Abstract
Objective:
New transapical minimally invasive artificial chordae implantation devices are a promising alternative to traditional open-heart repair, with the potential for decreased postoperative morbidity and reduced recovery time. However, these devices can place increased stress on the artificial chordae. We designed an artificial papillary muscle to alleviate artificial chordae stresses and thus increase repair durability.
Methods:
The artificial papillary muscle device is a narrow elastic column with an inner core that can be implanted during the minimally invasive transapical procedure via the same ventricular incision site. The device was 3-dimensionally printed in biocompatible silicone for this study. To test efficacy, porcine mitral valves (n = 6) were mounted in a heart simulator, and isolated regurgitation was induced. Each valve was repaired with a polytetrafluoroethylene suture with apical anchoring followed by artificial papillary muscle anchoring. In each case, a high-resolution Fiber Bragg Grating sensor recorded forces on the suture.
Results:
Hemodynamic data confirmed that both repairs—with and without the artificial papillary muscle device—were successful in eliminating mitral regurgitation. Both the peak artificial chordae force and the rate of change of force at the onset of systole were significantly lower with the device compared with apical anchoring without the device (P < .001 and P < .001, respectively).
Conclusions:
Our novel artificial papillary muscle could integrate with minimally invasive repairs to shorten the artificial chordae and behave as an elastic damper, thus reducing sharp increases in force. With our device, we have the potential to improve the durability of off-pump transapical mitral valve repair procedures.
Keywords: minimally invasive surgery, device development, mitral valve repair, 3D-printing, biomechanics
Graphical Abstract
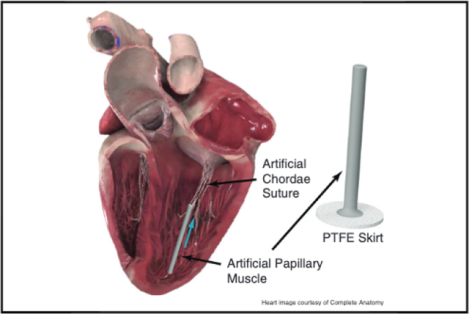
Design of an artificial papillary muscle device for off-pump mitral valve repair.
CENTRAL MESSAGE
A novel artificial papillary muscle device was developed to mitigate high artificial chordae stresses in off-pump transapical mitral valve repair.
Mitral valve procedures are increasingly prioritizing repair over replacement, as repair has been shown to be associated with superior patient survival, left ventricular function, and freedom from reoperation.1–7 Thus, although minimally invasive technology for valve replacement has previously been a focal point for a number of medical device companies, many are now prioritizing the development of minimally invasive technologies to perform these repair operations. In particular, multiple companies are developing new off-pump mitral valve repair devices that take a minimally invasive approach to implant artificial chordae to repair a prolapsing, regurgitant valve. The NeoChord (NeoChord, St Louis Park, Minn) and HARPOON (Edwards Lifesciences Corporation, Irvine, Calif) devices both have been CE marked, and preliminary human trials have been completed. NeoChord has undergone a 1-year European study,8 and a United States randomized trial is currently underway9; the HARPOON device has been preliminarily evaluated in a 30-patient prospective, nonrandomized multicenter study.10 Both devices provide a promising alternative to traditional open-heart repair with the potential to decrease postoperative morbidity and recovery time, presenting an attractive option for high-risk, elderly patients.11
Traditional open-heart surgery to repair chordal insufficiency includes the implantation of an expanded polytetrafluoroethylene (ePTFE) suture to act as an artificial chordae tendineae, spanning from the leaflet to the papillary muscle tip. To facilitate an off-pump minimally invasive incision with direct access to the mitral leaflets, these new devices employ a transapical/transventricular approach to implant the artificial chordae suture. This anchoring method results in a longer chord that extends from the leaflet to a point at or near the ventricular apex, parallel to the plane of coaptation. We previously used an ex vivo heart simulator to examine the effect of this increased suture length coupled with an altered anchoring position and found an elevated rate of loading as well as greater peak forces, which could predispose apical repair to early failure.12 This conclusion was in line with an in vivo porcine study that found elevated tension fluctuations in apical artificial chordae.13 In addition, there have been early clinical instances of leaflet rupture at the implanted artificial chordae attachment point as well as ePTFE rupture or detachment at the epicardium,8,10,14–16 all of which would point to high suture force relative to tissue strength. While adjustments are being implemented to mitigate the risk of failure, including implanting additional chordae to distribute the load, shifting the apical access site further off-apex, and narrowing patient selection criteria,16–19 innovations that lower artificial chordae suture stresses are one of the essential factors to expand the impact of these minimally invasive repair devices and unlock their full potential to provide durable repairs across a wide patient population. We hypothesized that an elastic artificial chordae anchor that shortened the suture length and dampened sharp increases in force would reduce artificial chordae stresses and mitigate leaflet rupture at the suture-valve interface. We designed and tested an artificial papillary muscle that functions as this elastic anchor and would seamlessly integrate with the current minimally invasive repair devices.
METHODS
Artificial Papillary Muscle Device Design
We designed an artificial papillary muscle device to alleviate stresses in transapical off-pump mitral valve repair (Figure 1, A). The artificial papillary muscle is a narrow elastic column implanted to act as the suture anchor during a minimally invasive transapical artificial chordae implantation. First, implantation of artificial chordae in the leaflet would be performed with a minimally invasive mitral valve repair device transapically. This implantation procedure has been described in detail for relevant devices currently in development.20,21 When all necessary sets of artificial chordae have been implanted and retrieved outside the ventricle, an eye needle is used to thread each suture end individually through the head of the artificial papillary muscle and down through its inner core; a metal insert can be used to facilitate this threading process by providing a rigid channel that prevents the needle from catching on the inner walls (Figure 1, B). Note that multiple sets of artificial chordae can be threaded through the device for anchoring. In addition, a polytetrafluoroethylene (PTFE) felt skirt is included at the base of the device for suturing apical purse-strings for hemostasis. For an off-pump mitral valve repair device that is inserted via an introducer near the ventricular apex,22 this introducer would be removed (and the apical purse strings tightened) before threading the suture ends through the artificial papillary muscle. The artificial papillary muscle is then inserted into the left ventricle through the same incision site and surrounding apical purse strings are tightened around it for hemostasis. Video 1 shows an animation of the implementation of this device to alleviate stresses in transapical mitral valve repair.
