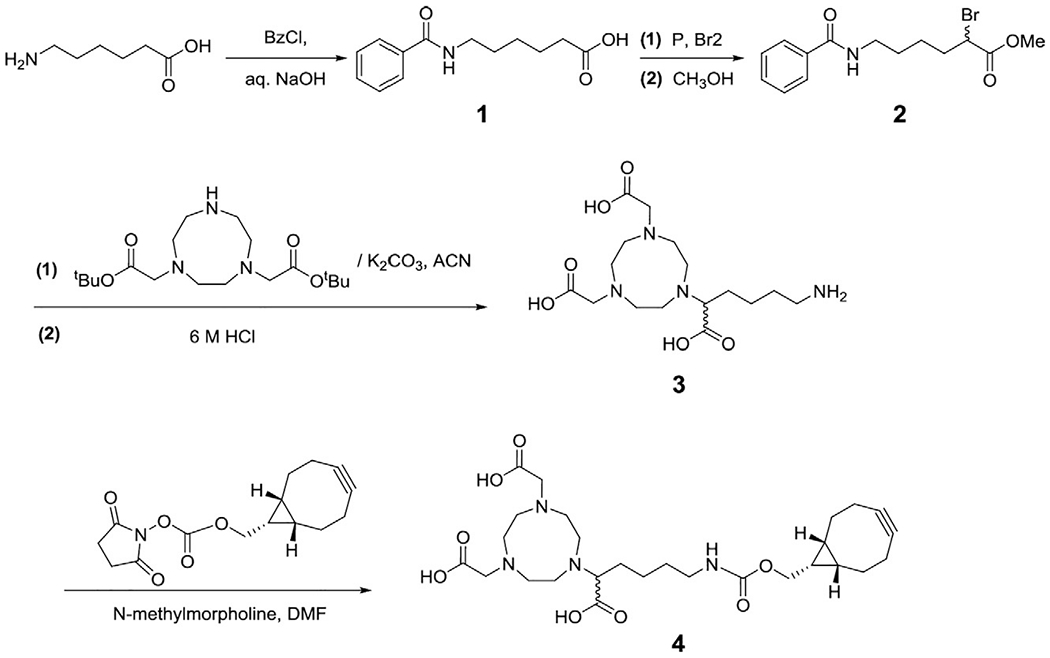
Synthetic scheme of 1,4,7-triazacyclononane-N,N′,N″-triacetic acid (NOTA) derivative with a (1R, 8S, 9S)-bicyclo[6.1.0]non-4-yn-9-ylmethylcarbamate (BCN) linker. Adapted with permission from Wissler, H. L., Ehlerding,E. B., Lyu, Z., Zhao, Y., Zhang, S., Eshraghi, A., et al. (2019). Site-specific immuno-PET tracer to image PD-L1, Molecular Pharmaceutics, 16, 2028—2036. Copyright (2019) American Chemical Society.
3.1. Equipment and materials
Chemical Fume Hood (SafeAire)
Stirring Hot Plate (Thermo Fisher)
Handheld UV Lamp (Chemglass)
Thin-layer chromatography plates (VMR International)
Rotary Evaporator System (Buchi R-200)
Agilent 1100 series, high-resolution LC–MS
400 and 500 MHz NMR (Bruker Advance)
Waters 1525 series HPLC equipped with a XBridge Prep C18 column (5 μm, 19 × 250 mm2)
LABCONCO FreeZone 4.5 Lyophilizer
Silica Gel (Fisher Scientific 50–60 Å pore diameter)
Round Bottom Flasks (ChemGlass)
3.2. Reagents
Organic solvents (methanol, ethyl acetate, hexane, diethyl ether, acetonitrile, N,N-dimethylformamide) (Fisher Scientific/VWR International)
HPLC-grade water (Fisher Scientific)
Sodium thiosulfate (Fisher Scientific)
Sodium bicarbonate (VWR International)
Potassium carbonate (VWR International)
N-methyl morpholine (VWR International)
Bromine liquid (EMD Millipore)
Phosphorus, red stabilized for synthesis (MilliporeSigma)
1,4,7-Triazacyclononane-1,4-bis(t-butyl acetate) (Macrocyclics, Inc)
Hydrochloric acid, 36.5–38.0% (Fisher Scientific)
BCN-succinimidyl ester (Sigma Aldrich)
Benzamidohexanoic acid (Fisher Scientific)
Benzoyl chloride (Fisher Scientific)
Sodium hydroxide (VWR International)
Hydrochloric acid, 36.5–38.0% (Fisher Scientific)
3.3. Protocol
The number of the following steps refers to the intermediates given in Fig. 3. Care should be taken when handling phosphorus (toxic, flammable) and liquid bromine (toxic, corrosive) in step 2. All steps should be performed in a chemical fume hood, with the wearing of appropriate personal protective equipment.
