ABSTRACT
Cerebral ischemia/reperfusion (I/R) can lead to serious brain function impairments. Long noncoding RNA (lncRNA) CCAAT enhancer binding protein α antisense RNA 1 (CEBPA-AS1) was shown to be upregulated in human ischemic stroke. This study investigated the function and mechanism of CEBPA-AS1 in I/R. An oxygen-glucose deprivation/reperfusion (OGD/R) model was used to induce I/R injury in SH-SY5Y cells in vitro. RT-qPCR examined the expression of CEBPA-AS1, microRNA 24-3p (miR-24-3p), and Bcl-2-related ovarian killer (Bok). The cell viability, apoptosis, oxidative stress in OGD/R-treated cells were detected using CCK-8, flow cytometry, Western blotting, and enzyme-linked immunosorbent assays. The relationship among genes was tested by RNA pulldown and luciferase reporter assays. We found that OGD/R upregulated CEBPA-AS1 expression in SH-SY5Y cells. Functionally, CEBPA-AS1 depletion ameliorated OGD/R-induced apoptosis and oxidative stress in SH-SY5Y cells by reducing reactive oxygen species production and superoxide dismutase and glutathione. Mechanistic investigations indicated that CEBPA-AS1 acts as a sponge for miR-24-3p, and miR-24-3p binds to BOK. Moreover, miR-24-3p upregulation or BOK downregulation antagonized the protective role of CEBPA-AS1 depletion in SH-SY5Y cells exposed to OGD/R. Overall, downregulation of CEBPA-AS1 exerts protective functions against OGD/R-induced injury by targeting the miR-24-3p/BOK axis.
KEYWORDS: CEBPA-AS1, miR-24-3p, BOK, I/R, OGD/R, CEBPA-AS1
INTRODUCTION
Stroke is a neurological disorder with cerebral ischemia and hemorrhagic injury as the main clinical manifestations (1). Ischemic stroke is a major ischemic cerebrovascular event as a consequence of cerebral blood circulation disorder, ischemia, and hypoxia (2), accounting for 80 to 90% of all strokes with an increasing incidence annually (3). Major risk factors of ischemic stroke include diabetes, alcohol, smoking, and hypertension (4). Recovery of the blood supply is an acceptable treatment strategy for ischemic stroke. Unfortunately, the reperfusion of blood in ischemic areas often leads to ischemia/reperfusion (I/R) injury (5, 6), which is a major cause of many complications related to stroke, traumatic brain injury, and myocardial infarction (7). I/R injury is accompanied by multiple pathological processes, including necrosis, nerve damage, and the apoptosis of neuronal cells stimulated by oxidative stress (8, 9). Although many treatment methods for neuroprotection and anticerebral ischemia have been applied to clinic, the therapeutic effect is still not ideal (10). Therefore, understanding of the mechanisms in I/R injury to develop effective therapeutic targets is key for the improvement of ischemic stroke treatment.
Long noncoding RNAs (lncRNAs) are ncRNAs more than 200 nucleotides and regulate gene expression transcriptionally and posttranscriptionally (11, 12). Evidence suggests that lncRNAs show important potential in regulating biological functions, such as epigenetic inheritance, gene translation, cell survival, differentiation, and cell death (13, 14). The roles of lncRNAs in neurodevelopment and neurological disease progression have been a research hot spot recently (15). For example, lncRNA Nespas exerts antiapoptotic and anti-inflammatory effects in oxygen-glucose deprivation (OGD)-treated microglial cells and in ischemic stroke mice (16). lncRNA Malat1 plays antiapoptotic and anti-inflammatory roles in brain microvasculature to reduce ischemic cerebral vascular and parenchymal damages (17). lncRNA SETD5-AS1 induces cell apoptosis and inhibits proliferation in mouse neuroblastoma N2a cells with OGD/R (18). Previous studies reported that lncRNA CEBPA-AS1 is upregulated in human ischemic stroke, indicating that CEBPA-AS1 may be a useful biomarker (19). However, the biological role of CEBPA-AS1 in ischemic stroke requires further investigation.
lncRNAs could function by interacting with various molecules or acting as sponges for microRNAs (miRNAs) (20). lncOGD-1006 attenuates brain injury induced by OGD by regulating the miRNA 184-5p (miR-184-5p)/CAAP1 axis (21). lncRNA SNHG16 plays a neuroprotective role in OGD/R-treated neurons by the miR-106b-5p/LIMK1 axis (22). miRNAs are small ncRNAs that affect protein translation and mRNA degradation by binding to the mRNA posttranscriptionally (23). Research suggests that miRNAs are involved in the pathophysiology of ischemic stroke by mediating oxidative stress, inflammation, and cell apoptosis (24, 25). In addition, differential expression of miRNAs was found in OGD/R cell models (26, 27). A previous research found that miR-24-3p can inhibit angiogenesis-related functions in intracranial atherosclerotic disease (28).
In the present study, we verified the functional interaction among CEBPA-AS1, miR-24-3p, and Bcl-2-related ovarian killer (BOK) in ischemic stroke in vitro, providing an innovative therapeutic insight into the functional improvement of I/R injury.
RESULTS
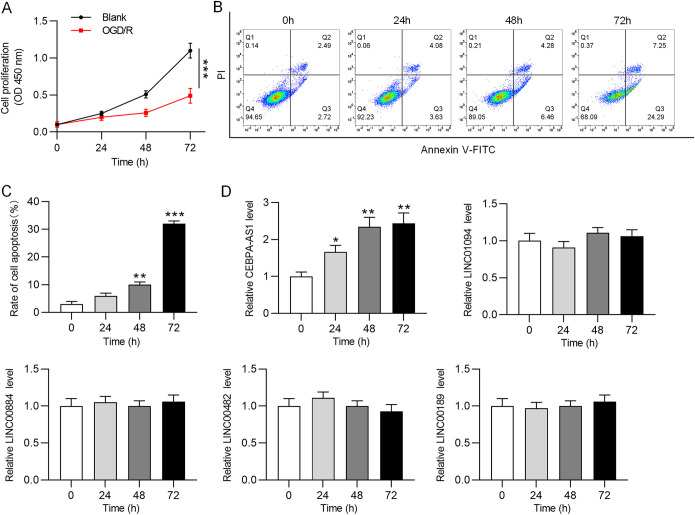
OGD/R induces cell injury and upregulates CEBPA-AS1 expression.