FIGURE 1.
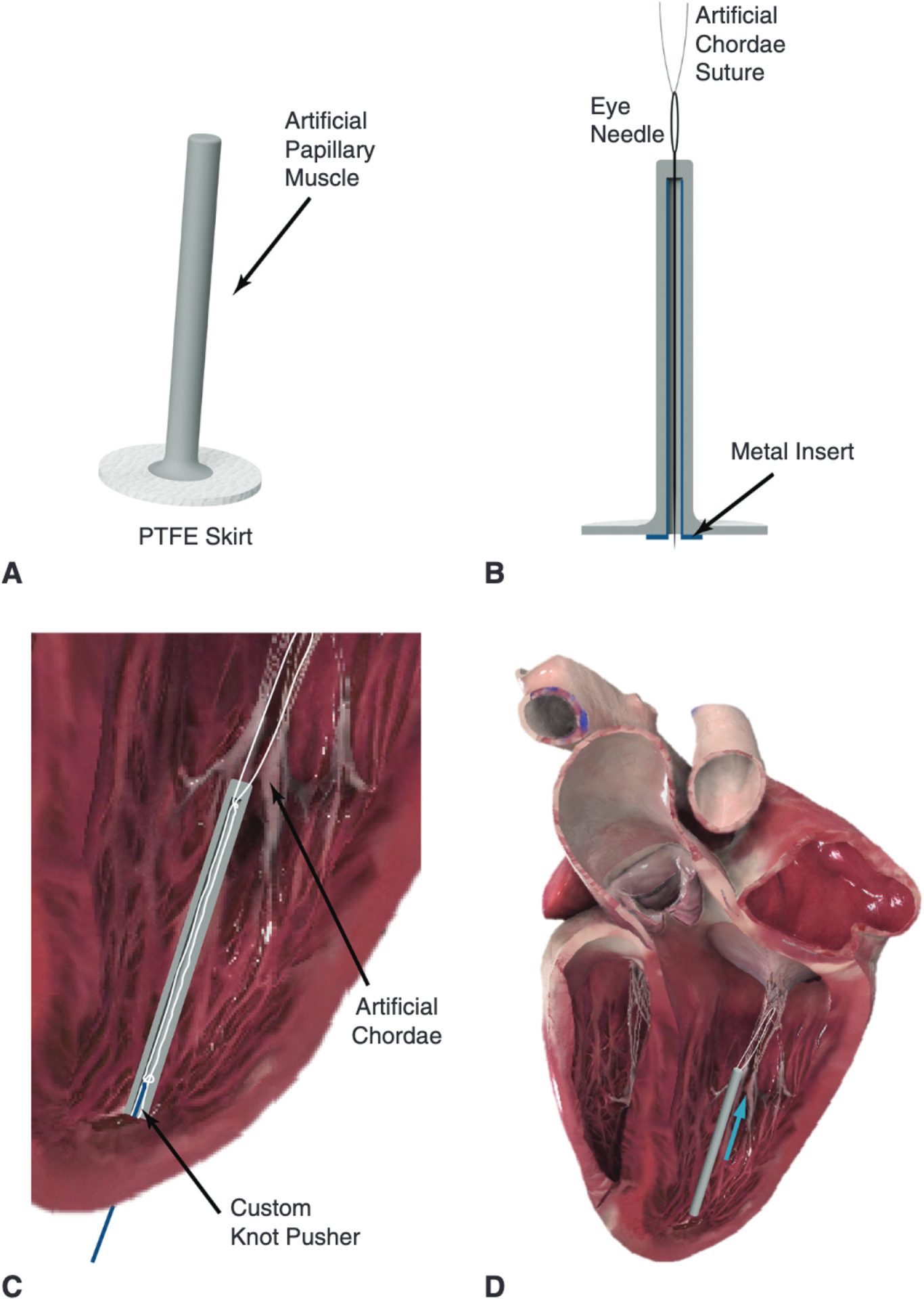
A, Labeled rendering of the artificial papillary muscle device to be used in conjunction with a minimally invasive transapical artificial chordae implantation device. Multiple pairs of chords can be anchored to this artificial papillary muscle. B, An eye needle is used to thread each artificial chordae suture end through the head of the artificial papillary muscle and down through its inner core; a metal insert can be used to facilitate this threading process. C, A cross-section of the artificial papillary muscle showing the suture length adjustment. The length for each pair of sutures would be determined under echo guidance and could be set with a low-profile knot pusher or any crimping device. D, The final implanted artificial papillary muscle serves to shorten the artificial chordae and attenuate high rate of change of force; blue arrow represents force from the suture. PTFE, Polytetrafluoroethylene. Heart images courtesy of Complete Anatomy.
VIDEO 1.
An animation illustrating the proposed use of an artificial papillary muscle device to serve as an artificial chordae anchor in minimally invasive transapical off-pump mitral valve repair, integrating with the new repair devices currently under trials. For clarity, only one set of artificial chordae sutures is pictured in the animation, though multiple sets can be implanted through a single artificial papillary muscle device. In ex vivo tests, the artificial papillary muscle dampened peak forces and rates of change of force, thus serving as a potential means for a more durable repair. Video available at: https://www.jtcvs.org/article/S0022-5223(20)33191-3/fulltext.
Finally, as shown in Figure 1, C, the inner core ensures that artificial chordae sutures are anchored at the tip of the device (closest to the valve). A square knot is tied on each set of sutures and pushed through the inner core to the head of the artificial papillary muscle using a low-profile knot pusher. Echo guidance is used to determine the proper suture length for each artificial chordae set and additional knots are then added to each suture to lock the artificial chordae length. Thus, the knots anchoring the sets of artificial chordae lie at the tip of the device rather than the base; this results in a shorter chord secured to an elastic anchor. Additional methods of anchoring at the device tip are possible, including low-profile clip or crimping devices. The elastic material of the device seals around the suture, but to further protect against leakage, an additional PTFE felt piece is secured over the opening of the device core.
