Abstract
The study evaluated the evolution of the incidence of infections with Klebsiella in the County Clinical Emergency Hospital of Craiova (SCJUC), Romania. Also, we monitored antibiotic resistance over more than two years and detected changes in resistance to various antimicrobial agents. Our study included 2062 patients (823 women and 1239 men) hospitalised in SCJUC during the period 1st of September 2017 to 30 June 2019. In 458 patients (22.21%) from the 2062 total patients, the collected samples (1116) were positive and from those, we isolated 251 strains of Klebsiella spp. We conducted a longitudinal analysis of the prevalence of Klebsiella spp. over calendar months, which showed a prevalence in surgical wards that ranged between 5.25% and 19.49% in June 2018, while in medical wards the variation was much wider, between 5.15% and 17.36% in April 2018. Klebsiella spp. strains showed significant resistance to Amoxicillin/Clavulanate, Aztreonam and Cephalosporins such as Ceftriaxone, Ceftazidime and Cefepime. We examined the possible link with the consumption of antibiotics in the same month by performing a multiple linear regression analysis. The evolution of antibiotic resistance in Klebsiella was correlated with the variation of resistance in other bacteria, which suggests common resistance mechanisms in the hospital environment. By performing the regression for dependency between antibiotic resistance and antibiotic consumption, we observed some correlations between antibiotic consumption and the development of antibiotic resistance after 1, 2 and even 3 months (e.g., resistance to meropenem was influenced by the consumption in the hospital ward of imipenem 1 month and two months before, but only 1 month before by the consumption of meropenem). The clustering of strains showed filiation between multiresistant Klebsiella spp. strains isolated from specific patients from the ICU. The evolution of prevalence and antibiotic resistance in Klebsiella correlated with the resistance in other bacteria, which suggest common resistance mechanisms in the hospital environment, and also with the consumption of antibiotics.
Keywords: multidrug-resistant bacteria, Klebsiella, antibiotic resistance
1. Introduction
The primary proposed mechanism underlying antibiotic disease associations is microbiota dysbiosis, which results in changes in gene expression, epigenetic alteration, and invasion by pathogenic bacteria, the development of biofilms, and immune regulation and inflammation. This connection between antibiotic exposure and disease risk contributes to an increasing amount of knowledge that antibiotic use has harmful long-term health consequences. Significant studies have found a correlation between antibiotic use and chronic disease in both paediatric and adult populations [1].
Nosocomial infections, due to their frequency and severity, are one of the major epidemiological problems of any inpatient, regardless of the number of beds or the profile of the ward [2]. In the USA alone, there were more than 1.7 million nosocomial infections, which resulted in the near 100,000 deaths [3]. Knowledge of the epidemiological process in these infections, with all particularities that the factors of this process may present depending on the etiological agent, clinical manifestations, type of hospital unit, largely conditions the effectiveness of practical actions of prophylaxis and control. The major factors causing the development of bacterial resistance include the indiscriminate use of antimicrobial agents in human and animal medicine, agriculture, and aquatic farming [1,4]. Different mechanisms or their combination are used by bacteria in developing resistance to antibiotics [5]. The presence of plasmids that contain one or more resistance genes, with each encoding a single antibiotic resistance (AR) phenotype, often causes the development of multiple AR (MAR) in bacteria [6]. These AR genes can transfer to other bacteria of the same or different species.
Klebsiella pneumoniae (K. pneumoniae) is the second most common gram-negative pathogen after Escherichia coli (E. coli) associated with a wide range of infections, such as urinary tract infection (UTI), pneumonia and pleurisy, intra-abdominal infections, bloodstream infections, meningitis, and pyogenic liver abscesses [7,8,9,10,11], especially in immunodeficient patients [12].
Klebsiella spp. are widespread in nature: in the atmosphere, where they arise from surface water, waste, soil, and plants [13,14], and the mucosal surfaces of mammals such as humans, horses, or swine, which they colonize. Klebsiella is similar to Enterobacter and Citrobacter in this sense, but not to Shigella spp. or E. coli, which are popular in humans but not in the ecosystem. K. pneumoniae is identified as a saprophyte in the nasopharynx and the gastrointestinal tract in humans. Carrier rates differ tremendously between studies [15,16].
In recent decades, rates of broad-spectrum cephalosporin-resistant K. pneumoniae that produce broad-spectrum β-lactamases (ESBL) have increased dramatically worldwide, and in most parts of the world, K. pneumoniae is the pathogen most commonly associated with the dissemination of ESBL and other horizontally transmissible resistance genes [17,18,19].
For K. pneumoniae, a high frequency of resistance to third-generation cephalosporins, fluoroquinolones, and aminoglycosides, has become evident in southern, central and Eastern Europe [18,20]. Many of these strains have gained resistance to all classes of antibiotics. Carbapenem resistance in K. pneumoniae isolates is on the rise in the European Union, except for Greece, where it is already established [20,21,22]. This is a particularly worrying phenomenon, as carbapenems are the ultimate antibiotics and treatment options for patients infected with this bacterium and other carbapenem-resistant bacteria are severely limited [23].
Because of the development of MAR strains, several Klebsiella sp. infections acquired in clinics raise a medical challenge. It was first reported in 1981 that Klebsiella strains were resistant to different generations of cephalosporins, especially the third generation; since then, these bacteria have become more resistant to antibiotics [23,24].
This study analyzed the evolution of the prevalence of infections with Klebsiella in the Intensive Care Unit (ICU), medical and surgical services of the County Clinical Emergency Hospital of Craiova (SCJUC), Romania, one of the largest hospitals in our country. We also wanted to determine the changes in the antibiotic resistance spectrum for two years and to observe correlations between resistances to different antibiotics over time. Also, we wanted to investigate a possible explanation for antibiotic resistance, namely the consumption of the antibiotic, which will help to adjust the hospital specific anti-biotherapy guidelines to minimize the development of resistant strains in our hospital. We also wanted to determine the degree of similarity of the strains based on antibiotic resistance profiles in an effort of epidemiologic tracing of the circulation of Klebsiella strains in our hospital.
2. Materials and Methods
For our study, we collected 2456 samples from 2062 patients (823 women and 1239 men) hospitalised in SCJUC during the period 1st of September 2017 to 30 June 2019, in the Intensive Care Unit (ICU), medical and surgical wards. The biological samples were joint fluid, bile, blood, catheter, pleural fluid, cerebrospinal fluid, purulent secretion, sputum, urine, tracheal aspirate, pharyngeal swab, nasal swab, puncture liquid, conjunctival secretion and ear discharges We first identified bacteria by classical microbiological methods, but then we verified the identification of Klebsiella with the automated analyzer Vitek 2 (Biomerieux, Marcy-l’Étoile, France). For most strains, antimicrobial susceptibility testing was performed by the disk diffusion Kirby-Bauer method, using the following antibiotics: Sulphametoxazole/Thrimetoprim (1.25/23.75 µg), Tigecyclin (15 µg), Ciprofloxacin (5 µg), Amikacin (30 µg), Meropenem (10 µg), Ceftriaxone (30 µg), Ceftazidime (30 µg), Cefepime (30 µg), Cefazolin (30 µg), Piperacillin/Tazobactam (100/10 µg) and Amoxicillin/Clavulanate (20/10 µg). The multiresistant strains were also tested on the automated analyzer Vitek 2 (which uses the microdilution method) and can provide Minimum Inhibitory Concentration (MIC) as a quantitative measure of antibiotic resistance.
Statistical Analysis
The patients, specimens collected, and isolated strains, along with antibiotic resistance profiles were registered in the WHONET bacteriological analysis software, provided by World Health Organization (WHO).