OGD/R could effectively induce I/R injury in vitro (29). SH-SY5Y cells were subjected to OGD for 4 h and subsequently reoxygenated for 24, 48, and 72 h to simulate I/R in vitro. As shown by CCK-8 assay, OGD/R reduced SH-SY5Y cell viability to approximately 50% at 72 h (Fig. 1A). In addition, the apoptosis rate of SH-SY5Y cells was significantly increased after OGD/R exposure (Fig. 1B). These data suggested that OGD/R successfully caused cell injury in vitro. Subsequently, RT-qPCR was used to analyze the expression of several lncRNAs (CEBPA-AS1, LINC01094, LINC00884, LINC00482, and LINC00189) that were reported to be upregulated in human ischemic stroke. It was found that the CEBPA-AS1 level was significantly elevated in OGD/R-treated SH-SY5Y cells in a time-dependent manner (Fig. 1C), while the other lncRNAs showed no significant changes. Therefore, we decided to study CEBPA-AS1. Overall, OGD/R induces cell injury and upregulates CEBPA-AS1 expression in SH-SY5Y cells.
FIG 1.
OGD/R induces cell injury and upregulates CEBPA-AS1 expression. SH-SY5Y cells were subjected to OGD for 2 h and then reoxygenated for 24, 48, and 72 h to simulate CI/R in vitro. (A) Cell viability was assessed by CCK-8 assay. (B) Cell apoptosis was analyzed by flow cytometry. (C) RT-qPCR was used to measure the expression of lncRNAs. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
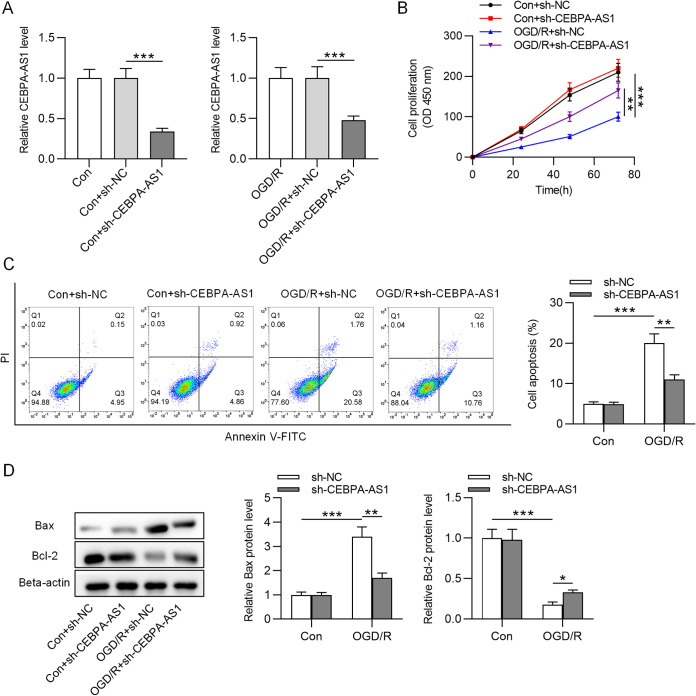
Knockdown of CEBPA-AS1 protects neurons against OGD/R-induced injury.
To determine the role of CEBPA-AS1 in CI/R injury in vitro, sh-CEBPA-AS1 or scrambled control (sh-NC) was transfected into SH-SY5Y cells. After transfection, the cells were subjected to OGD for 4 h and reoxygenated for 72 h. The CEBPA-AS1 level was lower in cells with sh-CEBPA-AS1 than in cells with sh-NC (Fig. 2A). CCK-8 assay indicated that the viability in sh-NC-transfected control cells had no significant difference compared to that in control cells, whereas, compared to sh-NC, sh-CEBPA-AS1 significantly increased cell viability in OGD/R-treated SH-SY5Y cells (Fig. 2B). In addition, the promotive effects of OGD/R on cell apoptosis were significantly reversed by CEBPA-AS1 knockdown (Fig. 2C). Furthermore, we tested the impact of CEPBA-AS1 knockdown on apoptotic markers (Bax and Bcl-2). Western blot indicated that OGD/R upregulated Bax and downregulated Bcl-2 in SH-SY5Y cells, but downregulated CEBPA-AS1 expression reversed these effects (Fig. 2D). Therefore, depletion of CEBPA-AS1 protects SH-SY5Y cells against injury induced by OGD/R.
FIG 2.
CEBPA-AS1 depletion protects SH-SY5Y cells against OGD/R-induced injury. SH-SY5Y cells were transfected with sh-CEBPA-AS1 or sh-NC. After transfection, the cells were subjected to OGD for 4 h and reoxygenated for 72 h. (A) RT-qPCR was used to measure the CEBPA-AS1 level. (B) Cell viability was evaluated by CCK-8 assay. (C) Cell apoptosis was detected by flow cytometry. (D) The levels of Bax and Bcl-2 were measured by Western blotting. **, P < 0.01; ***, P < 0.001.
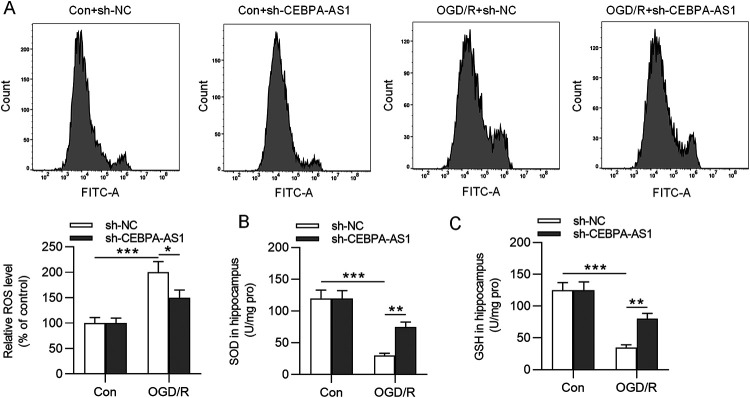
Knockdown of CEBPA-AS1 ameliorates OGD/R-induced oxidative stress.
Whether CEBPA-AS1 could affect oxidative stress in OGD/R-treated SH-SY5Y cells was next analyzed. Here, we assessed the intracellular production of reactive oxygen species (ROS) and the concentrations of superoxide dismutase (SOD) and glutathione (GSH) in SH-SY5Y cells. Flow cytometry and enzyme-linked immunosorbent assay (ELISA) demonstrated that OGD/R stimulated the production of ROS and reduced the concentrations of SOD and GSH (Fig. 3), suggesting that OGD/R induced oxidative stress. However, CEBPA-AS1 knockdown antagonized the effects of OGD/R on oxidative stress, as shown by decreased ROS levels and increased SOD and GSH levels (Fig. 3). These results showed that silencing CEBPA-AS1 could reduce OGD/R-induced oxidative stress in neurons.
FIG 3.