Add 6-aminohexanoic acid (4 g, 0.0341 mol) to a round-bottom flask and dissolve it with 1 M NaOH in water (41 mL). Place the reaction mixture into an ice bath. Add benzoyl chloride (4.76 mL, 0.0410 mol) dropwisely and stir the reaction mixture overnight. Once completed, wash the aqueous layer with diethyl ether (2 × 40 mL) to extract unreacted benzoyl chloride. Acidify the aqueous layer with 1 M HCl (~45 mL) to pH ~3. Extract the product with ethyl acetate (3 × 40 mL) and vacuum concentrate the organic layer to obtain compound 1 (70% yield, 5.29 g) as a white solid.
Mix compound 1 (3 g, 0.0128 mol) and dry red phosphorus (0.53 g, 0.0172 mol) in a flame-dried 100 mL round-bottom flask. Cool the mixture down to −15 °C in an ice-salt bath. Add liquid bromine (8.16 g, 0.102 mol) dropwisely, remove the ice bath and reflux the reaction mixture in a silicon oil bath overnight at 100 °C. Once completed, remove the flask from the oil bath and cool the mixture to room temperature and then 0 °C, followed by adding 20 mL of methanol dropwisely to quench the reaction. To form the desired methyl ester (compound 2), reflux the reaction mixture at 100 °C for 30 min. Upon reaction completion as monitored by TLC, vacuum concentrate, and re-suspend the mixture in 100 mL of ethyl acetate. Wash the organic phase with saturated sodium bicarbonate (1 × 30 mL), saturated sodium thiosulfate (2 × 30 mL), and brine (1 × 30 mL). After washing, concentrate the organic layer to a crude oil via rotary evaporation and purify the product via flash column chromatography (5:1, hexane:ethyl acetate) to obtain compound 2 at an 85% yield (3.57 g).
Add compound 2 (82.3 mg, 0.243 mmol) along with 1,4,7-triazacyclononane-1,4-bis(t-butyl acetate) (72.6 mg, 0.203 mmol), and K2CO3 (33.6 mg, 0.243 mmol) to 5 mL dry acetonitrile in a flame-dried round bottom flask under nitrogen atmosphere. Reflux the reaction for 48 h, and then concentrate the mixture under reduced pressure. Redissolve the crude mixture in 4 mL of 6 M aqueous HCl and reflux the solution for 24 h. Purify the hydrolyzed product via HPLC to yield compound 3 in ~50% yield (38 mg) over two steps.
To a flame-dried flask, dissolve compound 3 (6.9 mg, 0.0185 mmol) and BCN-Succinimidyl ester (5.4 mg, 0.0185 mmol) in anhydrous DMF. Add n-methylmorpholine (3.75 mg, 0.037 mmol) dropwisely and then stir the reaction mixture for 4 h at room temperature, at which point the succinimidyl ester should be fully converted. Concentrate the reaction mixture under vacuum, and purify the product via HPLC to afford 3.2 mg of final compound 4 (NOTA-BCN, 31% yield) as a white solid.
4. Site-specific fab conjugation and purification
Site-specific conjugation between the UAA on the Fab proteins and the linker compound is performed under mild reaction conditions and can be classified into two major categories: SPAAC for pAzF incorporated antibodies at neutral pH, or Oxime coupling for pAcF incorporated antibodies under mildly acidic pH. For both reactions, at most 10% of DMSO can be added into the final reaction buffer in order to promote the solubility of chemical linkers. Addition of any reducing agents may risk the stability of disulfide bonds within the protein structure. Lastly, the concentration of linker compound should be restrained (usually ~10 equivalents relative to the protein) to prevent non-specific side reactions, and to facilitate follow-up purification.
4.1. Equipment and materials
Allegra Centrifuge (X-14R) (Beckman Coulter)
Fisherbrand AccuSpin Micro 17R Microcentrifuges (Fisher Scientific)
NanoDrop Lite Spectrometer (Thermo Scientific)
Heratherm Microbiological Incubator (Thermo Scientific)
Amicon Centrifugal Filter (EMD Millipore)
AKTA fast protein liquid chromatography (FPLC) system (Amersham Biosciences)
Superdex 200 Increase 10/300 GL (GE Healthcare)
Zeba Spin Desalting Column (Thermo Scientific)
Dulbecco’s phosphate-buffered saline (DPBS), without calcium and magnesium (Corning)
Sodium acetate (VWR International)
4.2. Protocol
Thaw the mutant Fab stock solution on ice. Measure the stock concentrations again by NanoDrop, as freezing and thawing sometimes may lead to protein loss. Concentrate and/or buffer exchange with the Fab fragments using an Amicon centrifugal filter. Since the storage buffer is PBS that is ideal for SPAAC conjugation, directly concentrate the Fab fragments to 2.5 mg/mL for coupling between pAzF and the NOTA-BCN compound. For conjugation of pAcF incorporated Fab with aminooxy-derivatized AF488 or BCN linker compound, exchange the PBS stock buffer into 50 mM NaOAc reaction buffer (pH 4.5).