Resistance phenotypes were generated for each isolate and statistically processed by the specialised module of the WHONET program. A single phenotype was considered for samples collected from the same patient if they were collected at a distance of fewer than 7 days. The SatScan module with the space-time permutation method was used to define transmission foci.
The database was then transferred to STATA. Continuous variables are presented as means ± standard deviation, and discrete variables as numbers and/or percentages. Most of the statistical analyses were performed using the STATA program (STATACORP, College Station, TX, USA, 2015). The monthly prevalences were calculated as the number of patients from which the species of interest was identified divided by the total number of patients analyzed in the respective month.
The MAR index is a reliable, accurate, and premium tool for documenting the origins of antibiotic-resistant bacteria. The MAR index is measured as the ratio of the number of antibiotics to which an organism is resistant and the total number of antibiotics to which the entity has been exposed. A MAR greater than 0.2 indicates that the high-risk source of infection is a region where drugs are widely used [25]. We used the MAR index to generate histograms for the distribution of the index in various wards to identify resistant and susceptible populations. Further, we performed one-way ANOVA on the MAR using the type of ward (ICU, surgical or medical) as a categorical variable to compare resistance levels in various types of wards.
To further explain the time variation of resistance in Klebsiella strains we correlated antibiotic resistance with antibiotic consumption in the same month and ward. The consumption of antibiotics was calculated in Defined Daily Doses (DDD) per 100 patient-days as follows: the consumption in tablets or vials of antibiotics ordered for a particular patient was obtained from the pharmacy, as well as the duration of treatment. We summed the doses administered obtaining the total quantity of antibiotic, that we then divided by the number of days and multiplied by 100 (to obtain DDD’s for 100 patient-days). For all patients in the ward, we averaged the DDDs.
The monthly resistances were calculated by averaging the MAR index of the Klebsiella strains analyzed in the month. The trending evolution was performed by testing the trend through the Chi2 test for trend.
We performed a time series analysis using the lagging antibiotic consumption as a predictor for the resistance.
The lineage of the strains was analyzed by the hierarchical clustering method in STATA software based on the MAR resistance index.
3. Results
After performing microbiological diagnosis by the classical and automated method (Vitek 2 analyzer for bacterial identification and antibiogram), we isolated 1503 bacterial strains from 1116 positive samples (45.44%) collected from 458 patients (22.21% from all analysed patients). The isolated bacteria included E. coli, Klebsiella spp., non-fermenter Gram-negative rods (NFR) (Pseudomonas aeruginosa, Acinetobacter spp.), Staphylococcus aureus, Streptococcus spp. and other species.
3.1. Prevalence of Klebsiella spp. Strains
In all samples, the prevalence of Klebsiella spp. was 10.22%, respectively 251 strains.
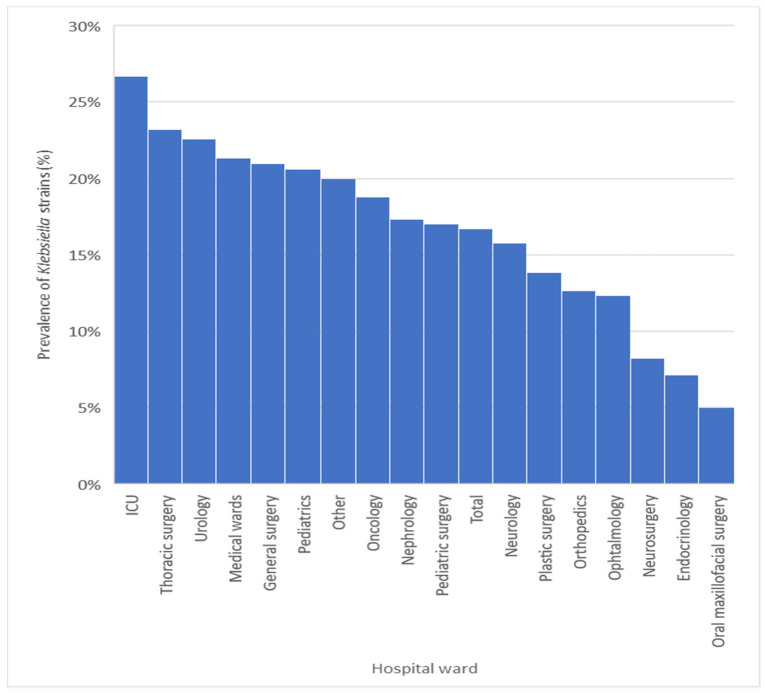
The prevalence of Klebsiella spp. had a relatively uniform distribution in all wards analysed (Figure 1), which suggests ubiquity in SCJUC. The maximum prevalence was recorded in the departments of ICU (26.67%), Thoracic surgery (23.22%), Urology (22.58%), medical wards (21.32%) and General surgery (20.98%), where it is a causative agent of decubitus pneumonia. The lowest prevalences were recorded in the Neurosurgery (8.25%), Endocrinology (7.00%) and oral maxillofacial surgery (5.00%) departments.
Figure 1.
Prevalence of Klebsiella strains by hospital ward. The bars heights are the percentages of prevalence in different wards.
The Klebsiella strains were isolated with the highest rate from ear discharges (42.86%), even only 7 such specimens were analysed, followed by sputum (27.34%) and tracheal aspirate (21.03%). The prevalence was between 10% and 20% in blood, purulent secretion, nasal swab, and conjunctival secretion (Table 1).
Table 1.
The prevalence of Klebsiella strains in the various clinical specimens.
| No. Samples | No. Strains | Percent | |
|---|---|---|---|
| Joint fluid | 8 | 0 | 0.00% |
| Bile | 5 | 0 | 0.00% |
| Blood | 130 | 21 | 16.15% |
| Catheter | 31 | 4 | 12.90% |
| Pleural fluid | 19 | 0 | 0.00% |
| Cerebrospinal fluid | 6 | 0 | 0.00% |
| Purulent secretion | 276 | 34 | 12.32% |
| Sputum | 128 | 35 | 27.34% |
| Urine | 1294 | 77 | 5.95% |
| Tracheal aspirate | 290 | 61 | 21.03% |
| Pharyngeal swab | 72 | 4 | 5.56% |
| Nasal swab | 38 | 5 | 13.16% |
| Puncture liquid | 120 | 3 | 2.50% |
| Conjunctival secretion | 32 | 4 | 12.50% |
| Ear discharges | 7 | 3 | 42.86% |
| total | 2456 | 251 | 10.22% |
3.2. Evolution of the Prevalence of Klebsiella Strains
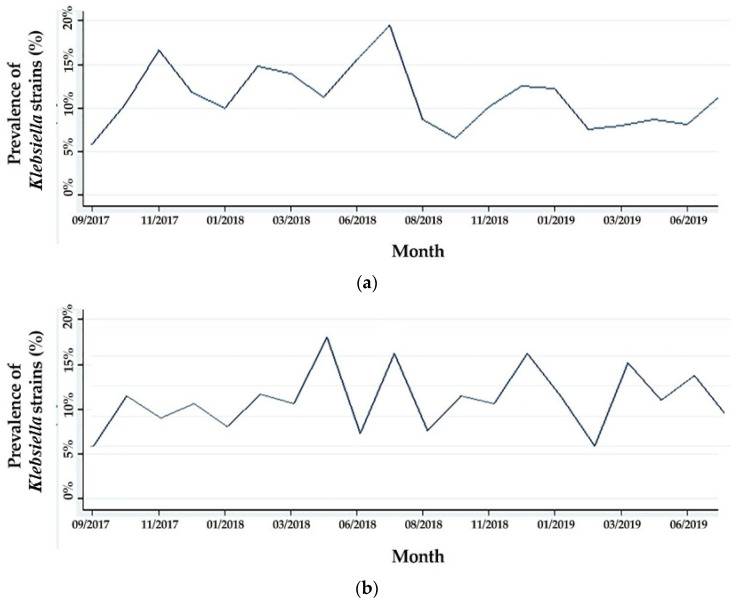
We conducted a longitudinal analysis of the prevalence of Klebsiella spp. over calendar months, which showed a prevalence in surgical wards that ranged between 5.25% and 19.49% in June 2018, while in medical wards, the variation ranged between 5.15% and 17.36% in April 2018 (Figure 2a,b).