The overall elasticity of the artificial papillary muscle device was calculated to correspond to native papillary muscle tissue. The longitudinal stress on a papillary muscle, given a cross-sectional area of π(5 mm)2 23 and a 1-N force, is 12.7 kPa. Note that the 1-N reference force was chosen to be on the order of the forces experienced by artificial chordae anchored at the apex of the heart.12,13 Using previously published biomechanics data on papillary muscle tissue, this stress corresponds to approximately 20% strain.24 We selected a 3-dimensional (3D)-printed biocompatible and tear-resistant silicone (SIL 30; Carbon, Redwood City, Calif); a 20% strain with this silicone corresponds to approximately 100 kPa,25 which in turn requires a cross-sectional area of approximately 10 mm2 at the same 1-N force. Thus, the outer diameter of the artificial papillary muscle was designed to be 4 mm and the inner diameter was designed to be 1.8 mm with an overall length of 40 mm. As a comparison, previous mechanical testing of Gore-Tex CV-5 ePTFE suture, commonly used for artificial chordae, showed a modulus of elasticity of approximately 4000 MPa,26 which is nearly 6000 times the modulus of elasticity of SIL 30.25
Left Heart Simulator Testing
Porcine hearts were obtained fresh from an abattoir (Animal Technologies, Tyler, Tex) in accordance with institutional guidelines, and their mitral valves (n = 6) were carefully explanted with papillary muscles intact. The valves were then mounted in a left heart simulator using a 3D-printed silicone sewing ring (SIL 30) as previously described.12,27 The left heart simulator (Figure 2, A) features a pulsatile linear piston pump (ViVitro Superpump, ViVitro Labs, Victoria, British Columbia, Canada) used to generate physiologic hemodynamics for the valve. The pump controller and software (ViVitest Software, ViVitro Labs) are programmed in accordance with ISO 5840 standards for in vitro valve testing.28 The heart simulator features an electromagnetic flow probe (Carolina Medical Electronics, East Bend, NC) positioned to record mitral valve flow; 0.9% normal saline was used as the test fluid to ensure proper transduction of the flow probe. Ventricular, aortic, and left atrial pressures were measured using pressure transducers (Utah Medical Products, Inc, Midvale, Utah). The linear piston pump was programmed to generate an effective stroke volume of 70 mL/beat at 70 bpm. The heart simulator also features 2 compliance chambers as well as adjustable peripheral resistance; both were titrated at baseline to produce a mean arterial pressure of 100 mm Hg (systolic 120 mm Hg, diastolic 80 mm Hg). For each test, hemodynamic data were collected and averaged across 10 cardiac cycles.
FIGURE 2.
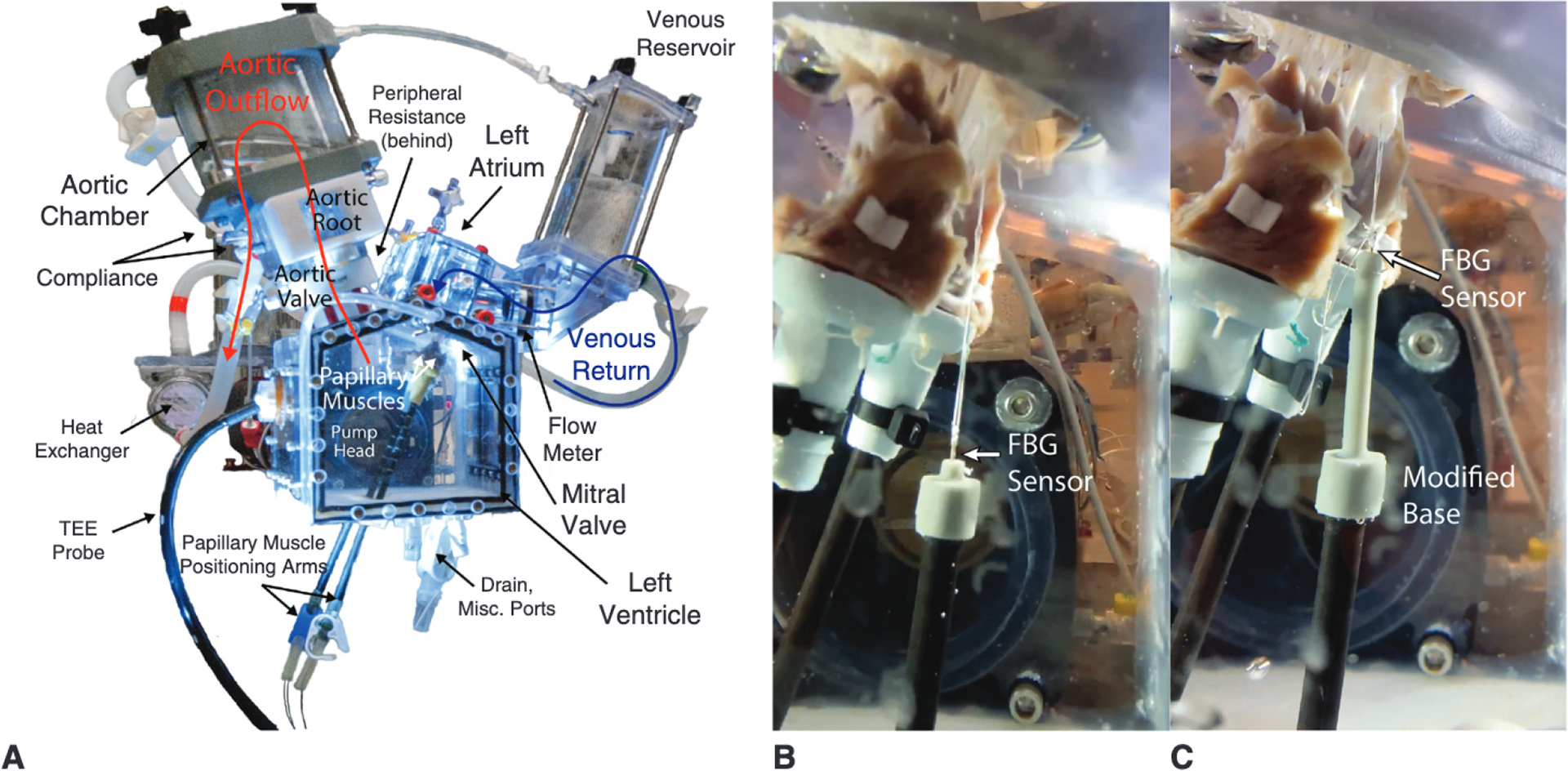
A, Labeled image of the heart simulator featuring compliance chambers and adjustable resistive elements, and including a left ventricular chamber affixed to a linear piston pump programmed to reproduce physiologic pressures and flows. B, Heart simulator testing of a mitral valve repaired with a Gore-Tex CV-5 suture anchored to a custom force-sensing post positioned to mimic apical placement. C, Without adjusting the post position, the same valve was tested with the artificial papillary muscle device serving as the suture anchor. A low-profile and high-resolution FBG sensor was used in each case to measure real-time artificial chordae forces. TEE, Transesophageal echocardiography; FBG, fiber bragg grating.
