The COVID-19 pandemic has caused unprecedented morbidity and mortality, overwhelming healthcare systems around the world. To prevent the spread of the virus, hospitals have had to severely reduce in-person visits by cancelling elective procedures and delaying routine patient visits. Recent surveys have shown a 45%–70% drop in patient visits across the USA, UK and other European nations.1 2 Simultaneously, the COVID-19 pandemic has forced providers to adopt telehealth solutions, dramatically accelerating the adoption of digital health platforms.
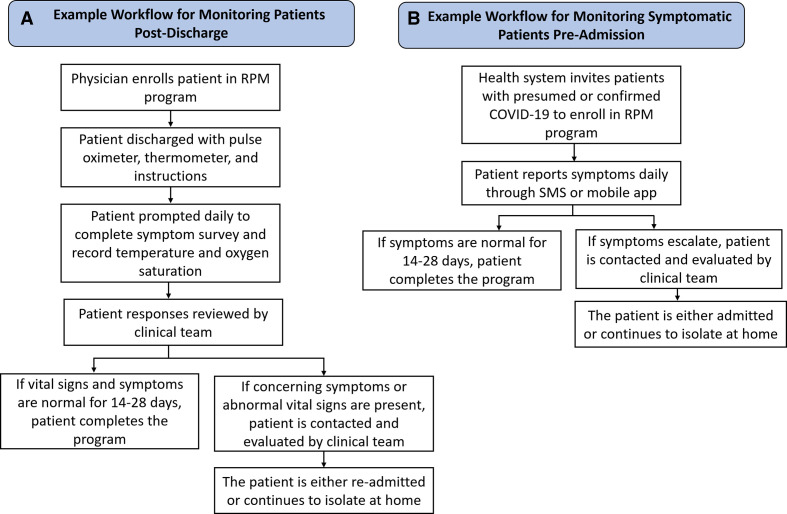
Remote patient monitoring (RPM) technologies are a type of digital health platform which enable patients to be evaluated outside of a typical clinical visit, in their home or in their community. RPM programmes collect data using symptom surveys, wearable sensors, and other medical devices and send this information to a healthcare practitioner to facilitate clinical assessment and decision-making. In the last decade, many studies have demonstrated the utility of RPM in improving the outcomes of patients with chronic health conditions.3 For example, patients with hypertension who monitored their blood pressure at home and shared readings with their healthcare provider achieved significantly better blood pressure control.4 Similarly, meta-analyses have found that monitoring blood glucose at home and sending measurements to the provider for clinical feedback yield significant improvements in glycaemic control.5 Further randomised controlled trials have shown RPM reduced mortality and readmissions in patients with heart failure and chronic obstructive pulmonary disease.6–8 In the context of COVID-19, RPM programmes could play an important role in strengthening healthcare delivery. During the past year, several hospital systems around the world have developed and implemented RPM platforms in response to the pandemic. Many of these programmes have focused on monitoring patients with COVID-19 after hospital discharge (figure 1A).
Figure 1.
(A) Example workflow of post-discharge remote patient monitoring (RPM) programme. (B) Example workflow of pre-admission RPM programme. SMS, short message service.
Throughout the COVID-19 pandemic, the capacity of hospitals has been severely strained, and RPM programmes provide a mechanism to reduce this strain. RPM platforms enable physicians to discharge patients early and monitor them remotely after leaving the hospital, freeing up inpatient beds. Furthermore, as the SARS-CoV-2 virus is highly transmissible, monitoring patients remotely can reduce the number of follow-up hospital visits needed, decreasing the spread of the virus. Recognising these potential benefits, five hospitals in the Mass General Brigham healthcare system implemented an RPM programme in which patients with COVID-19 being discharged were provided with a pulse oximeter and thermometer and self-reported their symptoms, oxygen saturation, and temperature daily through a mobile app.9 Triage nurses reviewed any concerning trends, performed a clinical evaluation, and if necessary, called the patient to develop a care plan. Patients enrolled in the RPM programme were significantly less likely to present to the emergency department (ED) or be readmitted to the hospital. Similarly, the New York-Presbyterian Brooklyn Methodist Hospital implemented an RPM programme where patients with COVID-19 were provided with a pulse oximeter post-discharge, and one nurse was assigned to monitor 50 patients.10 Post-programme surveys revealed high patient satisfaction, with over 91% of participants indicating they would recommend it to others. Similarly, the St Antonius Hospital in the Netherlands developed an RPM programme which employed at-home oxygen saturation, temperature and symptom tracking to monitor patients with severe COVID-19 after hospital discharge, including those receiving oxygen therapy at home.11 The mean reduction in length of hospitalisation was 5.0±3.8 days per patient, and 97% of patients reported the programme was user friendly.11 The Cleveland Clinic and hospitals in the UK’s National Health Service (NHS) have also implemented RPM programmes which use pulse oximeters and structured telephone interviews to monitor patients post-discharge reporting comparable results.12 13 These studies have revealed the effectiveness of RPM in reducing hospital readmissions, increasing patient satisfaction and enabling early discharge in patient populations with COVID-19.