Figure 2.
Evolution of the incidence of Klebsiella strains: (a) in the surgical wards; (b) in medical wards.
3.3. Resistance to Antibiotics of the Isolated Bacterial Species
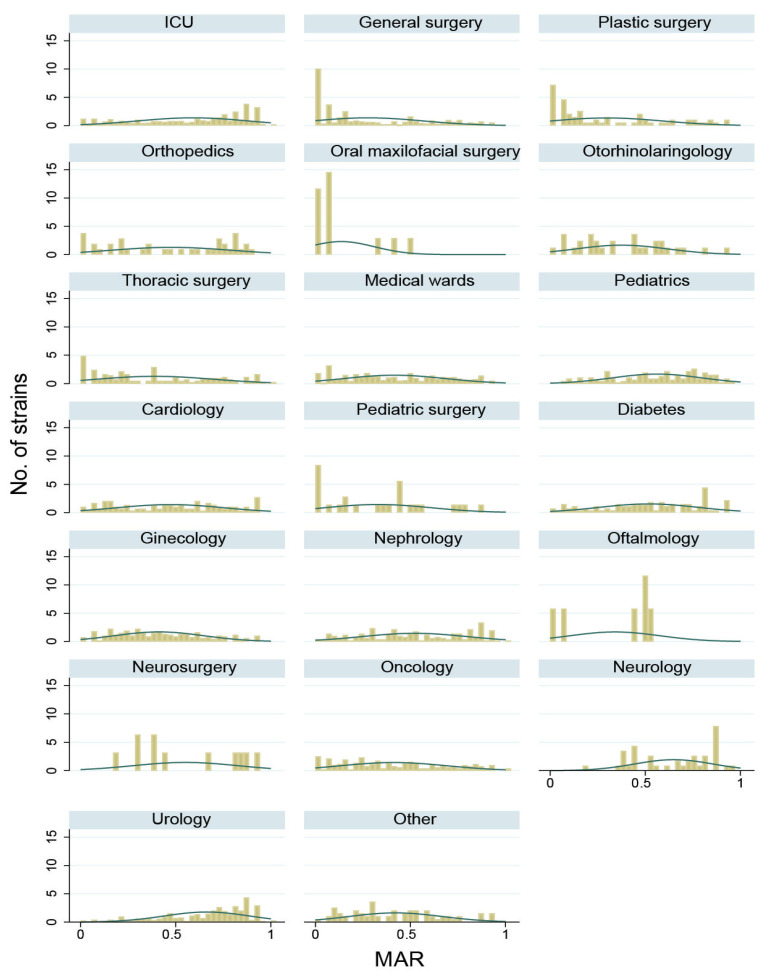
By performing the analysis of the antibiotic susceptibility spectrum (Figure 3), we found that the highest resistance index was found in the ICU ward (58.20%), followed by surgical departments (44.69%) and the medical wards with an index of 47.32%. Overall, the mean index was 50.75%, with a significant variation depending on the type of section (one-way ANOVA test, p < 0.001). The post-hoc Dunnet test identified a significantly lower resistance in surgical wards (−16.41%) and medical wards (−12.37%).
Figure 3.
Histogram of the distribution of MAR index in various wards from SCJUC. The columns heights are the number of strains for which MAR falls in a specific interval (bin). The green line is a smoothing line of the heights of the bars.
By analysing the distribution of Klebsiella spp. resistance in the SCJUC wards, a population of multidrug-resistant strains with MAR > 0.8 was present in General Surgery, Plastic Surgery, and Pediatric Surgery wards. We observed two populations of Klebsiella spp. into the ICU, one with a low MAR, and another with a high MAR, corresponding to community and hospital strains.
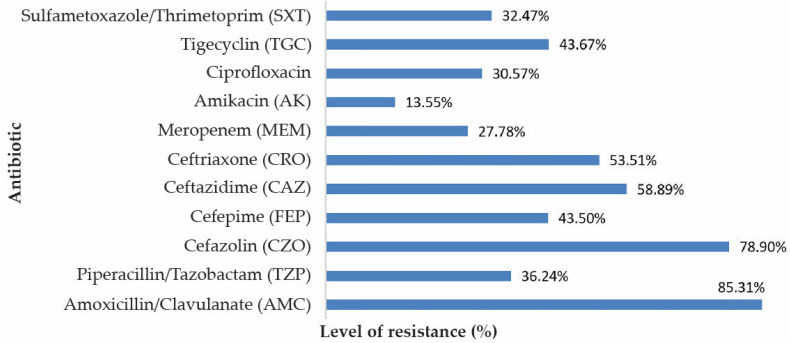
Klebsiella strains showed significant resistance to cephalosporins due to hospital circulation of ESBL strains in the hospital. Thus, we observed increased resistance of Klebsiella to Amoxicillin/Clavulanate (85.31%), Cefazoline (78.90%), Ceftazidime (58.89%), Ceftriaxone (53.51%) and Cefepime (43.50%). Note the resistance to carbapenems due to the circulation of plasmids encoding carbapenemase-producing genes. Thus, the resistance to Meropenem in Klebsiella was 27.78%. Resistance to quinolones (Ciprofloxacin) in Klebsiella strains was 30.57% (Figure 4).
Figure 4.
Resistance to antimicrobial substances of the isolated Klebsiella strains.
3.4. Analyse the Variation in Time of the Antibiotic Resistance
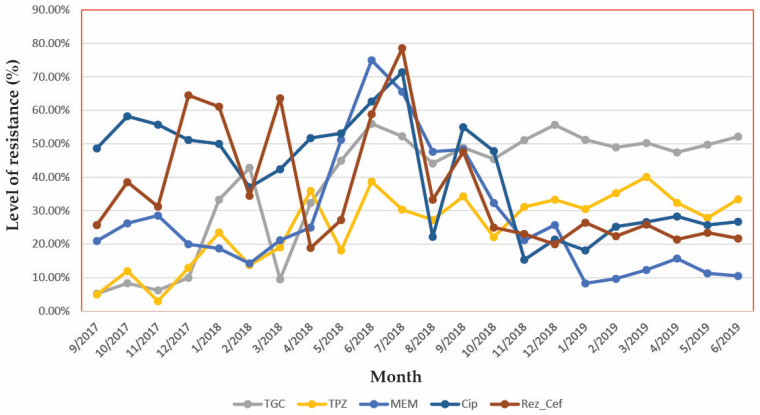
We used Ceftriaxone resistance as a marker for beta-lactams resistance. The longitudinal analysis of the monthly resistance index is presented in Figure 5. The resistance to 3rd generation cephalosporins oscillated between 60% and 90% in an undulant manner. We observed that the Tigecycline resistance began to increase in our hospital.
Figure 5.