To test device efficacy, the valves were mounted in the heart simulator, and P2 primary chordae were cut to induce isolated mitral valve regurgitation as previously described.12,29 Each valve was repaired with an ePTFE CV-5 suture anchored to a custom force-sensing post positioned to mimic apical placement (Figure 2, B). Without adjusting the post position, the device was then affixed to the post and the artificial chordae was adjusted by the exact length of the artificial papillary muscle extension length (Figure 2, C). Note that the force on the artificial chord suture is entirely transmitted from the leaflet attachment to the force-sensing post; thus, by measuring the force on the suture we are simultaneously examining the force at the leaflet attachment point. The artificial papillary muscle device was modified at its base to mount on a carbon fiber post with 3 degrees of freedom; the modifications did not affect the geometry of the operative portion of the device. The device was 3D-printed with the modified base using the elastic biocompatible Carbon SIL30 silicone. In addition, a modified high-resolution Fiber Bragg Grating (FBG) sensor (DTG-LBL-1550, 125 μm; FBGS International, Geel, Belgium) was implanted at the head closest to the valve to directly measure force on the artificial chordae suture.30 For consistency, the force-sensing post for apical anchoring was designed in a comparable manner with a 3D-printed silicone (SIL30) mount affixing the FBG sensor to the carbon fiber rod. The FBG optical strain sensors were calibrated to correlate strain to load using an Instron 5848 Microtester (Norwood, Mass) with a 20-N load cell as previously described.31 FBG measurements were recorded at a sampling frequency of 1000 Hz with an optical interrogator (Micron Optics si255 with ENLIGHT; Micron Optics, Atlanta, Ga), and peak forces on the artificial chordae suture as well as the rate of change of force with respect to time were analyzed in MAT-LAB (MathWorks, Natick, Mass).
Statistical Analysis
Continuous variables were tested for normality and found to be non-normally distributed; these variables are thus reported as median (interquartile range). The nonparametric Friedman test was used for significance tests as it accounts for the non-normally distributed hemodynamic and force data as well as the multistaged experimental design. The test reports one significance value to identify significance across multiple treatment groups, and then pairwise comparisons were used to compare continuous variables between groups.
RESULTS
Hemodynamic data confirmed that cutting P2 primary chordae successfully induced mitral regurgitation. The mean arterial pressure was reduced from 99.82 (99.66–99.83) mL to 70.61 (69.76–73.99) mL (P < .001) from the baseline to the prolapse states (Figure 3, A). As shown in Figure 3, C, the mitral valve leakage volume was significantly greater for prolapse compared with baseline: 19.89 (16.66 to 21.80) mL versus 0.71 (0.59 to 1.08) mL (P < .001). Pressure and flow tracings over a cardiac cycle for the repair stages of the experiment (apical repair and artificial papillary muscle repair) are shown in Figure 3, B and D, respectively, with the shaded regions representing standard deviation. Both repairs—with and without the artificial papillary muscle device—were successful in eliminating the regurgitation. Mitral valve leakage volume was reduced from 19.89 (16.66–21.80) mL to 2.38 (1.46-mL (P = .001) with the device, and to 1.40 (0.91-mL (P = .003) without the device. Pressures were also restored for both repairs, with no significant difference found in the mean arterial pressure between the repairs and baseline levels. No significant difference in leakage volume or arterial pressure was found between the 2 repairs.
FIGURE 3.