Knockdown of CEBPA-AS1 ameliorates OGD/R-induced oxidative stress. (A) ROS production was analyzed by flow cytometry. (B and C) The concentrations of SOD and GSH in the supernatants of OGD/R-treated SH-SY5Y cells were measured by ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
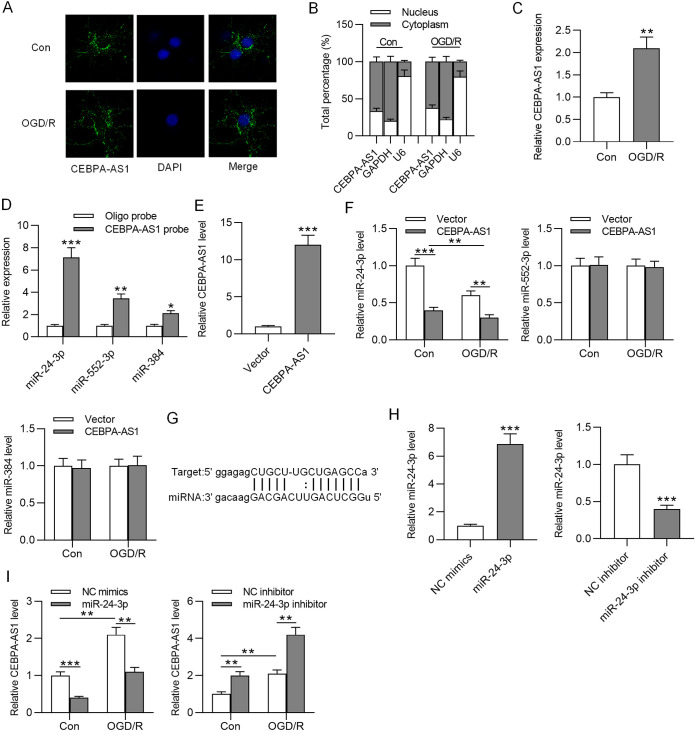
CEBPA-AS1 binds to miR-24-3p.
To explore how CEBPA-AS1 exerts its function, we assessed its subcellular localization. The results from FISH and RT-qPCR suggested that CEBPA-AS1 was mainly located in the cytoplasm of both control and OGD/R-treated SH-SY5Y cells (Fig. 4A and B). These results indicated that CEBPA-AS1 may function as a competing endogenous RNA (ceRNA) of miRNAs. The copy number of CEBPA-AS1 in control or OGD/R-treated SH-SY5Y cells was also detected and shown to be significantly increased under OGD/R treatment (Fig. 4C). Bioinformatics analysis was performed to identify the miRNAs that potentially bind to CEBPA-AS1. We found 12 miRNAs that have binding sites for CEBPA-AS1 at the starBase website (listed in Table 1). RNA pulldown assay tested the interaction of miRNAs with CEBPA-AS1. RT-qPCR showed that three miRNAs (miR-24-3p, miR-552-3p, and miR-384) were significantly enriched in the CEBPA-AS1 probe (Fig. 4D). Next, CEBPA-AS1 was overexpressed by transfection of CEBPA-AS1 (Fig. 4E). As the RT-qPCR results show in Fig. 4F, miR-24-3p exhibited higher expression in OGD/R-treated cells than in control cells, and CEBPA-AS1 overexpression significantly downregulated miR-24-3p expression in both OGD/R-treated cells and control cells. In addition, miR-24-3p expression was significantly lower in OGD/R-treated cells transfected with CEBPA-AS1 than in control cells transfected with CEBPA-AS1. However, there were no changes in the expression of miR-552-3p and miR-384. The binding site of miR-24-3p at the CEBPA-AS1 sequence is displayed in Fig. 4G. We then overexpressed miR-24-3p in SH-SY5Y cells using miR-24-3p and knocked down miR-24-3p using miR-24-3p inhibitor. RT-qPCR analysis demonstrated the successful transfection (Fig. 4H). Further, the effects of overexpressing and antagonizing miR-24-3p on the levels of endogenous CEBPA-AS1 was detected. The results revealed that overexpression of miR-24-3p caused a reduction in CEBPA-AS1 expression in both OGD/R-treated cells and control cells, and downregulation of miR-24-3p had an opposite result (Fig. 4I). Overall, CEBPA-AS1 could act as a sponge for miR-24-3p.
FIG 4.
CEBPA-AS1 binds to miR-24-3p. (A and B) FISH and subcellular fractionation assays were used to identify the localization of CEBPA-AS1 in control and OGD/R-treated SH-SY5Y cells. (C) Copy number of CEBPA-AS1 in control or OGD/R-treated SH-SY5Y cells. (D) The expression of miRNAs in the beads pulled down by CEBPA-AS1 probe was measured by RT-qPCR. (E) Overexpression efficiency of CEBPA-AS1 was verified by RT-qPCR. (F) The expression of miR-24-3p in control or OGD/R-treated SH-SY5Y cells transfected with vector or CEBPA-AS1 was measured by RT-qPCR. (G) Predicted binding site of miR-24-3p on CEBPA-AS1. (H) Overexpression efficiency of miR-24-3p and knockdown efficiency of miR-24-3p inhibitor were verified by RT-qPCR. (I) The expression of CEBPA-AS1 in control or OGD/R-treated SH-SY5Y cells transfected with miR-24-3p or miR-24-3p inhibitor was measured by RT-qPCR. **, P < 0.01; ***, P < 0.001.
TABLE 1.