Evolution of antibiotic resistance in Klebsiella strains. MAR = Multiple Antibiotic Resistance index. Note: here were presented the resistance for typical antibiotics from each chemical class, however, because multiple cephalosporins were used we calculated MAR index specific for cephalosporins. TGC: Tigecyclin; TPZ: Piperacillin/Tazobactam; MEM: Meropenem; CIP: Ciprofloxacin; Rez_Cef: Resistance index to cephalosporins.
One can easily observe some parallelisms between evolutions of resistances (this parallel variation is analyzed by the time series procedures further in the text). For example, we can observe that between April and October 2018 a parallel evolution of resistance to cephalosporins and Ciprofloxacin. In early 2019, the resistance to all antibiotics remained mainly unchanged.
Next, we compared the evolution of resistance to various classes of antibiotics between Klebsiella strains and other Gram-negative bacilli (E. coli and non-fermenters) and Gram-positive cocci (Staphylococcus aureus), to identify correlations that can be explained by consumption-driven resistance and shared resistance genes.
The resistance of Klebsiella to quinolones represented by Ciprofloxacin correlated significantly with that of Staphylococcus aureus (r = 0.4850, p = 0.0302) (Table 2).
Table 2.
Correlations between monthly ciprofloxacin resistance of Staphylococcus aureus, E. coli, Klebsiella and NFR.
|
Staphylococcus
aureus |
E. coli | Klebsiella | NFR | |
|---|---|---|---|---|
| Staphylococcus aureus | 1 | |||
| E. coli | 0.3472 p = 0.1336 |
1 | ||
| Klebsiella | 0.4850 * p = 0.0302 |
0.2924 p = 0.2109 |
1 | |
| NFR | −0.1583 p = 0.5051 |
0.2769 p = 0.2372 |
0.1359 p = 0.5679 |
1 |
* p < 0.05.
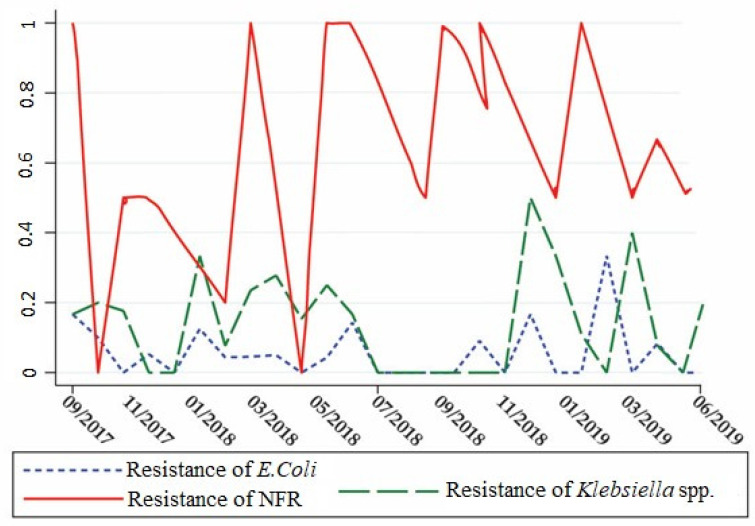
As for the resistance of Gram-negative rods to carbapenems, represented by Ertapenem, that was slightly ascending in the last year for the non-fermenters and stationary for Klebsiella. From Figure 6, we can also observe the synchronous evolution of carbapenem resistance rates in Klebsiella and E. coli, although the correlation coefficient was non-significant, suggesting both consumption-driven resistance and shared resistance genes. Interestingly, it can also be seen from Figure 6 that the increase of resistance in E. coli seems to precede that of Klebsiella.
Figure 6.
Monthly evolution of Meropenem resistance in E. coli, Klebsiella, and Gram-negative non-fermenter rods (NFR).
Taking into account the monthly resistance index, Klebsiella resistance correlated with E. coli (r = 0.5107, p < 0.0214), as Klebsiella is a known plasmid collector of resistance from other enterobacteria, and also we observed a negative correlation between Pseudomonas aeruginosa and Staphylococcus aureus (r = −0.4089, p = 0.0422) (Table 3).
Table 3.
Correlation between values of the monthly resistance index of E. coli, Klebsiella, Pseudomonas aeruginosa, non-fermenter Gram-negative rods (NFR) and Staphylococcus aureus.
| Species | E. coli | Klebsiella | Ps. aeruginosa | NFR |
Staphylococcus
aureus |
|---|---|---|---|---|---|
| E. coli | r = 1.0000 | ||||
| Klebsiella | r = 0.5107 * p = 0.0214 |
r = 1.0000 | |||
| Pseudomonas aeruginosa, | r = 0.3184 p = 0.1841 |
r = 0.2755 p = 0.2536 |
r = 1.0000 | ||
| NFR | r = 0.1906 p = 0.4209 |
r = 0.1661 p = 0.4839 |
r = 0.3599 p = 0.1302 |
r = 1.0000 | |
| Staphylococcus aureus | r = 0.1972 p = 0.4046 |
r = 0.3500 p = 0.1303 |
r = −0.4089 * p = 0.0422 |
r = 1.0000 |
* p < 0.05.
To understand the sources of variation in antibiotic resistance of Klebsiella strains from our hospital we examined the possible link of resistance index with consumption of antibiotics (Table 4) in the same month by performing a multiple linear regression analysis (Table 5) using Klebsiella MAR as the outcome variable and the consumption of various antibiotics (expressed in DDDs administered in the same ward over the current month) as predictors. We chose the option to display the standardized coefficients to be able to compare the relative influence of antibiotics on resistance.
Table 4.
Medium annualized antibiotic consumption in the hospital wards.
| No. Patients | AK (g) | AMC (g) | CRO (g) | CZO (g) | FEP (g) | CIP (g) | CAZ (g) | SXT (g) | IPM (g) | MEM (g) | TPZ (g) | TGC (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DDD/100 patient-days (WHO) | 1 | 3 | 2 | 3 | 1.8 | 0.5 | 0.24 | 1 | 2 | 2 | 14 | 0.1 | |
| ICU | 1646 | 1.01 | 0.9 | 12 | 3.5 | 1.27 | 0.16 | 0.11 | 0.35 | 1.83 | 6.5 | 23 | 0.04 |
| Medical wards | 1521 | 0.01 | 1.54 | 11.1 | 0.85 | 0 | 0.07 | 0.55 | 0 | 1.16 | 5.15 | 5.01 | 0.08 |
| Nephrology | 1003 | 0.08 | 0.7 | 16 | 1.49 | 1.25 | 0.03 | 0.08 | 0.36 | 2.83 | 7.4 | 12.29 | 0.02 |
| Neurology | 436 | 0.18 | 0.07 | 15 | 0 | 0.06 | 0.14 | 0.02 | 1.55 | 0 | 0.55 | 1.16 | 0 |
| Oncology | 804 | 0 | 0.95 | 9 | 0.44 | 0.29 | 0 | 0.04 | 0.07 | 0.32 | 2.47 | 0 | 0 |
| Cardiology | 219 | 0.09 | 12.9 | 16.17 | 0.13 | 0.3 | 0.49 | 0.4 | 0.25 | 2.45 | 2.54 | 1.24 | 0 |
| Pediatrics | 199 | 0.38 | 0.43 | 13.3 | 2.29 | 0 | 0.08 | 1.45 | 0 | 10.14 | 1.01 | 1.75 | 0 |
| Pediatric surgery | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neurosurgery | 1768 | 0.49 | 0.84 | 26 | 1.6 | 0.59 | 0.03 | 0.2 | 0 | 0 | 12.4 | 6.7 | 0 |
| Ophthalmology | 1696 | 0 | 0.15 | 15.8 | 0.04 | 0 | 0 | 0.11 | 0 | 0.05 | 0.21 | 0.77 | 0 |
| Orthopaedics | 2824 | 0 | 0.01 | 15.9 | 0.008 | 0.08 | 0.47 | 0 | 0 | 0 | 0.27 | 0 | 0 |
| Urology | 1927 | 0.98 | 0.06 | 18.8 | 0 | 0.03 | 0.02 | 0.58 | 0.06 | 3.63 | 1.17 | 0 | 0.003 |
| General surgery | 1329 | 0.6 | 0 | 17.6 | 6.32 | 0.009 | 0.12 | 0.12 | 1.7 | 1.44 | 3.61 | 1.49 | 0.003 |
| Thoracic surgery | 1131 | 0.52 | 0.12 | 19.3 | 0 | 2.28 | 0.26 | 0.55 | 0.08 | 0.25 | 4.22 | 9.75 | 0 |
| Plastic surgery | 34 | 0.39 | 0 | 15.5 | 0 | 0 | 1.3 | 0.33 | 0.07 | 0.35 | 0.6 | 1.86 | 0 |
| Oral maxillofacial surgery |
287 | 0 | 0 | 0 | 0 | 0 | 0 | 0.44 | 0 | 0 | 0 | 3.18 | 0 |
AK: Amikacin; AMC: Amoxicillin/Clavulanate; CAZ: Cephtazidime; CRO: Ceftriaxiaxone; CIP: Ciprofloxacin; CZO: Cephazolin; FEP: Cephepime; MEM: Meropenem; SXT: Sulphametoxazole/Trimethoprim; TGC: Tigecycline; TPZ: Piperacillin/Tazobactam.