Transfer the concentrated and/or buffer exchanged antibody into an Eppendorf tube. Add 10 equivalents of the desired linker compound. For oxime coupling, add to the mixture acetyl hydrazide (1000 equivalents) as a catalyst. Mix the reaction mixture gently by pipetting or flipping.
Incubate the reaction mixture at 37 °C for 6 h or 24 h, for pAzF or pAcF-mediated conjugation, respectively.
Before work-up, ESI-MS characterization of the crude mixture is necessary to ensure the complete reaction conversion, since it is challenging to separate conjugates with unconjugated antibodies. Purify the fully converted mixture by FPLC. Carefully collect the eluted fractions to avoid contamination by unreacted linker compounds. Alternatively, pass the mixture through at least two rounds of Zeba spin column with PBS as the equilibrium buffer. The purified conjugates will need to be revalidated by ESI-MS, followed with either subsequent in vitro and in vivo assays or snap-freezing in liquid nitrogen for long time storage.
5. In vitro characterization of the fab conjugates
With the site-specific Fab conjugates prepared in Section 4, a series of in vitro assays (Fig. 4) can be performed to evaluate their purity (by sodium dodecyl sulfate—polyacrylamide gel electrophoresis (SDS-PAGE)), identity (by electrospray ionization—mass spectrometry (ESI-MS)), and binding affinity (by enzyme-linked immunosorbent assay (ELISA) and/or flow cytometry). It is important to compare the antigen binding affinity between the wild type Fab and the mutants, as well as the Fab conjugates, in order to make sure that neither the incorporation of UAA nor the payload conjugation compromises the antibody’s binding.
Fig. 4.

In vitro characterization of the site-specific Fab conjugates. (A) SDS-PAGE analysis of αPD-L1 Fab wild type (lane 1), αPD-L1 Fab mutant (HC K129X, X = pAzF) (lane 2), and αPD-L1 Fab-NOTA conjugate (HC K129X, X = pAzF-BCN-NOTA) (lane 3); (B) ESI-MS analysis of αPD-L1 Fab before (left) and after conjugation (right) with the BCN-NOTA linker; (C) ELISA analysis of the binding affinities of αPD-L1 Fab and Fab conjugates toward antigen PD-L1. EC50 was determined by GraphPad Prism to be around 7 × 10−10 M for Wt, the mutant (HC K129X), and the NOTA conjugate. (D) Flow cytometry analysis of cancer cell detection by GCN4-mediated switchable Fab conjugates. Peak traces from top to bottom represent cells treated with the mixture of the αGCN4 Fab AF488 conjugate and GCN4-fused αHER2 IgG at 25 nM (pink) and 5 nM (deep green);or the mixture of the same Fab conjugate but with GCN4-tagged αCD19 IgG at 25 nM (green) and 5 nM (orange). The cells without treatment were used as the negative control (red). Adapted with permission from Wissler, H. L., Ehlerding,E. B., Lyu, Z., Zhao, Y., Zhang, S., Eshraghi, A., et al. (2019). Site-specific immuno-PET tracer to image PD-L1, Molecular Pharmaceutics, 16, 2028–2036; Lyu, Z., Kang, L., Buuh, Z. Y., Jiang, D., McGuth, J. C., Du, J., et al. (2018). A switchable site-specific antibody conjugate, ACS Chemical Biology, 13, 958–964. Copyright (2018, 2019) American Chemical Society.
5.1. Equipment and materials
Direct-Q 3 UV Water Purification System (Millipore Sigma)
Mini Gel Tank (Thermo Fisher)
KJ-201BD Orbital Shaker (KangJian)
Q Exactive Plus Orbitrap Mass Spectrometer (Thermo Fisher)
Synergy H1 Plate Reader (BioTek)
Allegra Centrifuge (X-14R) (Beckman Coulter)
LSR Fortessa Flow Cytometer (BD Biosciences)
NuPAGE Precast Gels (Thermo Fisher)
PageRuler Prestained Protein Ladder (Thermo Fisher)
LDS Sample Buffer (Novex)
MES SDS Running Buffer (Novex)
Coomassie Brilliant Blue (CBB) R-250 (Thermo Scientific)
Methanol (Fisher Scientific)
Glacial acetic acid (Fisher Scientific)
Flat-bottom 96-well plates (Corning)
Phosphate-buffered saline (PBS) buffer (Corning)
Bovine serum albumin (Thermo Fisher)
QuantaBlu Fluorogenic Peroxidase Substrate Kit (Thermo Fisher)
Anti-Human Lambda Light Chain Antibody HRP-Labeled (MilliporeSigma)
Anti-Human Kappa Light Chain Antibody HRP-Labeled (MilliporeSigma)
Recombinant Human ErbB2/Her2 Fc Antigen (R&D Systems)
Recombinant Human CD19 Fc Antigen (R&D Systems)
SK-BR-3 Breast Cancer Cell Line (ATCC)
Ramos Burkitt’s Lymphoma Cell Line (ATCC)
DMEM Cell Culture Medium (Corning Cellgro)
RPMI-1640 Cell Culture Medium (Corning Cellgro)
Fetal bovine serum (Fisher Scientific)
Antibiotic-Antimycotic 100× Mix (Corning)
0.05% trypsin, 0.53 mM EDTA solution (Corning)
32% paraformaldehyde solution (Electron Microscopy Sciences)
5.2. Protocol
5.2.1. SDS-PAGE and ESI-MS
Prepare samples for SDS-PAGE in Eppendorf tubes. For samples without the use of Dithiothreitol (DTT) as a reductant, add 2 μg of the desired conjugate, 5 μL of LDS sample loading buffer (4×) and add 13 μL of Milli-Q water to dilute the sample to a total volume of 20 μL. For samples with DTT, add 3 μg of the desired Fab conjugate, 2 μL of 100 μM DTT, 5 μL of LDS sample loading buffer, and 10 μL of Milli-Q to dilute the sample to a total volume of 20 μL. All samples are mixed thoroughly via pipetting and heated at 90 °C for 2–5 min prior to loading.