Twelve miRNAs that have binding sites for CEBPA-AS1 at the starBase website
| miRNA ID | miRNA name | Gene ID | Gene name | Gene type | Chromosome | Start | End | miRNA sequence | Target sequence |
|---|---|---|---|---|---|---|---|---|---|
| MIMAT0015016 | hsa-miR-3145-3p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795102 | 33795129 | guuaaGGUUUGUG-AGU- - -UUUAUAGa | aaaaaCCAGAUACGUUAAAUAAAUAUCc |
| MIMAT0000080 | hsa-miR-24-3p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795141 | 33795161 | gacaagGACGACUUGACUCGGu | ggagagCUGCU-UGCUGAGCCa |
| MIMAT0003215 | hsa-miR-552-3p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795173 | 33795195 | aacAG-AU-UGGUCAGUGGACAa | cauUCAUAUAGCAUUCACCUGUg |
| MIMAT0001075 | hsa-miR-384 | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795268 | 33795287 | auacuuguuaaaGAUCCUUa | ggcuuuuccuacCUAGGAAc |
| MIMAT0004780 | hsa-miR-532-3p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795315 | 33795339 | acGUUCGGA-ACC-C-ACACCCUCc | ucCCGGCCUGUGGAGUUGUGGGAGg |
| MIMAT0014990 | hsa-miR-3127-5p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795778 | 33795800 | gaaggguaagguguuCGGGACUa | augaagagaaguccuGCCCUGAa |
| MIMAT0022726 | hsa-miR-1306-5p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795828 | 33795850 | accugcAAAC-GUCCCCUCCACc | aucagaUUUGUCUGCGGAGGUGu |
| MIMAT0004784 | hsa-miR-455-3p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795841 | 33795860 | caCAUAUACGGGUACCUGACg | gcGGAGGUG-UUAUGGACUGa |
| MIMAT0015011 | hsa-miR-3142 | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795877 | 33795898 | agacuuccaagucUUUCCGGAa | aaauuccuauguuGAAGGCCUa |
| MIMAT0019950 | hsa-miR-1245-5p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795877 | 33795898 | aaauucacUA-GAUUUCCGGAu | aaauuccuAUGUUGAAGGCCUa |
| MIMAT0002826 | hsa-miR-515-5p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795901 | 33795924 | gucuUUCACGAAAGAAAACCUCUu | ccccAUGUGACUGUAUUUGGAGAu |
| MIMAT0002828 | hsa-miR-519e-5p | ENSG00000267296 | CEBPA-AS1 | Antisense | chr19 | 33795903 | 33795924 | cuUUCACGAGGGAAAACCUCUu | ccAUGUGACUGUAUUUGGAGAu |
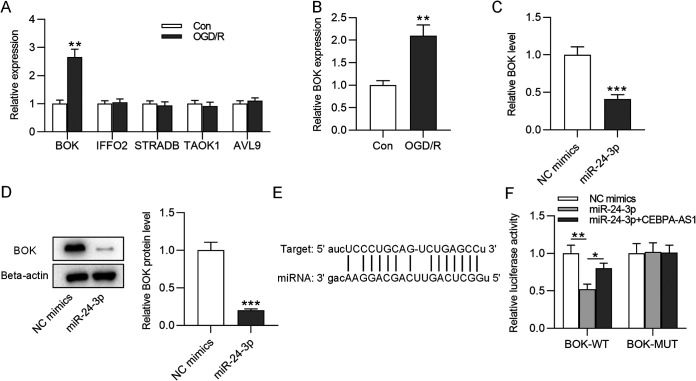
miR-24-3p targets BOK.
Next, the target genes of miR-24-3p were identified using the miRDB database. We selected the five mRNAs (BOK, IFFO, STRADB, TAOK1, and AVL9) according to the target rank and tested their expression levels in OGD/R-treated SH-SY5Y cells. The data indicated only BOK was significantly upregulated after OGD/R treatment (Fig. 5A). Thus, BOK was chosen from candidate mRNAs. The copy number of BOK in control or OGD/R-treated SH-SY5Y cells was found to be increased under OGD/R treatment (Fig. 5B). As RT-qPCR and Western blotting showed, BOK expression at mRNA and protein levels in SH-SY5Y cells was reduced by miR-24-3p overexpression (Fig. 5C and D). In Fig. 5E, the binding site at the BOK 3′ untranslated region is potentially targeted by miR-24-3p. To verify their targeted relationship, a luciferase reporter assay was conducted. Decreased luciferase activity of the BOK-WT vector was observed in the miR-24-3p group, and overexpression of CEBPA-AS1 restored the luciferase activity of the BOK-WT vector (Fig. 5F), suggesting that miR-24-3p binds to BOK. These findings indicated that CEBPA-AS1 functions as a regulator of BOK expression by controlling miR-24-3p availability.
FIG 5.
miR-24-3p binds to the BOK. (A) The expression of mRNAs in OGD/R-treated SH-SY5Y cells was measured by RT-qPCR. (B) Copy number of BOK in control or OGD/R-treated SH-SY5Y cells. (C and D) The expression of BOK mRNA and protein in SH-SY5Y cells transfected with miR-24-3p was detected by RT-qPCR and Western blot. (E) Predicted binding site of miR-24-3p on BOK. (F) A luciferase reporter assay in SH-SY5Y cells was conducted to assess the luciferase activity of BOK-WT or BOK-MUT vector. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
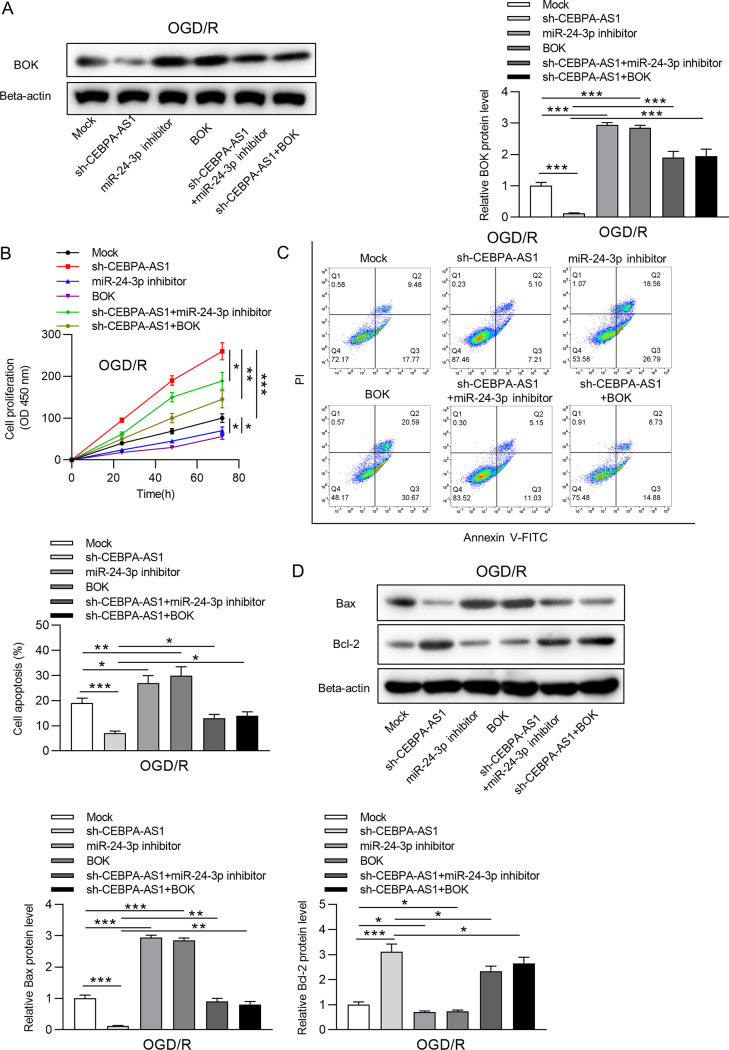
CEBPA-AS1 knockdown alleviates OGD/R-induced injury by the miR-24-3p/BOK axis.