Table 5.
Linear regression analysis of correlations between antibiotic resistance in Klebsiella and antibiotic consumption.
| Consumption of Antibiotics | Standardized Coefficient |
Std. Err. | t | p | 95% Confidence Interval |
|---|---|---|---|---|---|
| Ciprofloxacin | −0.0021 | 0.0008975 | −2.34 | 0.101 | [−0.0049579, 0.0007546] |
| Tigecycline | 0.0118279 | 0.0032538 | 3.64 | 0.036 * | [0.001473, 0.0221829] |
| Piperacillin/ Tazobactam |
−0.0027939 | 0.0008836 | −3.16 | 0.050 * | [−0.0056061, 0.0000182] |
| Meropenem | 0.003232 | 0.000955 | 3.38 | 0.043 * | [0.0001927, 0.0062714] |
| _constant | −0.3240754 | 0.1989285 | −1.63 | 0.202 | [−0.9571546, 0.3090037] |
* p < 0.05.
The antibiotic consumption showed marked differences between the wards, as in almost any speciality it is possible to find patients with severe infections that require high-dose antibiotherapy. We observed that Ceftriaxone is a popular antibiotic being used at dosages up to ten times than those recommended by the WHO in the wards of ICU, Neurosurgery, and other surgical wards. Also, Piperacillin/Tazobactam was used in double quantities in ICU, as Amoxicillin/Clavulanate in Cardiology.
We observed a significant effect on the antibiotic resistance index of consumption of Tigecycline (1.18%), Piperacillin/Tazobactam (−0.28%), and Meropenem (0.32%). The constant term signifies the resistance index predicted by the model that is not dependent on antibiotic consumption (intrinsic resistance).
The results of the time series analysis of the effect of antibiotic consumption on antibiotic resistance are presented in Table 6.
Table 6.
Time-series regression analysis of correlations between meropenem and Ciprofloxacin resistance in Klebsiella and antibiotic consumption.
| Outcome Used | R Sq. | Predictors in the Model | Coefficient | 95% Confidence Interval |
p |
|---|---|---|---|---|---|
| Resistance to Meropenem | 0.627 | Consumption of Imipenem 1 month before | 0.0243 | [−0.0043, 0.0529] | 0.078 |
| Consumption of Meropenem 1 month before | −0.0016 | [−0.0057, 0.0025] | 0.341 | ||
| Resistance to Meropenem | 0.753 | Consumption of Imipenem 2 months before | 0.0075 | [0.0011, 0.0130] | 0.032 * |
| Consumption of Meropenem 2 months before | −0.0042 | [−0.0077, 0.0081] | 0.027 | ||
| Resistance to Ciprofloxacin | 0.753 | Consumption of Imipenem 2 months before | 0.0010 | [0.0007, 0.0014] | <0.001 * |
| Consumption of Meropenem 2 months before | −0.0002 | [−0.0001, 0.0010] | 0.094 | ||
| Consumption of Ciprofloxacin 2 months before | 0.0004 | [0.0001, 0.0007] | 0.0047 * | ||
| Resistance to Ciprofloxacin | 0.537 | Consumption of Imipenem 3 months before | 0.0064 | [0.0004, 0.0009] | <0.001 |
| Consumption of Meropenem 3 months before | 0.0001 | [−0.0002, 0.0004] | 0.571 | ||
| Consumption of Ciprofloxacin 3 months before | −0.0001 | [−0.0004, 0.0001] | 0.330 |
* p < 0.05.
We observed a marginally significant correlation between the resistance to Meropenem and the consumption of Imipenem 1 month before (p = 0.078), but surprisingly not with the consumption of Meropenem. Nevertheless, the resistance to Meropenem strongly correlated with the consumption of imipenem 2 months before and also with the consumption of Meropenem.
We also observed that the resistance to Ciprofloxacin was strongly correlated with the consumption of Imipenem 2 and 3 months before (p < 0.001 for both correlations), but only with the consumption of Ciprofloxacin 2 months before (p = 0.005) and not 3 months before.
3.5. Analysis of Strains Relatedness Based on Clustering on MAR Index
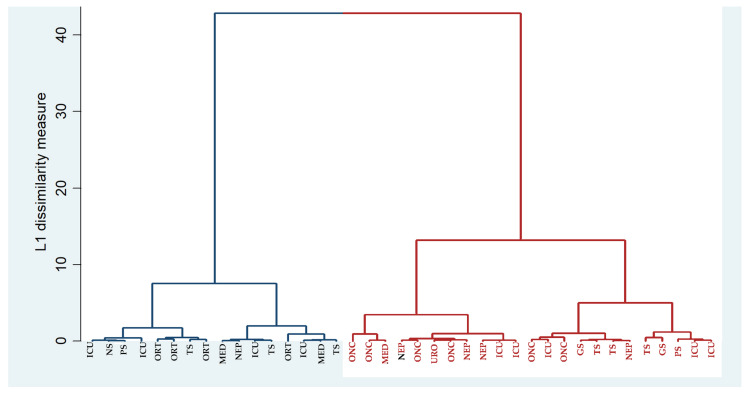
Klebsiella presented two main clusters corresponding to the community and hospital strains (which had low resistance (blue) and high resistance (red)) (Figure 7), each with two secondary clusters of low/high resistance. Most surgical strains had a medium resistance, but the high-resistance strains were found in the Thoracic Surgery ward, Orthopaedics and ICU. It can be observed from Figure 7 that the ICU strains are divided into 3 subclusters each with 2 strains. 3 strains from Orthopaedics belong in the same cluster and Oncology strains were divided into 2 subclusters each with 2 strains.
Figure 7.
Clustering analys1is of the isolated Klebsiella strain using hospital ward as a label. ICU: Intensive Care Unit; NS: Neurosurgery; PS: Plastic Surgery; ORT: Orthopaedics; TS: Thoracic Surgery; MED: Medical wards; NEP: Nephrology; ONC: Oncology; URO: Urology; GS: General Surgery. Blue lines: low resistance cluster, red lines: high resistance cluster.