Insert a piece of 4–12% Bis-Tris Plus, 10-well pre-cast gel into a Mini Gel Tank. Add 1× MES SDS running buffer to fully submerge the gel.
Load the PageRuler™ Prestained protein ladder, followed by each antibody or antibody conjugate sample into individual wells.
Close the lid to Mini Gel Tank and run gel electrophoresis at 150 V for approximately 50 min or until the target molecular weight on the ladder has moved halfway down the gel.
Remove the gel from the Mini Gel Tank and place it in a container for CBB gel staining.
Prepare the CBB staining solution that contains 1.0 g of CBB R-250 per 400 mL with 10% glacial acetic acid, 45% methanol, and 45% H2O.
Fully submerge the gel in the CBB staining solution and incubate at room temperature for 20 min on the orbital shaker.
Decant the staining solution and rinse the gel with 100 mL of Milli-Q water. Incubate the gel in Milli-Q water with moderate shaking. Repeat the washing step until the gel has been fully destained. At this point, the CBB stained protein bands corresponding to Fab conjugates should be clearly observed. The non-reduced Fab or Fab conjugate should migrate as a single band, with >95% purity and a molecular mass of ~45–50 kDa (Fig. 4A). In the lane for samples treated by DTT, two distinct bands can be observed at ~20–30 kDa, corresponding to the reduced heavy chain and light chain, respectively.
After validation of purity, the protein conjugates are also analyzed by ESI-MS. This part is usually handled by the staff at the mass spectrometry core. Briefly, samples will be diluted with 0.1% formic acid. About 2 μL of the diluted Fab conjugates at a concentration of 1 pmol/μL will be injected onto a custom-made Poros 10R2 column, and reverse phased into a Q Exactive Plus Orbitrap Mass Spectrometer. The mass raw data will be extracted by Thermo Xtract program, and the protein deconvolution will be carried out using Thermo Promass for Xcalibur. A sample ESI-MS spectra is shown in Fig. 4B.
5.2.2. ELISA
ELISA was used to determine binding affinity, comparing the Fab conjugates and Fab mutations incorporating the UAA against the wild type. Briefly, the antigen is coated onto a well and the Fab or Fab conjugates specific to that antigen is added. A detection antibody conjugated with horse radish peroxidase (HRP) is then added, which will recognize the antigen captured Fab. The HRP on the detection antibody will then react with a highly-sensitive fluorogenic substrate to emit a stable and robust fluorescent signal (excitation: 325 nm/emission: 420 nm) that can be detected and analyzed. For measuring different classes of Fab antibodies (e.g., αGCN4 Fab has a kappa light chain (Lyu et al., 2018) while αPD-L1 Fab has a lambda light chain (Wissler et al., 2019)), different detection antibodies were used (e.g., anti-human kappa light chain antibody HRP-labeled for detecting αGCN4 Fab, anti-human lambda light chain antibody HRP-labeled for detecting αPD-L1 Fab).
Add 100 μL of antigen (1–10 μg/mL) in PBS solution to each well of a flat-bottom 96-well black plate and let them sit overnight for coating.
Wash with PBS buffer three times, and block each well with 300 μL of PBS buffer/1% BSA at room temperature for 1.5 h.
Serially dilute Fab/Fab mutants/Fab conjugates in the blocking buffer to desired concentrations. Then add these at 100 μL/well in triplicate and incubate the plate at room temperature for 2 h.
Wash all the wells with washing buffer (PBS/0.05% Tween) for three times.
Dilute HRP-labeled detection antibody at an appropriate concentration (usually 1000–10,000-fold) in blocking buffer, add them to wells and incubate for another 2 h.
- Wash wells extensively with washing buffer a further five times.