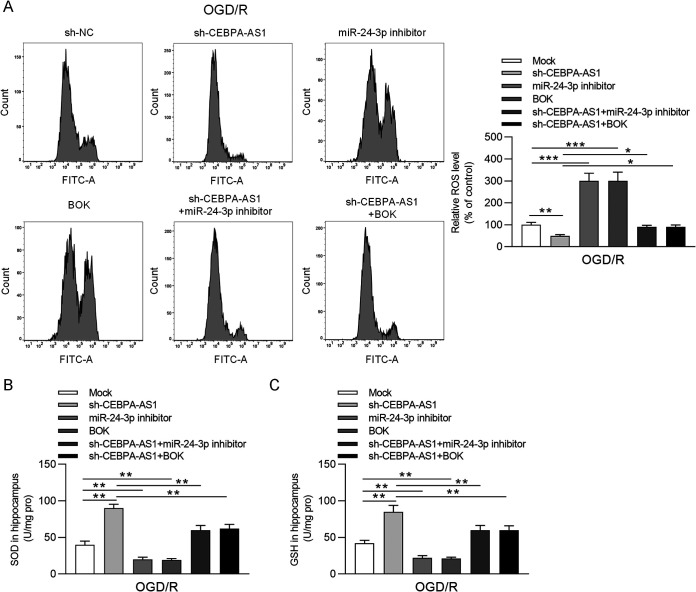
We explored whether the miR-24-3p/BOK axis is involved in the regulation of CEBPA-AS1 in OGD/R-induced injury. The OGD/R-treated SH-SY5Y cells were transfected with sh-NC, sh-CEBPA-AS1, miR-24-3p inhibitor, BOK, sh-CEBPA-AS1+miR-24-3p inhibitor, and sh-CEBPA-AS1+BOK. RT-qPCR and Western blotting showed that downregulation of miR-24-3p or overexpression of BOK restored the BOK expression reduced by knockdown of CEBPA-AS1 in OGD/R-treated SH-SY5Y cells (Fig. 6A). As shown in Fig. 6B to D, miR-24-3p silencing or BOK upregulation significantly inhibited cell viability and promoted apoptosis. CCK-8 assay suggested that increased cell viability by CEBPA-AS1 knockdown was inhibited by miR-24-3p silencing or BOK upregulation (Fig. 6B). Moreover, downregulation of miR-24-3p or upregulation of BOK restored the cell apoptosis inhibited by knockdown of CEBPA-AS1 in OGD/R-induced SH-SY5Y cells (Fig. 6C). The effects of sh-CEBPA-AS1 on Bax and Bcl-2 expression were also antagonized by miR-24-3p inhibitor or sh-BOK (Fig. 6D). In addition, reduced ROS production in OGD/R-induced SH-SY5Y cells transfected with sh-CEBPA-AS1 was restored after transfection of miR-24-3p inhibitor or BOK (Fig. 7A). ELISA further that verified that miR-24-3p silencing or BOK upregulation decreased the concentrations of SOD and GSH increased by CEBPA-AS1 knockdown (Fig. 7B and C). Overall, downregulation of CEBPA-AS1 exerts protective functions against OGD/R-induced injury in SH-SY5Y cells by targeting the miR-24-3p/BOK axis.
FIG 6.
CEBPA-AS1 depletion alleviates OGD/R-induced cell apoptosis by the miR-24-3p/BOK axis. (A) The BOK level in OGD/R-treated SH-SY5Y cells transfected with Mock, sh-CEBPA-AS1, miR-24-3p inhibitor, BOK, sh-CEBPA-AS1+miR-24-3p inhibitor, or sh-CEBPA-AS1+BOK was measured by RT-qPCR and Western blotting. (B) Cell viability was evaluated by CCK-8 assay. (C) Cell apoptosis was detected by flow cytometry. (D) The levels of Bax and Bcl-2 were measured by Western blotting. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 7.
CEBPA-AS1 depletion alleviates OGD/R-triggered oxidative stress by the miR-24-3p/BOK axis. (A) ROS production was analyzed by flow cytometry. (B and C) The concentrations of SOD and GSH in the supernatants of OGD/R-treated SH-SY5Y cells were measured using ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Cerebrovascular diseases represent a serious hazard to human health and life (30). Understanding the mechanisms in I/R injury in order to develop effective therapeutic targets is crucial for the improvement of ischemic stroke treatment. Studies suggest that lncRNAs could function as diagnostic biomarkers and therapeutic targets for the treatment of ischemic stroke (31). lncRNA Malat1 plays antiapoptotic and anti-inflammatory roles in brain microvasculature to reduce ischemic cerebral vascular and parenchymal damages (17). lncRNA SETD5-AS1 induces cell apoptosis and inhibits proliferation in mouse neuroblastoma N2a cells with OGD/R (18). These reports demonstrate the important functions of lncRNAs in ischemic stroke. CEBPA-AS1 was found upregulated in human ischemic stroke (19). However, the biological role of CEBPA-AS1 in ischemic stroke remains unclear. We learned that CEBPA-AS1 acts as a biomarker of poor prognosis in several cancers (32, 33). In the present study, CEBPA-AS1 had a high expression in OGD/R-treated SH-SY5Y cells. Silencing CEBPA-AS1 attenuated SH-SY5Y cell injury induced by OGD/R, suggesting that CEBPA-AS1 inhibition may have neuroprotective effects against OGD/R-induced injury.
Evidence reveals that OGD/R can stimulate I/R injury in vitro, leading to upregulation of proapoptotic factors and overproduction of ROS (34). Furthermore, OGD induces ROS generation that is related to oxidative stress, which results in neuronal damage (35). RMST knockdown restrains neuronal apoptosis by inhibiting oxidative stress in N2a cells exposed to OGD/R (36). AK005401 knockdown attenuates apoptosis and oxidative stress in OGD/R-treated neurons (37). Here, CEBPA-AS1 inhibition prevented OGD-stimulated oxidative stress in SH-SY5Y cells through reducing the production of ROS and increasing the concentrations of SOD and GSH. In addition, silencing CEBPA-AS1 elevated Bcl-2 expression and reduced Bax expression in SH-SY5Y cells exposed to OGD/R, suggesting that silencing CEBPA-AS1 prevented cell apoptosis. Overall, CEBPA-AS1 downregulation could attenuate OGD/R-induced apoptosis and oxidative stress in SH-SY5Y cells.