3.6. Epidemiologic Tracing of the Antibiotic Resistance Phenotypes
We used antibiotics for which the resistance was common on Klebsiella: Aztreonam (ATM) Ceftazidime (CAZ), Cefepime (FEP) Amoxicillin/Clavulanate (AMC) TPZ: Piperacillin/Tazobactam.), Ertapenem (ETP), Ciprofloxacin (CIP) Imipenem (IMP), Amikacin (AMK) to search for repeated resistance profiles. Next, we analyzed the frequencies of each resistance profile to the above-mentioned antibiotics, as multiple strains with the same resistance profile are probably related and can represent an epidemiologic transmission event.
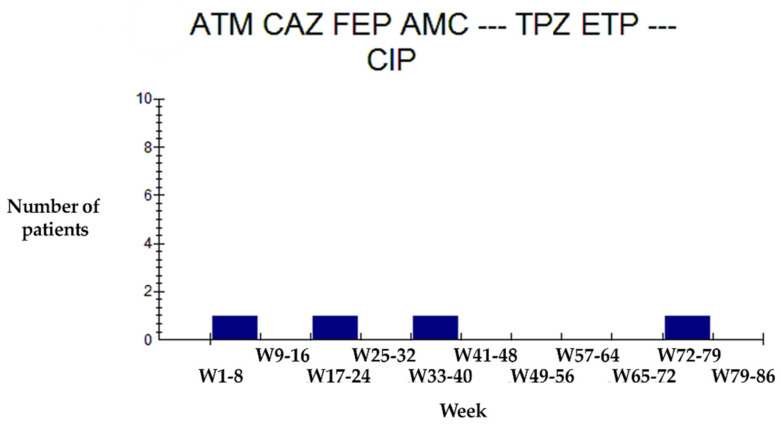
In this regard, we observed that the Klebsiella phenotype with ATM-CAZ-FEP-ACM-TPZ-ETP-CIP resistance profile appeared sporadically every two weeks, from week 32 of 2018 to week 36 of the same year, to reappear at week 41 (Figure 8), suggesting a possible resistance transmission chain.
Figure 8.
Evolution of the Klebsiella phenotype ATM-CAZ-FEP-AMC-TZP-ETP-CIP. Aztreonam (ATM) Ceftazidime (CAZ), Cefepime (FEP), Amoxicillin/Clavulanate (AMC) TPZ: Piperacillin/Tazobactam, Ertapenem (ETP) and Ciprofloxacin (CIP).
4. Discussion
Chronically underreported, present anywhere in the world, nosocomial infections (IN) are and continue to be a challenge in inpatient or outpatient medicine, even in this century, due to weak spots of hygiene in hospitals, negligence of the medical staff, outdated antibiotic therapies, and other reasons. These infections are one of the leading causes of death in hospitals worldwide [2,3].
We assist in recent years with an increase in the number of K. pneumoniae isolates, especially antibiotic-resistant strains, particularly in high-risk wards as ICUs. The dissemination of hospital strains into the community can lead to problems of public health that should not be taken lightly [18,24,26]. Particularly in ICUs, often more than half of the isolated strains are antibiotic-resistant [27,28]. These resistant strains complicate the management of infections and particular clones spread in the hospital environment. In our hospital, Klebsiella strains were 26.67% of the strains isolated from the ICU department, followed by a prevalence of 23.22% in Thoracic Surgery and Urology (22.58%). We observed pretty high prevalences of Klebsiella in many wards, which suggests the ubiquity of Klebsiella in our hospital.
The transmission of Klebsiella strains through the air is supported by the high prevalence in specimens collected from the respiratory tract: sputum (27.34%), tracheal aspirate (21.03%) and nasal swabs (13.16%). Other studies incriminated also hospital air as a route of transmission for Klebsiella strains [29].
4.1. Prevalence of Klebsiella
Our study showed an undulant evolution of the Klebsiella prevalence in our hospital, without an ascending or descending trend (Chi2 test for trend p = 0.654). This can be regarded as a positive result because numerous studies show an increase in prevalence rates of K. pneumoniae, while other studies show a decrease in prevalence after applying infection control measures [30,31]. In our hospital, infection control was greatly improved in recent years. Still, the lack of a descending trend in Klebsiella prevalence can suggest an actual increase in incidence that was kept under control by the increase of preventive measures. The lack of seasonal variability of the prevalence of Klebsiella infections in our hospital can also suggest that a great percentage of these infections is actually iatrogenic in nature.
4.2. Antibiotic Resistance of Klebsiella
By analysis of MAR, we detected that the strains isolated from the ICU had the highest resistance, presumably due to the higher dosage of antibiotics used on these patients which often has severe infections due to lower immunity. We can also suppose in the ICU there is the transmission of multiresistant strains established as hospital multidrug-resistant strains. The histogram analysis showed in the ICU a low-resistance Klebsiella population with a low MAR, which also had MIC determined by the Vitek2 system to various antibiotics below epidemiological breakpoints (wild-type strains without detectable resistance mechanisms), representing about half of the Klebsiella strains isolated from this ward. These community strains were present in most wards, coming from recently hospitalised patients who did not fall within the definition of nosocomial infection (infection occurring more than 2 days after the date of hospitalisation). There are two more populations of Klebsiella in ICU, one with moderate resistance and one with increased resistance, corresponding to hospital strains.
All over the globe, the prevalence of Klebsiella strains and especially of multiresistant ones is increased in settings in which patients are often under antibiotic treatment. These include the ICUs where there are patients with septic shock that are massively treated with antibiotics and some surgical wards in which wounds sometimes get infected and need prolonged antibiotic treatment with doses higher than usual [27]. Our study showed the presence of resistant Klebsiella populations in General surgery, Plastic surgery, Pediatric surgery and ICU wards.
The multiresistant Klebsiella strains isolated from hospitals possess various resistance mechanisms as ESBLs, AmpC (that give resistance to penicillin and first-generation cephalosporins) [27], or carbapenemases (that confer resistance to carbapenems and 3rd generation cephalosporins) [32] that reflect in the antibiotic resistance pattern. In our study, we also observed multiple antibiotic resistances in Klebsiella strains, particularly high resistances were encountered to Amoxicillin/Clavulanate, 1st generation cephalosporins and to a lesser extent to the third-generation cephalosporins, which is due to the transmission of plasmids encoding resistance genes, in particular ESBL [33,34,35].
In hospitals, there is a high probability of horizontal gene transfers of resistance genes that can explain the increased resistance to Amoxicillin/Clavulanate, Ceftazidime and Ceftriaxone observed by us. Previous studies have shown an increased prevalence of OXA-48 carbapenemases in Klebsiella strains from SCJUC [8,36].
Due to the presence of beta-lactam resistance genes, the resistance to 3rd generation cephalosporins oscillated between 60% and 90% in an undulant manner that can be related to the periodic changes in antibiotic pre-surgery prophylaxis protocols in surgical wards, as part of infection control efforts in our hospital, which are probably the explanation to a marked decrease in resistance to Meropenem observed in the second year of the study.
The explanation of correlations between antibiotic resistances first resides in the usage of the same antibiotics in different wards, according to infection control protocols. Ciprofloxacin resistance correlation between Klebsiella and Staphylococcus is explained by the rapid development of AR to quinolones in patients with mixed infections [37]. Also, the correlation between the resistance of E. coli rods and Klebsiella is that carbapenem treatment induces resistance in both bacteria, alongside horizontal gene transfer of carbapenemases’ genes which were captured by the Enterobacteriaceae from Pseudomonas and other non-fermenter Gram-negative rods (NFR) [27,38,39]. In our results, the increase of resistance in E. coli seems to precede that of Klebsiella spp. (Figure 6), suggesting that the later acquired resistance genes from E. coli, as Klebsiella is a well-known collector of resistance genes [34,40].