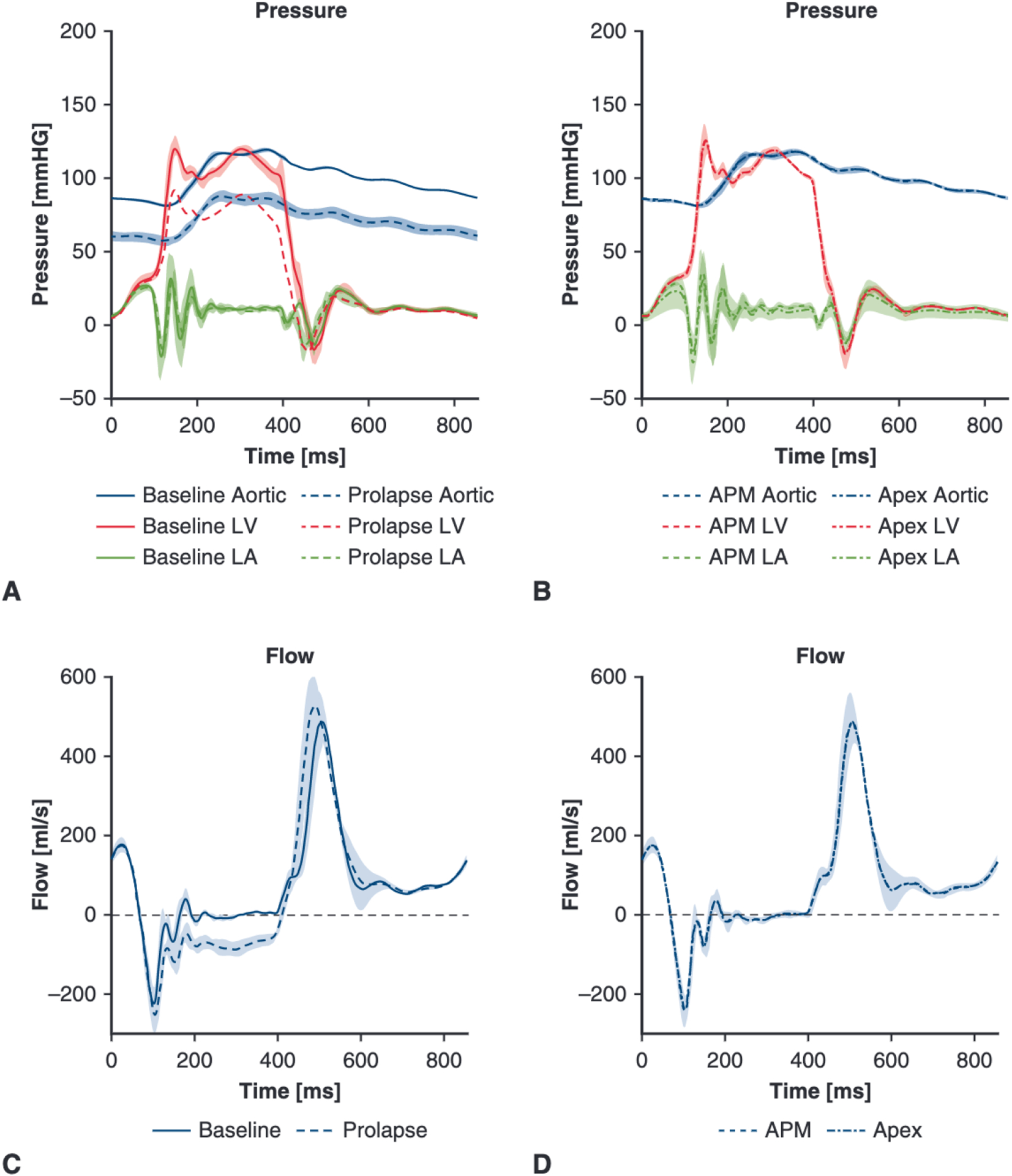
A, Mean pressure tracings across the 6 valves for baseline and prolapse states. The mean arterial pressure was significantly lower in the prolapse state compared to baseline: 70.61 (69.76–73.99) mL versus 99.82 (99.66–99.83) mL (P < .001). B, Mean pressure tracings for apical repair and APM repair. Pressures were restored for both repairs with no significant difference found in the mean arterial pressure between the repairs and baseline levels; additionally, there was no significant difference between repairs. C, Mean flow tracings confirmed successful generation of regurgitation with a mitral valve leakage volume of 19.89 (16.66–21.80) mL in the prolapse state versus 0.71 (0.59–1.08) mL at baseline (P < .001). D, Mean flow tracings for the repairs show that leakage volume was reduced from 19.89 (16.66–21.80) mL to 2.38 (1.46–3.34) mL (P = .001) in the APM repair, and to 1.40 (0.91–3.34) mL (P = .003) in the apical repair. No significant difference in leakage volume was found between repairs. Shaded regions represent standard deviation. LV, Left ventricle; LA, left atrial; APM, artificial papillary muscle.
Figure 4, A shows artificial chordae forces over the course of a cardiac cycle. Note that the exact timing of the force tracing peaks will necessarily differ slightly between valves due to variation in the progression of coaptation and transfer of force to the repair suture, so the composite graph across the 6 valves will consequently marginally smooth the tracings. Figure 4, B and C, shows box plots with the median and interquartile range for the peak suture force and the maximum rate of change of force on the suture, respectively. Peak suture forces (Figure 4, B) with artificial papillary muscle device fixation were significantly lower than apical fixation without the device: 0.30 (0.23–0.36) N versus 0.51 (0.43–0.53) N, P < .001. The apical anchoring suture forces without the device were similar to those previously observed in ex vivo analysis of apical anchoring and correlated to forces slightly greater than primary chordae forces.12 In addition, the maximum rate of change of force (Figure 4, C) was significantly lower with the artificial papillary muscle device than without: 2.89 (2.80 to 4.32) N/s versus 7.92 (7.49 to 9.23) N/s (P < .001).
FIGURE 4.