- Add 100 μL QuantaBlu fluorogenic ELISA substrate to each well. After 20 min of signal development, run fluorescent test on a plate reader (excitation: 325 nm/emission: 420 nm).
Process the data by Graphpad Prism software. Plot the results using the nonlinear regression equation “Log(agonist) vs. response—variable slope” to determine the EC50 values (half-maximal binding constant). A sample ELISA results was shown in Fig. 4C.
5.2.3. Flow cytometry
For certain applications (GCN4-mediated switchable antibody conjugate (Lyu et al., 2018)), we also gauged if the switch GCN4 can mediate Fab conjugates’ detection of different cancer cell lines, which serves as a solid tool to predict the feasibility of follow-up in vivo experiments. Thus, flow cytometry experiments were carried out using GCN4 fused primary antibody (αHER2 or αCD19) and αGCN4-AF488 conjugate, which demonstrated the targeted detection of corresponding cancer cell lines (αHER2 combo mix bound HER2+ SK-BR-3 cell line, while αCD19 combo mix bound CD19+ Ramos cell line).
Harvest SK-BR-3 and Ramos cells. The adherent cell line SK-BR-3 was separated from the culture plate by 0.05% trypsin, 0.53 mM EDTA solution. Wash cells by repeated suspending in 4 °C PBS and spinning at 1000 rpm for 5 min.
Add 4% paraformaldehyde solution to the cell pellets. Pipette up and down to mix well. Incubate at room temperature for 15 min to allow for complete fixing of cells.
Wash cells with PBS and block with 2% BSA/PBS buffer for 1 h at 1 × 106 cells/mL.
Mix cells with a GCN4-tagged antibody (either αCD19 or αHER2) and the AF488 conjugated αGCN4 Fab at a molar ratio of 1:2 in the blocking buffer. Shake the tube or plate mildly at room temperature for 2 h.
Spin the cells down at 1000 rpm for 5 min and decant the solution.
Wash cells with cold PBS three times.
Analyze cell samples with a flow cytometer and detect the signals in the FITC channel. Process the results with FlowJo software (TreeStar) (Fig. 4D).
6. In vivo imaging using fab conjugates
Depending on the payloads, the site-specific Fab conjugates prepared in previous sections can be utilized for different in vivo imaging applications. Conjugation with the 1,4,7-triazacyclononane-N,N′,N″-triacetic acid (NOTA) chelator allows for the radiolabeling of the antibody using 64Cu. Following procedures similar to literature reports (D’Souza et al., 2017; Hettich et al., 2016), the radiolabeled conjugates can be used for immunoconjugate-based positron emission tomography (immuno-PET) (Wissler et al., 2019) (Fig. 5A). Conjugation with Cy7 allows for a safe means for the optical imaging of organs and tumor tissues in the near-infrared region, without interference by background tissues’ autofluorescence and light scattering (Yi, Wang, Qin, Yang, & Yuan, 2014; Zhang, Bloch, Akers, & Achilefu, 2012) (Fig. 5B). Note: All animal experiments were performed in accordance with the National Institute of Health (NIH) guidelines and conducted under the approval by University Institutional Animal Care and Use Committee.
Fig. 5.

Representative in vivo imaging using site-specific Fab conjugates. (A) PET imaging scans at 15 and 45 min post-injection of 64Cu-αHER2-NOTA (left) or 64Cu-α PD-L1-NOTA (right) in nude mice. The yellow arrow indicates adipose tissue while the white arrow points to the spleen;(B) NIRF imaging of SKBR3 and Ramos tumor bearing xenografts after the administration of premixed αHER2-GCN4 fusion: αGCN4-Cy7(LC-S155X, HC-K132X, X = pAcF) or αCD19-GCN4 fusion: αGCN4-Cy7(LC-S155X, HC-K132X, X = pAcF). Adapted with permission from Wissler, H. L., Ehlerding,E. B., Lyu, Z., Zhao, Y., Zhang, S., Eshraghi, A., et al. (2019). Site-specific immuno-PET tracer to image PD-L1, Molecular Pharmaceutics, 16, 2028–2036; Lyu, Z., Kang, L., Buuh, Z. Y., Jiang, D., McGuth, J. C., Du, J., et al. (2018). A switchable site-specific antibody conjugate, ACS Chemical Biology, 13, 958–964. Copyright (2018, 2019) American Chemical Society.
6.1. Equipment and materials
Inveon microPET/CT scanner (Siemens)
Automated gamma counter (PerkinElmer)
IVIS Spectrum for NIRF imaging (PerkinElmer)
Orbital Shaker (Thermo Fisher)
Athymic (nude) mice (Envigo)
C57BL/6 mice (Envigo)
CB17-SCID mice (Envigo)
Matrigel matrix to re-suspend cancer cells (Invitrogen)
Cell Biology Grade PBS buffer (Corning)
2% isoflurane (Millipore Sigma)
64CuCl2 in 0.1 N HCl (University of Wisconsin-Madison)
PD-10 column (GE Healthcare)
DPBS Buffer, Cell Biology Grade (Corning)
6.2. Protocol
All mice were purchased between the ages of 4 and 6 weeks. For tumor implantation, harvest and re-suspend cancer cells in a matrigel matrix at 5 × 106 cells/well. Inject ~200 μL cancer cells into the lower right flank of each mouse. Monitor mice health (including body weight) and measure tumor volumes every other day. Once the tumor size grows to a diameter of ~1 cm, mice will be sorted, grouped (n = 3–5), and subjected to imaging experiments.