By analysing the evolution in time of the resistance index of Klebsiella spp. strains, we observed that the graph resembles the evolution for Pseudomonas strains because both Gram-negative bacteria share common resistance genes. The resistance genes can be horizontally transmitted between hospital strains [18,34,35]. Thus, the virulent strain may persist for long periods and disseminate in clinical settings, because they survive to usual antibiotic treatments applied to patients according to various guidelines.
4.3. The Link between the Antibiotic Resistance of Klebsiella and the Consumption of Antibiotics
One of the well-known causes of the continuously increasing antibiotic resistance of bacteria worldwide is the over-prescription and consumption of antibiotics. We investigated this possible cause by performing two separated regression analyses: a multiple regression which examined the link between resistance and consumption of antibiotics in the same month and a time-series analysis which examined the link between resistance and consumption of antibiotics in the previous months, accounting for relationships between different points in time within a single series. That type of analysis should be more sensitive than the simple regression which does not account for the temporal nature of data.
By performing the regression for dependency between antibiotic resistance and antibiotic consumption, we observed, interestingly that entering the consumption of Ampicillin and Teicoplanin in the regression equation renders their regression coefficients significant, i.e., the correlation is now statistically significant. That is because of collinearity (correlation between two explanatory variables, used to explain the output variable). Indeed, both antibiotics are used in treating Gram-positive infections and their consumption is therefore correlated. Also, the consumption of Ampicillin correlates with the consumption of Meropenem, introducing further collinearity into the equation. Meropenem consumption is also correlated with imipenem consumption because the 2 antibiotics are of the same class and can be replaced with each other. For these reasons, we decided to drop Ampicillin and Teicoplanin resistances from the multiple regression models.
We observed some correlations between antibiotic consumption and the development of antibiotic resistance after 1, 2 and even 3 months. For example, resistance to Meropenem was influenced by the consumption in the hospital ward of imipenem 1 month and two months before, but only 1 month before for the consumption of Meropenem, suggesting that imipenem is a stronger and for a longer time inducer of carbapenem resistance compared with Meropenem. Indeed, in vitro studies showed that subinhibitory concentrations of imipenem can induce resistance to beta-lactam antibiotics by inducing the beta-lactamase production because its chemical structure is more related to the original penem nucleus than Meropenem [27].
Phenotypic clustering of bacterial strain relatedness can substitute genetic-based clustering for epidemiological tracing for infection control purposes [32]. We successfully realized a phenotypical clustering in the premiere for our hospital, which will have found a basis for future infection control efforts. As expected, the clustering showed filiation e.g., between multiresistant Klebsiella strains isolated from specific patients from the ICU, which can greatly improve the effectiveness of infection control measures. So the clustering based on MAR can at least orient the epidemiologist to the transmission events, although it cannot substitute to molecular methods.
The epidemiologic filiation tree of strains circulating within the hospital can be constructed by monitoring and linking in clusters specific resistance phenotypes. We managed to do that using the WHONET software capabilities. Thus, the resistance data from routine antimicrobial susceptibility testing performed in each clinical microbiology laborawe put limitations as a title of this sectiontory is a major resource for resistance surveillance that can be used to contribute to surveillance networks [41].
The time-series analysis can also be used for forecasting. Because antibiotic resistance can be induced by antibiotic consumption in previous months, it is possible to forecast the resistance for one or two months based on the antibiotic consumption in a certain month.
Antibiotic resistance testing can be influenced by a variety of laboratory or clinical factors. Although we analyzed all specimens collected, there is the possibility that bacteria did not grow from multiple causes like the quality of laboratory media or treatments with antibiotics. A factor that can influence the results of the study is the presence of uncultured bacteria which may be reluctant to be grown under laboratory conditions.
5. Conclusions
This work showed the importance of continuous monitoring of antibiotic resistance in hospitals to identify epidemiological transmission chains, adjust the antibiotherapy in various wards and improving the cure rate of infections treated in the hospital.
This can be very useful for infections that can change the guidelines of antibiotic prescription in a healthcare setting.
Author Contributions
Conceptualization, A.E.G., A.I.D., E.N.Ț., O.M.Z., M.P., D.C. (Daniela Calina) and R.C.; methodology, C.M.V., A.-L.U. and C.G.Ț.; software, A.-L.U., A.E.G. and O.M.Z.; validation, A.E.G., A.I.D. and R.C.; formal analysis, A.-L.U., O.M.Z., V.P. and R.C.; resources, A.-I.S., M.P., A.M. and D.C. (Dan Cȃrţu); data curation, C.M.V., A.M. and D.C. (Dan Cȃrţu); writing—original draft preparation, C.M.V., A.E.G., D.C. (Daniela Calina), E.N.Ț. and A.I.D.; writing—review and editing, A.E.G., M.P., O.M.Z. and V.P.; supervision, A.I.D., A.E.G. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Usha P.T.A., Jose S., Nisha A.R. Antimicrobial Drug Resistance-A global concern. Vet. World. 2010;3:138–139. [Google Scholar]
- 2.Al-Tawfiq J.A., Tambyah P.A. Healthcare associated infections (HAI) perspectives. J. Infect. Public Health. 2014;7:339–344. doi: 10.1016/j.jiph.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Klevens R.M., Edwards J.R., Richards C.L., Horan T.C., Gaynes R.P., Pollock D.A., Cardo D.M. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan M.M., El Zowalaty M.E., Lundkvist Å., Järhult J.D., Khan Nayem M.R., Tanzin A.Z., Badsha M.R., Khan S.A., Ashour H.M. Residual antimicrobial agents in food originating from animals. Trends Food Sci. Technol. 2021;111:141–150. doi: 10.1016/j.tifs.2021.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Tella D., Tamburro M., Guerrizio G., Fanelli I., Sammarco M.L., Ripabelli G. Molecular epidemiological insights into colistin-resistant and carbapenemases-producing clinical Klebsiella pneumoniae isolates. Infect. Drug Resist. 2019;12:3783–3795. doi: 10.2147/IDR.S226416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson D.J., Moehring R.W., Sloane R., Schmader K.E., Weber D.J., Fowler V.G.J., Smathers E., Sexton D.J. Bloodstream infections in community hospitals in the 21st century: A multicenter cohort study. PLoS ONE. 2014;9:e91713. doi: 10.1371/journal.pone.0091713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlatian O., Balasoiu A.T., Balasoiu M., Cristea O., Docea A.O., Mitrut R., Spandidos D.A., Tsatsakis A.M., Bancescu G., Calina D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp. Ther. Med. 2018;16:4499–4510. doi: 10.3892/etm.2018.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perl T.M., Cullen J.J., Wenzel R.P., Zimmerman M.B., Pfaller M.A., Sheppard D., Twombley J., French P.P., Herwaldt L.A., Team M.A.T.R.O.S.A.S., et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 2002;346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 10.Calina D., Staicus C., Rosu L., Rosu A.F. Involvement of microbial flora in aetiology of surgical site infections; Proceedings of the Clinical Pharmacy Congress; London, UK. 22–23 April 2016. [Google Scholar]