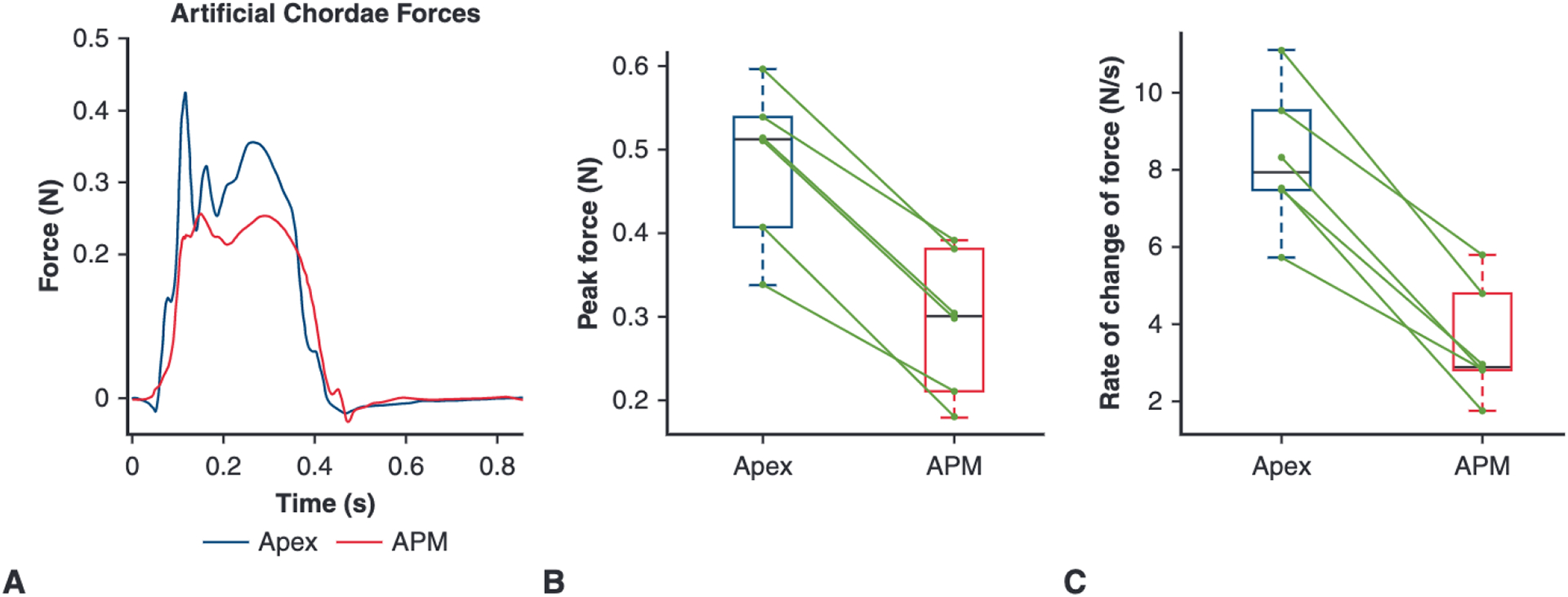
A, Composite force tracings for the artificial chordae over one cardiac cycle for both repairs: apical fixation and APM fixation. B, Box plot showing the peak artificial chordae force for the apical versus APM repair; green lines connect data points from the same valve illustrating the paired relationship. The peak force was significantly greater for apical anchoring compared to the APM anchoring: 0.51 (0.43–0.53) N versus 0.30 (0.23–0.36) N, P < .001. C, Box plot of the rate of change of artificial chordae force (dF/dt) for the apical versus APM repair. The rate of change of force was also significantly greater for apical anchoring compared with the APM anchoring: 7.92 (7.49 to 9.23) N/s versus 2.89 (2.80 to 4.32) N/s, P < .001. APM, Artificial papillary muscle.
DISCUSSION
We developed and analyzed a novel artificial papillary muscle prototype that would integrate with transapical off-pump mitral valve repair devices to serve as the anchor for implanted artificial chordae. Figure 5 depicts a summary of this study and its implications. We used an ex vivo left heart simulator to examine the forces experienced by the artificial chordae suture when positioned with apical fixation compared with the forces experienced with the addition of our artificial papillary muscle device. Both repairs—with and without the device—were successful in eliminating regurgitation; the artificial papillary muscle device lowered peak forces as well as the rate of loading on the suture compared to apical fixation. By shortening the effective length of the artificial chordae and incorporating an elastic anchor, the artificial papillary muscle device behaves as a damper to reduce the sharp increases in force at the onset of systole. Mitigating these force fluctuations and decreasing high force peaks is key to keeping stresses low, which in turn can limit instances where high stress on the artificial chordae ultimately causes failure, either via rupture of the suture itself or detachment at the suture anchoring points.
FIGURE 5.
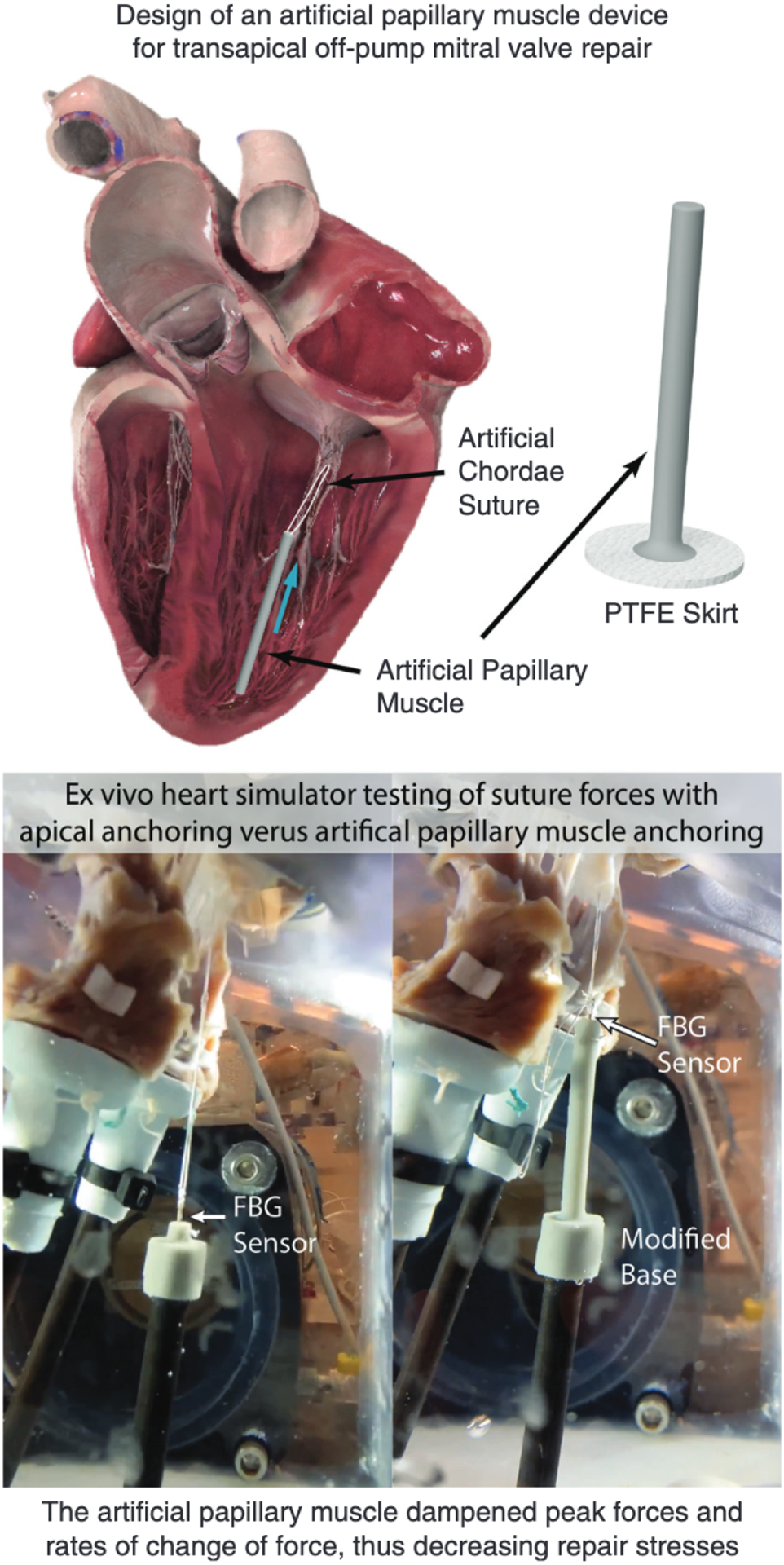
The development and biomechanical analysis of an artificial papillary muscle device prototype to serve as an anchor for artificial chordae in minimally invasive transapical off-pump mitral valve repair. Six porcine valves were tested in an ex vivo heart simulator with both apical anchoring and artificial papillary muscle anchoring configurations; a FBG sensor was used in each case to measure real-time suture forces. The artificial papillary muscle dampened peak forces and rates of change of force, thus serving as a potential means for a more durable repair. PTFE, Polytetrafluoroethylene; FBG, fiber bragg grating.