6.2.1. PET imaging
In vivo PET imaging has been performed with αPD-L1 Fab-NOTA conjugate and control conjugates such as αHER2 Fab-NOTA. Radiolabeling of the immunoconjugate is required before proceeding to the treatment of mice. Given the limited half-life (12.7 h) of the 64Cu isotope, the radiolabeled protein conjugate is usually immediately administered to mice after purification. Caution should be taken when performing the series of experiments involved with radioisotope materials. The 64CuCl2 stock should be stored behind 2 inch thick lead bricks. All the experiments should be performed in the lab area clearly outlined for radioactive material use. Disposable gloves, lab coats, and safety glasses as the minimum PPE are required. The persons working in the area should perform experiments behind L-block shields, and always wear radiation dosimetry monitoring badges (body and ring). All the experiment steps need to be planned ahead to minimize exposure to radiation. Lastly, potentially contaminated PPE should be removed and carefully disposed prior to leaving the area.
Radiolabeling of αPD-L1 Fab-NOTA was performed similar to previously reported methods (Ehlerding et al., 2017; Ferreira et al., 2018).
Dilute 64CuCl2 in 150–300 μL of sodium acetate buffer (pH ~5).
Add 50 μg of αPD-L1 Fab-NOTA or αHER2 Fab-NOTA to 1 mCi (37 MBq) 64Cu. Then incubate for 1 h at 37 °C with constant shaking.
Purify radiolabeled samples with a PD-10 column using PBS as the mobile phase. Note that all injectable solutions were prepared in sterile PBS.
Anesthetize mice with 3%/1% isoflurane via inhalation.
Intravenously inject the mice with 50–70 μCi of one of the radiolabeled 64Cu-NOTA conjugates, either the αPD-L1 or αHer2.
Perform PET imaging with an Inveon microPET/CT scanner at 5, 15, 45, and 90 min post-injection. At each of the scanning time points, 20 million counts per mouse was obtained. Reconstruct the PET images using a 3D ordered subsets expectation maximization reconstructions algorithm (Fig. 5A).
To quantify the Fab conjugate accumulation in organs and tissues, use the Inveon Research Workspace software to analyze the mean percent injected dose per gram (%ID/g) at the regions-of-interest (ROI) following the equation: %ID/g = [activity in tissue (μCi/g)/administered activity (μCi)] × 100%
6.2.2. NIRF imaging
In vivo NIRF imaging has been performed with GCN4-mediated switchable antibody conjugates, during which either the GCN4-fused primary antibody and the αGCN4 Fab-Cy7 can be co-injected or the primary antibody can be injected hours ahead for pretargeted imaging.
Premix GCN4-tagged primary antibody (either αCD19 or αHER2) with αGCN4 Fab-Cy7 conjugate at a molar ratio of 1:2 for 2 h.
Intravenously inject the antibody mixture into three mice with a dosage of 60 μg of primary antibody per mouse.
Anesthetize mice using 2% isoflurane at set post-injection time intervals: 1, 4, 8, 16, 24, 48, and 72 h.
After each anesthetization, place the mouse on the IVIS Spectrum stage with the tumor side facing the CCD camera. Perform NIRF imaging using an excitation wavelength of 745 nm and an emission wavelength of 800 nm. To prevent image saturation, use automatic scanning time.
Place regions of interests (ROIs) on the 2D NIRF image to encompass the entire NIRF signal (Fig. 5B).
Pre-targeted NIRF Imaging
Inject a GCN4-tagged primary antibody (either αCD19 or αHER2) intravenously into the SK-BR-3 mouse model at a dosage of 60 μg of primary antibody per mouse for pre-targeting.
Intravenously inject the αGCN4 Fab-Cy7 conjugate at a dosage of 35 μg/mouse 4 h after the primary antibody injection.
The control group mice were injected with PBS buffer and then 4 h later with the same dosage of αGCN4 Fab-Cy7 conjugate.
Perform the NIRF imaging using the same procedures as above.
7. Summary and conclusions
The methods presented here provide an efficient and reliable approach to develop site-specific antibody Fab fragment conjugates. The UAA-based site-specific protein conjugation approach has been thereby illustrated as a general strategy to endow versatility to antibodies, making homogeneous conjugates with various types of small molecules of distinct functions. Further efforts may include site-by-side comparisons of the in vivo properties and activity of this class of conjugates with random conjugates and cysteine-based site-specific conjugates. Nevertheless, future applications of these imaging probes into clinical settings would be expected to facilitate image-guided therapy in multiple disease areas.