lncRNAs could function as ceRNAs that sponge miRNAs to regulate gene expression posttranscriptionally (20). Bioinformatics analysis revealed that CEBPA-AS1 has potential binding sites for some miRNAs, including miR-24-3p. We then verified the interaction of CEBPA-AS1 with miR-24-3p. Our findings showed that OGD/R reduced miR-24-3p expression. Mechanistically, the miR-24-3p decay induced by CEBPA-AS1 may be related to the occurrence of a target-directed miRNA degradation mechanism (TDMD) (38, 39). In the process of TDMD, specific RNAs could bind to target miRNAs and promote the degradation of miRNAs (40). miR-24-3p also downregulated the expression of CEBPA-AS1. Moreover, miR-24-3p depletion antagonized the effects of CEBPA-AS1 depletion in OGD/R-induced SH-SY5Y cells. BCL-2-related ovarian killer (BOK) was targeted by miR-24-3p. BOK is an evolutionarily conserved BCL-2 family member that resembles the apoptotic effectors BAK and BAX in sequence and structure (41). As previously reported, depletion of Bok leads to mitochondrial fragmentation by decreasing mitochondrial fusion rate (42), and mitochondria are major areas of ROS generation and their dysfunction could induce the mitochondrion-mediated apoptosis signaling (43). Moreover, BOK overexpression significantly facilitates cell apoptosis in OGD/R-treated N2a cells (44). A study showed the upregulation of BOK expression after OGD stress (45). Consistent with previous findings, BOK expression here was found upregulated by OGD/R in SH-SY5Y cells. Overexpression of miR-24-3p downregulated BOK expression. Results of rescue assays showed that upregulated BOK expression eliminated the protective effects of CEBPA-AS1 depletion in OGD/R-induced SH-SY5Y cells.
Lack of clinical samples and in vivo experimental data are two shortcomings of our present study. We decided to investigate the role of CEBPA-AS1 in clinical samples or animal study in our future study. However, the present study still provides an elucidation of the mechanism governing CEBPA-AS1 in OGD/R-induced SH-SY5Y cell injury. The CEBPA-AS1/miR-24-3p/BOK axis is just one of multiple molecular pathways. Finding novel molecular pathways may contribute to providing more theoretical basis for the treatment of disease.
MATERIALS AND METHODS
Cell culture and treatment.
SH-SY5Y cells were purchased from ATCC (Manassas, VA) and incubated in Dulbecco modified Eagle medium (DMEM; Gibco, New York, NY) at 37°C with 5% CO2. The medium was added with 10% fetal bovine serum (FBS; Gibco) and 100 U/ml antibiotics (Gibco). For OGD/R model establishment, SH-SY5Y cells were maintained for 4 h in a serum/glucose-free medium under a low-oxygen atmosphere with 5% CO2 and 95% N2. After 4 h, the cells were transferred to complete DMEM with 10% FBS and 5 g/liter glucose for reoxygenation for 24, 48, and 72 h at 37°C (5% CO2 and 95% air).
Cell transfection.
Short hairpin RNA targeting CEBPA-AS1 (sh-CEBPA-AS1), and sh-NC were constructed by Ribobio (Guangzhou, China). The miR-24-3p mimics (miR-24-3p), miR-24-3p inhibitor, NC mimics, and NC inhibitor were obtained from Ribobio. Full-length CEBPA-AS1 or BOK was inserted into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA). Before OGD/R treatment, SH-SY5Y cells were seeded in 6-well plates (3 × 104 cells/well). When they reached 80% confluence, the cells were transfected with the indicated plasmids using Lipofectamine 2000 reagent (Invitrogen). After 48 h, the cells were subjected to OGD/R.
RT-qPCR.
Total RNAs from SH-SY5Y cells were extracted using a TRIzol kit (Invitrogen). The cDNA was synthesized using an EntiLink first-strand cDNA synthesis kit (Aspen Biotechnology Co., Ltd., Wuhan, China). Next, cDNA was used for real-time PCR on a 7500 FAST real-time PCR system (Applied Biosystems, Foster City, CA) with a SYBR Premix Ex Taq kit (TaKaRa, Tokyo, Japan). Primers for CEBPA-AS1, miR-24-3p, and BOK were provided by Sangon (Shanghai, China). Relative expression was calculated by the 2–ΔΔCT method. GAPDH or U6 was as an internal control for mRNA or miRNA. The primers used were as follows: CEBPA-AS1, forward (5′-CCCTCGCATTCTTTACCG-3′) and reverse (5′-TTACTCCCTCCCGTCCAA-3′); GAPDH, forward (5′-TGAAGGTCGGTGTGAACGGATTTGG-3′) and reverse (5′-ACGACATACTCAGCACCAGCATCAC-3′); miR‐24‐3p, forward (5′-UGGCUCAGUUCAGCAGGAACAG-3′) and reverse (5′-GUUCCUGCUGAACUGAGCCAUUU6-3′); and U6, forward (5′-CTCGCTTCGGCAGCACAT-3′) and reverse (5′-AACGCTTCACGAATTTGCGT-3′).
Western blotting.
The proteins from SH-SY5Y cells were extracted using RIPA buffer (Beyotime, Nanjing, China). The concentration of proteins was measured by a BCA kit (Beyotime). Next, 30-μg protein samples were separated by 12% SDS-PAGE and then transferred to polyvinylidene difluoride membranes. After blocking with 5% defatted milk, the membranes were incubated overnight at 4°C with the following primary antibodies: Bax (ab81083; Abcam), Bcl-2 (ab238041), BOK (ab233072), and beta-actin (ab213162). Next, horseradish peroxidase-conjugated secondary antibody (Abcam) was incubated with the membranes for 2 h at room temperature. Protein blots were visualized with an enhanced chemiluminescent substrate kit (Solarbio, Beijing, China) and quantified by ImageJ software (NIH, Bethesda, MD).
CCK-8.
Cell viability was assessed using CCK-8 kit (Beyotime). SH-SY5Y cells were seeded in 96-well plates (4 × 104 cells/well), followed by OGD/R treatment. Subsequently, 10 μl of CCK-8 reagent was added to each well for a 2-h incubation at 37°C. Later, the optical density was examined using a microplate reader (Bio-Rad, Hercules, CA) at a wavelength of 450 nm.
Flow cytometry.
An annexin V-fluorescein isothiocyanate (FITC)–phosphatidylinositol (PI) detection kit (BD Biosciences, USA) was used for the assessment of SH-SY5Y cell apoptosis. In brief, 5 × 104 SH-SY5Y cells were washed with cold phosphate-buffered saline (PBS), followed by incubation with annexin V-FITC and PI (Thermo Fisher Scientific) at room temperature for 15 min in the darkness. The apoptosis was analyzed by a FACSCalibur flow cytometry (BD Biosciences).
ELISA.