Beyond following up with patients after hospital discharge, RPM has also been employed to monitor symptomatic patients prior to admission (figure 1B). The University of Pennsylvania health system developed ‘COVIDWatch’, an RPM programme which used automated twice-daily short message service questionnaires to track symptoms.14 Patients who reported worsening symptoms were referred to the ED or to an on-call team of telemedicine physicians. Patient ratings indicated a high degree of satisfaction, with a net promoter score of 80. Similar RPM initiatives from the Northwestern University and University of Minnesota health systems demonstrated the effectiveness of these technologies in supporting patients who were managing COVID-19 symptoms at home.15 16 Patients reported that the programme provided a sense of safety and a direct pathway for them to rapidly access COVID-19-specific medical care.
This begs the question, if COVID-19 RPM programmes have demonstrated potential improve patient care, reduce readmissions and facilitate early discharge, why are they not more widely used? The gap lies in healthcare system integration. For these RPM platforms to meaningfully improve patient outcomes, they must be deeply embedded within a framework for healthcare delivery. Technological infrastructure needs to be built so that data obtained from at-home monitoring can be seamlessly and securely shared with a patient’s care provider. Implementation science frameworks (such as RE-AIM) should be used to translate RPM technology into clinical practice and could help facilitate widespread adoption of these tools. Additionally, to date, the majority of RPM programmes have been implemented in high-income countries, and there is a lack of RPM deployment in low/middle-income countries (LMICs). Pilot studies of RPM programmes in Southeast Asia and Africa have shown promise, but substantial cost and regulatory barriers remain.17–19
The deployment of an RPM intervention requires sufficient monitoring devices, clinical providers and information technology personnel, which necessitate significant upfront investment. However, studies have shown that RPM programmes can be cost-effective in the long term. For example, the costs of the COVID-19 post-discharge RPM programme in the Netherlands’ St Antonius Hospital were approximately fourfold less than the estimated costs of the saved patient-days, and a similar RPM programme at an NHS hospital resulted in a significant reduction of operational costs.11 20 Additionally, while the USA has implemented regulatory changes and Medicare reimbursement frameworks to support the use of telemedicine and RPM in response to the COVID-19 pandemic, this has not been the case in other countries.21 22 Policy and legislation must be developed to regulate the safety, privacy and reimbursement of RPM technologies globally.23 Additionally, further research is required to characterise the implementation and effectiveness of RPM programmes in LMICs and resource-limited settings before deploying RPM in these regions.
Ultimately, COVID-19 has illustrated that we need to expand the reach of the health system beyond the hospital and clinic setting. RPM technologies provide an avenue to do exactly that, and recent studies have demonstrated the effectiveness of these platforms in increasing patient satisfaction, reducing readmissions and optimising healthcare system efficiency. There is a pressing need to expand the usage of evidence-based RPM technologies, particularly as COVID-19 cases continue to rise with the spread of new variants. Overall, RPM technologies can strengthen care delivery, enabling symptomatic patients to be supported prior to hospital admission and providing post-discharge patients with continuity of care.
Footnotes
Twitter: @sreekarmantena
Contributors: SM and SK drafted and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Mehrotra A, Chernew M, Linetsky D. What impact has COVID-19 had on outpatient visits? The Commonwealth Fund. Available: https://www.commonwealthfund.org/publications/2020/apr/impact-covid-19-outpatient-visits [Accessed 16 May 2020].
- 2.Colclough G, Dash P, van der Veken L. The hidden health crisis of COVID-19 in Europe. McKinsey & Company, 2020. Available: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/understanding-and-managing-the-hidden-health-crisis-of-covid-19-in-europe# [Accessed 22 Jun 2020].