Acknowledgments
We thank Dr. Hsin-Yao Tang and Mr. Thomas Beer at the Wistar Institute, Proteomics and Metabolomics Facility for performing the ESI-MS analysis service. This work was supported by Temple University (start-up funds to R.E.W.), The American Cancer Society (#15-175-22 to R.E.W.), and the National Institutes of Health Grants (1R35GM133468-01 to R.E.W., P30CA014520 to collaborators at the University of Wisconsin-Madison). Support for the NMR facility at Temple University by a CURE grant from the Pennsylvania Department of Health is also gratefully acknowledged.
References
- Adumeau P, Sharma SK, Brent C, & Zeglis BM (2016a). Site-specifically labeled Immunoconjugates for molecular imaging—Part 1: Cysteine residues and Glycans. Molecular Imaging and Biology, 18, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adumeau P, Sharma SK, Brent C, & Zeglis BM (2016b). Site-specifically labeled Immunoconjugates for molecular imaging—Part 2: Peptide tags and unnatural amino acids. Molecular Imaging and Biology, 18, 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, et al. (2012). Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proceedings of the National Academy of Sciences of the United States of America, 109, 16101–16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badescu G, Bryant P, Bird M, Henseleit K, Swierkosz J, Parekh V, et al. (2014). Bridging disulfides for stable and defined antibody drug conjugates. Bioconjugate Chemistry, 25, 1124–1136. [DOI] [PubMed] [Google Scholar]
- Cao Y, Axup JY, Ma JS, Wang RE, Choi S, Tardif V, et al. (2015). Multiformat T-cell-engaging bispecific antibodies targeting human breast cancers. Angewandte Chemie International Edition, 54, 7022–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Lesniak WG, Gabrielson M, Lisok A, Wharram B, Sysa-Shah P, et al. (2016). A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget, 7, 10215–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommerholt J, van Rooijen O, Borrmann A, Guerra CF, Bickelhaupt FM, & van Delft FL (2014). Highly accelerated inverse electron-demand cycloaddition of electron-deficient azides with aliphatic cyclooctynes. Nature Communications, 5, 5378. [DOI] [PubMed] [Google Scholar]
- D’Souza JW, Hensley H, Doss M, Beigarten C, Torgov M, Olafsen T, et al. (2017). Cerenkov luminescence imaging as a modality to evaluate antibody-based PET radiotracers. Journal of Nuclear Medicine, 58, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlerding EB, England CG, Majewski RL, Valdovinos HF, Jiang D, Liu G, et al. (2017). ImmunoPET imaging of CTLA-4 expression in mouse models of non-small cell lung cancer. Molecular Pharmaceutics, 14, 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England CG, Jiang D, Ehlerding EB, Rekoske BT, Ellison PA, Hernandez R, et al. (2018). (89)Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. European Journal of Nuclear Medicine and Molecular Imaging, 45, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CA, Hernandez R, Yang Y, Valdovinos HF, Engle JW, & Cai W (2018). ImmunoPET of CD146 in a murine hindlimb ischemia model. Molecular Pharmaceutics, 15, 3434–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P, Forouzandeh M, Rahbarizadeh F, Ramezani R, & Rasaee MJ (2006). Production of anti-digoxigenin antibody HRP conjugate for PCR-ELISA DIG detection system. Journal of Immunoassay and Immunochemistry, 27, 303–318. [DOI] [PubMed] [Google Scholar]
- Hernandez R, Sun H, England CG, Valdovinos HF, Ehlerding EB, Barnhart TE, et al. (2016). CD146-targeted immunoPET and NIRF imaging of hepatocellular carcinoma with a dual-labeled monoclonal antibody. Theranostics, 6, 1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heskamp S, Hobo W, Molkenboer-Kuenen JD, Olive D, Oyen WJ, Dolstra H, et al. (2015). Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies. Cancer Research, 75, 2928–2936. [DOI] [PubMed] [Google Scholar]
- Hettich M, Braun F, Bartholoma MD, Schirmbeck R, & Niedermann G (2016). High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics, 6, 1629–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins BM, Kazane SA, Staflin K, Forsyth JS, Felding-Habermann B, Smider VV, et al. (2011). Selective formation of covalent protein heterodimers with an unnatural amino acid. Chemistry & Biology, 18, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Atkinson J, Guevara CI, Zhang C, Kery V, Moon SJ, et al. (2014). In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates. PLoS One, 9, e83865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazane SA, Sok D, Cho EH, Uson ML, Kuhn P, Schultz PG, et al. (2012). Site-specific DNA-antibody conjugates for specific and sensitive immuno-PCR. Proceedings of the National Academy of Sciences of the United States of America, 109, 3731–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Axup JY, Lawson BR, Yun H, Tardif V, Choi SH, et al. (2013). Bispecific small molecule-antibody conjugate targeting prostate cancer. Proceedings of the National Academy of Sciences of the United States of America, 110, 17796–17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kularatne SA, Deshmukh V, Ma J, Tardif V, Lim RK, Pugh HM, et al. (2014). A CXCR4-targeted site-specific antibody-drug conjugate. Angewandte Chemie International Edition, 53, 11863–11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. (2008). Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Research, 68, 9280–9290. [DOI] [PubMed] [Google Scholar]