The commercial ELISA kits were used to measure the concentrations of SOD and GSH (catalog no. A001-3 and A005-1; Jiancheng, Bioengineering Institute, Jiangsu China) from the supernatants of SH-SY5Y cells according to the manufacturer’s instructions.
ROS detection.
Cellular ROS was measured using 2′,7′-dichlorofluorescin diacetate (DCFH-DA; KeyGen, Ltd., Jiangsu, China). A 20 μM working substrate solution was used to stain SH-SY5Y cells in the dark for 30 min at 37°C. After washing, the production was analyzed using a FACSCalibur flow cytometer.
Luciferase assay.
Wild type or mutant BOK fragment at the binding site of miR-24-3p was inserted into the pmirGLO luciferase Vector (Promega, Madison, WI). SH-SY5Y cells were cotransfected with wild-type BOK or mutant BOK and then transfected with miR-24-3p or NC mimics using Lipofectamine 2000 (Invitrogen). Luciferase activity was determined by a luciferase reporter assay system (Promega) with renilla luminescence as an endogenous control.
RNA pulldown.
CEBPA-AS1 was biotin-labeled using T7/SP6 RNA polymerase (Life Technologies, Carlsbad, CA) and biotin RNA labeling mix (Sigma-Aldrich, St. Louis, MO). After 48 h, the SH-SY5Y cells were lysed, and Dynabeads M-280 Streptavidin (Life Technologies) was added to the lysates, followed by incubation for 30 min at room temperature. The RNAs attached to the beads were extracted. The expression of miRNAs was measured using RT-qPCR.
FISH.
CEBPA-AS1 probes were designed and synthesized by RiboBio (Guangzhou, China). The probe signals were detected using fluorescence in situ hybridization (FISH; RiboBio) according to the manufacturer’s instructions. In brief, SH-SY5Y cells were fixed with 4% formalin for 15 min. After prehybridization in PBS, the cells were hybridized in hybridization solution for 30 min at 37°C. The cell nuclei were then counterstained by DAPI staining. Images were captured using a fluorescence microscope (Leica, Wetzlar, Germany).
Subcellular fractionation assay.
The nuclear and cytoplasmic fractions of SH-SY5Y cells were separated and purified according to the cytoplasmic and nuclear RNA purification kit manual (Norgen BioTek, Thorold, Canada). The isolated RNA (CEBPA-AS1, GADPH, and U6) was analyzed by RT‐qPCR.
Statistical analysis.
Each assay was repeated at least three times. Statistical analysis was conducted using Prism 7.0 software (GraphPad, La Jolla, CA). All data are expressed as means ± the standard deviations and were analyzed using Student t tests or one-way analyses of variance. P < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (grant 81901343), Suzhou Science and Technology Project (SYS2020067), the Nature Science Research Project of Anhui province (1908085QH356), and the Funding of “Peak” Training Program for Scientific Research of Yijishan Hospital (GF2019G01).
We declare that there are no conflicts of interest regarding the publication of this paper.
Contributor Information
Hua Liu, Email: wacilii@163.com.
Min Xu, Email: xumin7788@126.com.
REFERENCES
- 1.Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. 2017. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 18:2135. 10.3390/ijms18102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. 2017. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135:e146–e603. 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. 2009. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8:355–369. 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 4.Du K, Zhao C, Wang L, Wang Y, Zhang KZ, Shen XY, Sun HX, Gao W, Lu X. 2019. MiR-191 inhibit angiogenesis after acute ischemic stroke targeting VEZF1. Aging (Albany, NY) 11:2762–2786. 10.18632/aging.101948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culman J, Nguyen-Ngoc M, Glatz T, Gohlke P, Herdegen T, Zhao Y. 2012. Treatment of rats with pioglitazone in the reperfusion phase of focal cerebral ischemia: a preclinical stroke trial. Exp Neurol 238:243–253. 10.1016/j.expneurol.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Riverol M, Becker JT, López OL, Raji CA, Thompson PM, Carmichael OT, Gach HM, Longstreth WT, Jr, Fried L, Tracy RP, Kuller LH. 2015. Relationship between systemic and cerebral vascular disease and brain structure integrity in normal elderly individuals. J Alzheimers Dis 44:319–328. 10.3233/JAD-141077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Chen Y, Liu P, Chen J, Song L, Tang Y, Wang Y, Liu J, Hu FB, Hui R. 2012. Variants on chromosome 9p21.3 correlated with ANRIL expression contribute to stroke risk and recurrence in a large prospective stroke population. Stroke 43:14–21. 10.1161/STROKEAHA.111.625442. [DOI] [PubMed] [Google Scholar]
- 8.Yan X, Yu A, Zheng H, Wang S, He Y, Wang L. 2019. Calycosin-7-O-β-d-glucoside attenuates OGD/R-induced damage by preventing oxidative stress and neuronal apoptosis via the SIRT1/FOXO1/PGC-1α pathway in HT22 cells. Neural Plast 2019:8798069. 10.1155/2019/8798069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong L, Tang Y, An R, Lin M, Chen L, Du J. 2017. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis 8:e3080. 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. 2016. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133:447–454. 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 11.Geisler S, Coller J. 2013. RNA in unexpected places: long noncoding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14:699–712. 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. 2013. Gene regulation by the act of long noncoding RNA transcription. BMC Biol 11:59. 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang KC, Chang HY. 2011. Molecular mechanisms of long noncoding RNAs. Mol Cell 43:904–914. 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer TR, Dinger ME, Mattick JS. 2009. Long noncoding RNAs: insights into functions. Nat Rev Genet 10:155–159. 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 15.Wan P, Su W, Zhuo Y. 2017. The role of long noncoding RNAs in neurodegenerative diseases. Mol Neurobiol 54:2012–2021. 10.1007/s12035-016-9793-6. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, Chen D, Wang L, Gao F, Jin B, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Miao Z. 2019. Silencing of long noncoding RNA Nespas aggravates microglial cell death and neuroinflammation in ischemic stroke. Stroke 50:1850–1858. 10.1161/STROKEAHA.118.023376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. 2017. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci 37:1797–1806. 10.1523/JNEUROSCI.3389-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao SY, Miao SM, Cui RT, Yu AL, Miao ZJ. 2018. SETD5-AS1 stimulates neuron death in stroke via promoting PTEN expression. Eur Rev Med Pharmacol Sci 22:6035–6041. 10.26355/eurrev_201809_15940. [DOI] [PubMed] [Google Scholar]