We previously hypothesized that the elevated rate of change of force during apical fixation compared with traditional papillary muscle fixation is due to a whipping phenomenon resulting from the increased suture length.12 With the artificial papillary muscle device, the resulting shorter chord in addition to the elasticity of the device could mitigate these sharp force changes that cause elevated stresses and thus increase repair durability. Early clinical results for the NeoChord device showed greater mitral repair failure rates than open-heart repair.11,32 However, given the evolving nature of novel medical device technology in early deployment stages, the device procedure underwent subsequent amendments as more information about the repairs were obtained. In particular, steps were taken to reduce failures due to operator error as well as improve patient selection criteria.11 Additionally, adjustments were made specifically to lower stresses on the valve, including increasing the number of artificial chordae as well as posterolateral shifting of the anchoring position.16–18 This posterolateral shift is in line with our previous biomechanical analysis of the forces on artificial chordae anchored to the posterior ventricular wall: artificial chordae in posterior anchoring positions can serve primarily to position the leaflet for coaptation rather than support a significant load.29 Nonetheless, long-term outcomes of these adjustments and their effect on the artificial chordae stresses remains unknown.
Precise length selection of artificial chordae is critical to the success of mitral valve repair, and it continues to represent a challenge in both open-heart and minimally invasive procedures given that sutures with nonoptimal lengths can result in stress concentrations.15,17 In addition, ventricular remodeling postrepair can cause a suture to become improperly tensioned over time, resulting in the recurrence of mitral regurgitation.33 One method of mitigating repair sensitivity to small changes in chordae length is to implant artificial chordae on the posterior ventricular wall in an open-heart repair, thus positioning the suture perpendicular to the plane of coaptation.34 However, with transapical implantation, the chord is parallel to the plane of coaptation and small changes in chordal length have significant effects on the suture forces, consequently impacting the repair durability. This sensitivity was directly observed in our preliminary experiments, thus requiring the exact matching of suture length that was implemented in our final experimental design. Interestingly, we observed in initial testing that the elasticity of the device appeared to facilitate less sensitivity of the artificial chordae force due to small length adjustments, though a robust study confirming this hypothesis would be necessary. Enabling minimally invasive repair to be more robust to nonoptimal suture length and ventricular remodeling would also be a significant advantage to improve repair success and durability. Additionally, an extension of this study that included an examination of native chordal forces would further elucidate the effect of the artificial papillary muscle device on the biomechanics of minimally invasive mitral valve repair.
In addition to large animal experiments to validate this study and facilitate the translation of this device to the clinic, further ex vivo experiments would also be recommended. In particular, additional heart simulator experiments could isolate and analyze the effects of the shortened suture versus the elastic anchor, as well as optimize the length and elasticity of the anchor. Although this study focused on a single artificial chord for a direct comparison with fewer confounding factors, future studies will explore anchoring multiple sets of artificial chordae to a single artificial papillary muscle device. Alternative designs, including a dual-headed artificial papillary muscle, could also be explored to further biomechanically optimize this repair procedure for multiple chordae. Given that minimally invasive repair devices use a spectrum of implantation sites off-apex, including posterolaterally16 and anterolaterally,10 the artificial papillary muscle device geometry could be similarly modified to both shorten and curve the device such that the anchor would be off-apex and individually optimized for each minimally invasive repair device. Finally, it is important to note that transapical minimally invasive techniques do not include an annuloplasty repair in contrast with typical open-heart mitral valve repair for similar disease states; this repair discrepancy could also be biomechanically analyzed ex vivo.
Limitations
As previously discussed, sensitivity to chordal length was a crucial obstacle that we sought to overcome in designing the final iteration of these experiments. Before each valve test, the suture loop was knotted at the exact distance of the extended artificial papillary muscle, thus enabling the transition from apical anchoring to artificial papillary muscle anchoring without any adjustment of the suture. Nonetheless, small discrepancies in the overall length may have occurred, which would introduce error in our measurements. It is also possible that the elastic material selected for our artificial papillary muscle device would change material or geometric properties over time. To ensure the chordal length and device operation remain stable, future analyses must be performed to examine how the device elasticity and length changes when exposed to the physiologic environment over many years. Furthermore, although the silicone used in this prototype has undergone biocompatibility tests,25 a more robust analysis must be performed to examine the long-term stability and inflammatory response when exposed to the bloodstream, as well as any wear or negative effects due to the interaction between the silicone and ePTFE suture. The device geometry is amenable to alternative manufacturing methods such as molding; thus, additional materials can also be explored to develop a second device iteration with improved biocompatibility and long-term material stability. Finally, the left heart simulator does not mimic ventricular motion. We control for this limitation by mounting the apical anchor and the artificial papillary muscle anchor at the same location, so any force discrepancy due to a stationary apex is kept constant in both cases. However, in vivo large animal experiments in ovine or porcine models would illuminate the extent of this discrepancy as well as serve to validate our ex vivo study.