- Liu CC, & Schultz PG (2010). Adding new chemistries to the genetic code. Annual Review of Biochemistry, 79, 413–444. [DOI] [PubMed] [Google Scholar]
- Lyu Z, Kang L, Buuh ZY, Jiang D, McGuth JC, Du J, et al. (2018). A switchable site-specific antibody conjugate. ACS Chemical Biology, 13, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir TW (2003). Semisynthesis of proteins by expressed protein ligation. Annual Review of Biochemistry, 72, 249–289. [DOI] [PubMed] [Google Scholar]
- Nastri HG, Iffland C, Leger O, An Q, Cartwright M, McKenna SD, et al. 2014. Anti-pd-l1 antibodies and uses thereof. United States, USPTO. [Google Scholar]
- Ricart AD (2011). Antibody-drug conjugates of calicheamicin derivative: Gemtuzumab ozogamicin and inotuzumab ozogamicin. Clinical Cancer Research, 17, 6417–6427. [DOI] [PubMed] [Google Scholar]
- Sano T, Smith CL, & Cantor CR (1992). Immuno-PCR: Very sensitive antigen detection by means of specific antibody-DNA conjugates. Science, 258, 120–122. [DOI] [PubMed] [Google Scholar]
- Schumacher D, Hackenberger CPR, Leonhardt H,& Helma J (2016). Current status: Site-specific antibody drug conjugates. Journal of Clinical Immunology, 36, S100–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, et al. (2014). A general approach to site-specific antibody drug conjugates. Proceedings of the National Academy of Sciences of the United States of America, 111, 1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wals K, & Ovaa H (2014). Unnatural amino acid incorporation in E. coli: Current and future applications in the design of therapeutic proteins. Frontiers in Chemistry, 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brock A, Herberich B, & Schultz PG (2001). Expanding the genetic code of Escherichia coli. Science, 292, 498–500. [DOI] [PubMed] [Google Scholar]
- Wang RE, Liu T, Wang Y, Cao Y, Du J, Luo X, et al. (2015). An immunosuppressive antibody-drug conjugate. Journal of the American Chemical Society, 137, 3229–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xie J, & Schultz PG (2006). Expanding the genetic code. Annual Review of Biophysics and Biomolecular Structure, 35, 225–249. [DOI] [PubMed] [Google Scholar]
- Warren DJ (2011). Preparation of highly efficient electrocompetent Escherichia coli using glycerol/mannitol density step centrifugation. Analytical Biochemistry, 413, 206–207. [DOI] [PubMed] [Google Scholar]
- Wissler HL, Ehlerding EB, Lyu Z, Zhao Y, Zhang S, Eshraghi A, et al. (2019). Site-specific immuno-PET tracer to image PD-L1. Molecular Pharmaceutics, 16, 2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu NJ, Kang CS, Sin I, Ren SY, Liu DJ, Ruthengael VC, et al. (2016). Promising bifunctional chelators for copper 64-PET imaging: Practical Cu-64 radiolabeling and high in vitro and in vivo complex stability. Journal of Biological Inorganic Chemistry, 21, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhu H, Zhang B, Liu F, Chen J, Wang Y, et al. (2016). Synthesis of site-specific radiolabeled antibodies for radioimmunotherapy via genetic code expansion. Bioconjugate Chemistry, 27, 2460–2468. [DOI] [PubMed] [Google Scholar]
- Yi X, Wang F, Qin W, Yang X, & Yuan J (2014). Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. International Journal of Nanomedicine, 9, 1347–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TS, & Schultz PG (2010). Beyond the canonical 20 amino acids: Expanding the genetic lexicon. Journal of Biological Chemistry, 285, 11039–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DD, Young TS, Jahnz M, Ahmad I, Spraggon G, & Schultz PG (2011). An evolved aminoacyl-tRNA synthetase with atypical polysubstrate specificity. Biochemistry, 50, 1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahnd C, Spinelli S, Luginbuhl B, Amstutz P, Cambillau C, & Pluckthun A (2004). Directed in vitro evolution and crystallographic analysis of a peptide-binding single chain antibody fragment (scFv) with low picomolar affinity. The Journal of Biological Chemistry, 279, 18870–18877. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bloch S, Akers W, & Achilefu S (2012). Near-infrared molecular probes for in vivo imaging. Current Protocols in Cytometry, 60, 12.27.1–12.27.20, [Chapter 12: Unit12 27]. [DOI] [PMC free article] [PubMed] [Google Scholar]