- 19.He W, Wei D, Cai D, Chen S, Li S, Chen W. 2018. Altered long non-coding RNA transcriptomic profiles in ischemic stroke. Hum Gene Ther 29:719–732. 10.1089/hum.2017.064. [DOI] [PubMed] [Google Scholar]
- 20.Ma H, Hao Y, Dong X, Gong Q, Chen J, Zhang J, Tian W. 2012. Molecular mechanisms and function prediction of long noncoding RNA. Sci World J 2012:541786. 10.1100/2012/541786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JY, Chen H, Li T, Yang L, Ye XM, Gao WY, Zhang SP, Zong L. 2020. LncRNA LncOGD-1006 alleviates OGD-induced ischemic brain injury regulating apoptosis through miR-184-5p/CAAP1 axis. Eur Rev Med Pharmacol Sci 24:12324–12333. 10.26355/eurrev_202012_24025. [DOI] [PubMed] [Google Scholar]
- 22.Pan H, Zhao F, Yang Y, Chang N. 2020. Overexpression of long noncoding RNA SNHG16 against cerebral ischemia-reperfusion injury through miR-106b-5p/LIMK1 axis. Life Sci 254:117778. 10.1016/j.lfs.2020.117778. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien J, Hayder H, Zayed Y, Peng C. 2018. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 9:402. 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge XL, Wang JL, Liu X, Zhang J, Liu C, Guo L. 2019. Inhibition of miR-19a protects neurons against ischemic stroke through modulating glucose metabolism and neuronal apoptosis. Cell Mol Biol Lett 24:37. 10.1186/s11658-019-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Ma Q, Li Y, Li B, Zhang L. 2018. Inhibition of microRNA-210 suppresses proinflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp Neurol 300:41–50. 10.1016/j.expneurol.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Huang Z, Meng J, Yu W, Yang H. 2020. MiR-202-5p attenuates neurological deficits and neuronal injury in MCAO model rats and OGD-induced injury in Neuro-2a cells by targeting eIF4E-mediated induction of autophagy and inhibition of Akt/GSK-3β pathway. Mol Cell Probes 51:101497. 10.1016/j.mcp.2019.101497. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Chen L, Chen B, Huang S, Zeng C, Wu H, Chen C, Long F. 2018. Increased serum exosomal miR-134 expression in the acute ischemic stroke patients. BMC Neurol 18:198. 10.1186/s12883-018-1196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Toscano JF, Song SS, Schlick KH, Dumitrascu OM, Pan J, Lyden PD, Saver JL, Gonzalez NR. 2019. Differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antiangiogenesis. Sci Rep 9:19429. 10.1038/s41598-019-54542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen Y, Yu Y, Fu X. 2017. LncRNA Gm4419 contributes to OGD/R injury of cerebral microglial cells via IκB phosphorylation and NF-κB activation. Biochem Biophys Res Commun 487:923–929. 10.1016/j.bbrc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Bai M, Liu B, Peng M, Jia J, Fang X, Miao M. 2019. Effect of Sargentodoxa cuneata total phenolic acids on focal cerebral ischemia reperfusion injury rats model. Saudi J Biol Sci 26:569–576. 10.1016/j.sjbs.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Hamblin MH, Yin KJ. 2019. Noncoding RNAs and stroke. Neuroscientist 25:22–26. 10.1177/1073858418769556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia P, Gu R, Zhang W, Sun YF. 2020. lncRNA CEBPA-AS1 overexpression inhibits proliferation and migration and stimulates apoptosis of OS cells via Notch signaling. Mol Ther Nucleic Acids 19:1470–1481. 10.1016/j.omtn.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Guo Y, Ma Y, Hu X, Song R, Zhu L, Zhong M. 2018. Long noncoding RNA CEBPA-AS1 correlates with poor prognosis and promotes tumorigenesis via CEBPA/Bcl2 in oral squamous cell carcinoma. Cancer Biol Ther 19:205–213. 10.1080/15384047.2017.1416276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, Hu F, Wu J, Zhang S. 2017. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol 11:577–585. 10.1016/j.redox.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen N, Zhou Z, Li J, Li B, Feng J, He D, Luo Y, Zheng X, Luo J, Zhang J. 2018. 3-n-Butylphthalide exerts neuroprotective effects by enhancing anti-oxidation and attenuating mitochondrial dysfunction in an in vitro model of ischemic stroke. Drug Des Devel Ther 12:4261–4271. 10.2147/DDDT.S189472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Zhang M, Yan F, Cong Y. 2021. Knockdown of RMST impedes neuronal apoptosis and oxidative stress in OGD/R-induced ischemic stroke via depending on the miR-377/SEMA3A signal network. Neurochem Res 46:584–594. 10.1007/s11064-020-03194-w. [DOI] [PubMed] [Google Scholar]
- 37.Wan H, Yang Y, Li M, Liu X, Sun Y, Wang K, Zhang C, Zheng Q, Wang C. 2019. Activation of AK005401 aggravates acute ischemia/reperfusion mediated hippocampal injury by directly targeting YY1/FGF21. Aging (Albany, NY) 11:5108–5123. 10.18632/aging.102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de la Mata M, Gaidatzis D, Vitanescu M, Stadler MB, Wentzel C, Scheiffele P, Filipowicz W, Grosshans H. 2015. Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep 16:500–511. 10.15252/embr.201540078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghini F, Rubolino C, Climent M, Simeone I, Marzi MJ, Nicassio F. 2018. Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nat Commun 9:3119. 10.1038/s41467-018-05182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheu-Gruttadauria J, Pawlica P, Klum SM, Wang S, Yario TA, Schirle Oakdale NT, Steitz JA, MacRae IJ. 2019. Structural basis for target-directed microRNA degradation. Mol Cell 75:1243–1255. 10.1016/j.molcel.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang SH, Perales O, Michaud M, Katz SG. 2019. BOK promotes erythropoiesis in a mouse model of myelodysplastic syndrome. Ann Hematol 98:2089–2096. 10.1007/s00277-019-03726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulman JJ, Szczesniak LM, Bunker EN, Nelson HA, Roe MW, Wagner LE, 2nd, Yule DI, Wojcikiewicz RJH. 2019. Bok regulates mitochondrial fusion and morphology. Cell Death Differ 26:2682–2694. 10.1038/s41418-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kantari C, Walczak H. 2011. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 1813:558–563. 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Hu X, Xiang Z, Zhang W, Yu Z, Xin X, Zhang R, Deng Y, Yuan Q. 2020. Protective effect of DLX6-AS1 silencing against cerebral ischemia/reperfusion induced impairments. Aging (Albany, NY) 12:23096–23113. 10.18632/aging.104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajima Y, Shimazawa M, Mishima S, Hara H. 2009. Neuroprotective effects of Brazilian green propolis and its main constituents against oxygen-glucose deprivation stress, with a gene-expression analysis. Phytother Res 23:1431–1438. 10.1002/ptr.2797. [DOI] [PubMed] [Google Scholar]