- 11.Calina D., Roșu L., Roșu A.F., Ianoşi G., Ianoşi S., Zlatian O., Mitruț R., Docea A.O., Rogoveanu O., Mitruț P., et al. Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleurisy. Farmacia. 2016;64:946–952. [Google Scholar]
- 12.Vading M., Nauclér P., Kalin M., Giske C.G. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0195258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills M.C., Lee J. The threat of carbapenem-resistant bacteria in the environment: Evidence of widespread contamination of reservoirs at a global scale. Environ. Pollut. 2019;255:113143. doi: 10.1016/j.envpol.2019.113143. [DOI] [PubMed] [Google Scholar]
- 14.Salehi B., Shivaprasad Shetty M., Anil Kumar N.V., Živković J., Calina D., Oana Docea A., Emamzadeh-Yazdi S., Sibel Kılıç C., Goloshvili T., Nicola S., et al. Veronica plants—Drifting from farm to traditional healing, food application, and phytopharmacology. Molecules. 2019;24:2454. doi: 10.3390/molecules24132454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorrie C.L., Mirceta M., Wick R.R., Edwards D.J., Thomson N.R., Strugnell R.A., Pratt N.F., Garlick J.S., Watson K.M., Pilcher D.V., et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017;65:208–215. doi: 10.1093/cid/cix270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungureanu A., Zlatian O., Mitroi G., Drocaş A., Ţîrcã T., Cǎlina D., Dehelean C., Docea A.O., Izotov B.N., Rakitskii V.N., et al. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol. Med. Rep. 2017;16:8771–8780. doi: 10.3892/mmr.2017.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Queenan A.M., Bush K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrik T.C., Voorintholt A.F., Vos M.C. Clinical and molecular epidemiology of extended-spectrum beta-lactamase- Producing Klebsiella spp: A systematic review and meta-analyses. PLoS ONE. 2015;10:e140754. doi: 10.1371/journal.pone.0140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iredell J., Brown J., Tagg K. Antibiotic resistance in enterobacteriaceae: Mechanisms and clinical implications. BMJ. 2016;352 doi: 10.1136/bmj.h6420. [DOI] [PubMed] [Google Scholar]
- 20.Afunwa R.A., Odimegwu D.C., Iroha R.I., Esimone C.O. Antimicrobial resistance status and prevalence rates of extended spectrum beta-lactamase producers isolated from a mixed human population. Bosn. J. Basic Med. Sci. 2011;11:91–96. doi: 10.17305/bjbms.2011.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pournaras S., Protonotariou E., Voulgari E., Kristo I., Dimitroulia E., Vitti D., Tsalidou M., Maniatis A.N., Tsakris A., Sofianou D. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 2009;64:348–352. doi: 10.1093/jac/dkp207. [DOI] [PubMed] [Google Scholar]
- 22.Galani I., Karaiskos I., Karantani I., Papoutsaki V., Maraki S., Papaioannou V., Kazila P., Tsorlini H., Charalampaki N., Toutouza M., et al. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Eurosurveillance. 2018;23:1700775. doi: 10.2807/1560-7917.ES.2018.23.30.1700775. [DOI] [PubMed] [Google Scholar]
- 23.Ripabelli G., Tamburro M., Guerrizio G., Fanelli I., Flocco R., Scutellà M., Sammarco M.L. Tracking multidrug-resistant Klebsiella pneumoniae from an Italian Hospital: Molecular epidemiology and surveillance by PFGE, RAPD and PCR-Based resistance genes prevalence. Curr. Microbiol. 2018;75:977–987. doi: 10.1007/s00284-018-1475-3. [DOI] [PubMed] [Google Scholar]
- 24.Phillips I.K.S. Class I β-lactamases induction and derepression. Drugs. 1989;37:402–407. doi: 10.2165/00003495-198937040-00002. [DOI] [PubMed] [Google Scholar]
- 25.Davis R., Brown P.D. Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 2016;65:261–271. doi: 10.1099/jmm.0.000229. [DOI] [PubMed] [Google Scholar]
- 26.Giacometti A., Siquini F.M., Cirioni O., Petroni S., Scalise G. Imipenem and meropenem induced resistance to beta-lactam antibiotics in Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13:315–318. doi: 10.1007/BF01974609. [DOI] [PubMed] [Google Scholar]
- 27.Rodloff A.C., Goldstein E.J.C., Torres A. Two decades of imipenem therapy. J. Antimicrob. Chemother. 2006;58:916–929. doi: 10.1093/jac/dkl354. [DOI] [PubMed] [Google Scholar]
- 28.Oberoi L., Singh N., Sharma P., Aggarwal A. ESBL, MBL and Ampc β Lactamases producing superbugs—Havoc in the Intensive Care Units of Punjab India. J. Clin. Diagn. Res. 2013;7:70–73. doi: 10.7860/JCDR/2012/5016.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M.J., Feng Y.S., Sung W.P., Surampalli R.Y. Quantification and analysis of airborne bacterial characteristics in a nursing care institution. J. Air Waste Manag. Assoc. 2011;61:732–739. doi: 10.3155/1047-3289.61.7.732. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber P.W., Sax H., Wolfensberger A., Clack L., Kuster S.P. The preventable proportion of healthcare-associated infections 2005-2016: Systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018;39:1277–1295. doi: 10.1017/ice.2018.183. [DOI] [PubMed] [Google Scholar]
- 31.Umscheid C.A., Mitchell M.D., Doshi J.A., Agarwal R., Williams K., Brennan P.J. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control Hosp. Epidemiol. 2011;32:101–114. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 32.Xu J., Shi C., Song M., Xu X., Yang P., Paoli G., Shi X. Phenotypic and genotypic antimicrobial resistance traits of foodborne Staphylococcus aureus isolates from Shanghai. J. Food Sci. 2014;79 doi: 10.1111/1750-3841.12405. [DOI] [PubMed] [Google Scholar]
- 33.Rozwandowicz M., Brouwer M.S.M., Fischer J., Wagenaar J.A., Gonzalez-Zorn B., Guerra B., Mevius D.J., Hordijk J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018;73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez M.S., Iriarte A., Reyes-Lamothe R., Sherratt D.J., Tolmasky M.E. Small Klebsiella pneumoniae plasmids: Neglected contributors to antibiotic resistance. Front. Microbiol. 2019;10:2182. doi: 10.3389/fmicb.2019.02182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schjørring S., Struve C., Krogfelt K.A. Transfer of antimicrobial resistance plasmids from Klebsiella pneumoniae to Escherichia coli in the mouse intestine. J. Antimicrob. Chemother. 2008;62:1086–1093. doi: 10.1093/jac/dkn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Călina D., Docea A.O., Rosu L., Zlatian O., Rosu A.F., Anghelina F., Rogoveanu O., Arsene A.L., Nicolae A.C., Drăgoi C.M., et al. Antimicrobial resistance development following surgical site infections. Mol. Med. Rep. 2017;15:681–688. doi: 10.3892/mmr.2016.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper D.C., Jacoby G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015;1354:12–31. doi: 10.1111/nyas.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berdal J.E., Bjørnholt J., Blomfeldt A., Smith-Erichsen N., Bukholm G. Patterns and dynamics of airway colonisation in mechanically-ventilated patients. Clin. Microbiol. Infect. 2007;13:476–480. doi: 10.1111/j.1469-0691.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 39.Tzouvelekis L.S., Markogiannakis A., Piperaki E., Souli M., Daikos G.L. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 2014;20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 40.Woodford N., Turton J.F., Livermore D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011;35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 41.Vatopoulos A.C., Kalapothaki V., Legakis N.J. An electronic network for the surveillance of antimicrobial resistance in bacterial nosocomial isolates in Greece. Bull. World Health Organ. 1999;77:595–601. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.