- 3.Vegesna A, Tran M, Angelaccio M, et al. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health 2017;23:3–17. 10.1089/tmj.2016.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis KL, Asche SE, Dehmer SP, et al. Long-Term outcomes of the effects of home blood pressure Telemonitoring and pharmacist management on blood pressure among adults with uncontrolled hypertension: follow-up of a cluster randomized clinical trial. JAMA Netw Open 2018;1:e181617. 10.1001/jamanetworkopen.2018.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitsiou S, Paré G, Jaana M, et al. Effectiveness of mHealth interventions for patients with diabetes: an overview of systematic reviews. PLoS One 2017;12:e0173160. 10.1371/journal.pone.0173160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedone C, Rossi FF, Cecere A, et al. Efficacy of a physician-Led multiparametric Telemonitoring system in very old adults with heart failure. J Am Geriatr Soc 2015;63:1175–80. 10.1111/jgs.13432 [DOI] [PubMed] [Google Scholar]
- 7.Ho T-W, Huang C-T, Chiu H-C, et al. Effectiveness of telemonitoring in patients with chronic obstructive pulmonary disease in Taiwan-A randomized controlled trial. Sci Rep 2016;6:23797. 10.1038/srep23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steventon A, Bardsley M, Billings J, et al. Effect of telehealth on use of secondary care and mortality: findings from the whole system Demonstrator cluster randomised trial. BMJ 2012;344:e3874. 10.1136/bmj.e3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon WJ, Henderson D, DeSharone A, et al. Remote patient monitoring program for hospital discharged COVID-19 patients. Appl Clin Inform 2020;11:792–801. 10.1055/s-0040-1721039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama R, Arora S, Anand S, et al. Reengineering the discharge transition process of COVID-19 patients using telemedicine, remote patient monitoring, and Around-the-Clock remote patient monitoring from the emergency department and inpatient units. Telemed J E Health 2020. 10.1089/tmj.2020.0459. [Epub ahead of print: 14 Dec 2020]. [DOI] [PubMed] [Google Scholar]
- 11.Grutters LA, Majoor KI, Mattern ESK, et al. Home telemonitoring makes early hospital discharge of COVID-19 patients possible. J Am Med Inform Assoc 2020;27:1825–7. 10.1093/jamia/ocaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell LC, Norris-Grey C, Luintel A, et al. Implementation and evaluation of a COVID-19 rapid follow-up service for patients discharged from the emergency department. Clin Med 2021;21:e57–62. 10.7861/clinmed.2020-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina M, Babiuch C, Card M, et al. Home monitoring for COVID-19. Cleve Clin J Med 2020;87:1–4. 10.3949/ccjm.87a.ccc028 [DOI] [PubMed] [Google Scholar]
- 14.Morgan AU, Balachandran M, Do D. Remote monitoring of patients with Covid-19: design, implementation, and outcomes of the first 3,000 patients in COVID Watch. NEJM Catalyst 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0342a [Google Scholar]
- 15.Annis T, Pleasants S, Hultman G, et al. Rapid implementation of a COVID-19 remote patient monitoring program. J Am Med Inform Assoc 2020;27:1326–30. 10.1093/jamia/ocaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kricke G, Roemer PE, Barnard C. Rapid implementation of an outpatient Covid-19 monitoring program. NEJM Catalyst 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0342a [Google Scholar]
- 17.Chau NVV, Hai HB, Greeff H, et al. Wearable remote monitoring for patients with COVID-19 in low-resource settings: case study. BMJ Innovations 2021;7:s12–15. 10.1136/bmjinnov-2021-000706 [DOI] [Google Scholar]
- 18.Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health Surveill 2020;6:e18810. 10.2196/18810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manyati TK, Mutsau M. Exploring the effectiveness of telehealth interventions for diagnosis, contact tracing and care of corona virus disease of 2019 (COVID19) patients in sub Saharan Africa: a rapid review. Health Technol 2021;11:341–8. 10.1007/s12553-020-00485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SS, Gvozdanovic A, Knight M, et al. Mobile App-Based remote patient monitoring in acute medical conditions: prospective feasibility study exploring digital health solutions on clinical workload during the COVID crisis. JMIR Form Res 2021;5:e23190. 10.2196/23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webster P. Virtual health care in the era of COVID-19. Lancet 2020;395:1180–1. 10.1016/S0140-6736(20)30818-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shachar C, Engel J, Elwyn G. Implications for telehealth in a Postpandemic future: regulatory and privacy issues. JAMA 2020;323:2375–6. 10.1001/jama.2020.7943 [DOI] [PubMed] [Google Scholar]
- 23.Gerke S, Shachar C, Chai PR, et al. Regulatory, safety, and privacy concerns of home monitoring technologies during COVID-19. Nat Med 2020;26:1176–82. 10.1038/s41591-020-0994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]