CONCLUSIONS
The artificial papillary muscle device represents a potential method of dampening sharp increases in artificial chordae suture forces, thus decreasing stress on the suture and suture-leaflet interface. With the implementation of this device, minimally invasive mitral valve repair devices could reach their full potential with improved durability of repair. Future experiments can optimize for artificial papillary muscle material elasticity and geometry, including the development and testing of an angled version that anchors the suture toward the posterior wall and thus positions the suture more perpendicular to the plane of coaptation. Furthermore, large animal experiments are essential in the next stage to analyze the device under in vivo conditions and validate the proposed implementation procedure.
PERSPECTIVE.
High stresses postrepair can dampen the potential of new minimally invasive artificial chordae implantation devices. Our novel artificial papillary muscle device would be seamlessly integrated into the current off-pump repair procedures, enabling a biomechanically optimized repair with reduced stresses. With this innovation, the full benefit of these promising repair devices can be fully realized.
Acknowledgments
This work was supported by the National Institutes of Health (NIH R01 HL152155 and NIH R01 HL089315-01, to Y.J.W.), the American Heart Association (17POST33410497, to M.J.P.), the National Science Foundation Graduate Research Fellowship Program (DGE-1656518, to A.M.I.), and a Stanford Graduate Fellowship (to A.M.I.). We also thank the generous donation by Dr D. Michael McKeough to support this research effort. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Abbreviations and Acronyms
- 3D
3-dimenional
- FBG
Fiber Bragg Grating
- PTFE
polytetrafluoroethylene
Footnotes
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Gillinov AM, Blackstone EH, Nowicki ER, Slisatkorn W, Al-Dossari G, Johnston DR, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg. 2008;135:885–93. 893.e1. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–89. [DOI] [PubMed] [Google Scholar]
- 4.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstone AB, Cohen JE, Howard JL, Edwards BB, Acker AL, Hiesinger W, et al. A “Repair-all” strategy for degenerative mitral valve disease safely minimizes unnecessary replacement. Ann Thorac Surg. 2015;99:1983–90; discussion 1990. [DOI] [PubMed] [Google Scholar]
- 6.Akins CW, Hilgenberg AD, Buckley MJ, Vlahakes GJ, Torchiana DF, Daggett WM, et al. Mitral valve reconstruction versus replacement for degenerative or ischemic mitral regurgitation. Ann Thorac Surg. 1994;58:668–75; discussion 675. [DOI] [PubMed] [Google Scholar]
- 7.Lee EM, Shapiro LM, Wells FC. Superiority of mitral valve repair in surgery for degenerative mitral regurgitation. Eur Heart J. 1997;18:655–63. [DOI] [PubMed] [Google Scholar]
- 8.Colli A, Manzan E, Aidietis A, Rucinskas K, Bizzotto E, Besola L, et al. An early European experience with transapical off-pump mitral valve repair with neochord implantation. Eur J Cardiothorac Surg. 2018;54:460–6. [DOI] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov. Randomized trial of the neochord DS1000 system versus open surgical repair (ReChord). Available at: https://clinicaltrials.gov/ct2/show/NCT02803957. Accessed July 19, 2020.
- 10.Gammie JS, Bartus K, Gackowski A, D’Ambra MN, Szymanski P, Bilewska A, et al. Beating-heart mitral valve repair using a novel ePTFE cordal implantation device: a prospective trial. J Am Coll Cardiol. 2018;71:25–36. [DOI] [PubMed] [Google Scholar]
- 11.Colli A, Manzan E, Rucinskas K, Janusauskas V, Zucchetta F, Zakarkaitė D, et al. Acute safety and efficacy of the neochord procedure†. Interact Cardiovasc Thorac Surg. 2015;20:575–80; discussion 580. [DOI] [PubMed] [Google Scholar]
- 12.Imbrie-Moore AM, Paulsen MJ, Thakore AD, Wang H, Hironaka CE, Lucian HJ, et al. Ex vivo biomechanical study of apical versus papillary neochord anchoring for mitral regurgitation. Ann Thorac Surg. 2019;108:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen H, Jensen MO, Waziri F, Honge JL, Sloth E, Fenger-Gron M, et al. Transapical neochord implantation: is tension of artificial chordae tendineae dependent on the insertion site? J Thorac Cardiovasc Surg. 2014;148:138–43. [DOI] [PubMed] [Google Scholar]
- 14.Gammie JS, Bartus K, Gackowski A, Szymanski P, Bilewska A, Kusmierczyk M, et al. Safety and performance of a novel transventricular beating heart mitral valve repair system: 1-year outcomes. Eur J Cardiothorac Surg. October 10, 2020 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Gammie JS, Wilson P, Bartus K, Gackowski A, Hung J, D’Ambra MN, et al. Transapical beating-heart mitral valve repair with an expanded polytetrafluoroethylene cordal implantation device: initial clinical experience. Circulation. 2016;134:189–97. [DOI] [PubMed] [Google Scholar]
- 16.Seeburger J, Rinaldi M, Nielsen SL, Salizzoni S, Lange R, Schoenburg M, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol. 2014;63:914–9. [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P, Radermecker M, Durieux R, Modine T, Oury C, Fattouch K. Transapical beating-heart chordae implantation in mitral regurgitation: a new horizon for repairing mitral valve prolapse. J Thorac Dis. 2016;8:E1665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colli A, Zucchetta F, Kliger C, Bellu R, Francone M, Sedati P, et al. CT for the Transapical off-pump mitral valve repair with neochord implantation procedure. JACC Cardiovasc Imaging. 2017;10:1397–400. [DOI] [PubMed] [Google Scholar]
- 19.Kiefer P, Meier S, Noack T, Borger MA, Ender J, Hoyer A, et al. Good 5-year durability of transapical beating heart off-pump mitral valve repair with neochordae. Ann Thorac Surg. 2018;106:440–5. [DOI] [PubMed] [Google Scholar]
- 20.Fiocco A, Nadali M, Speziali G, Colli A. Transcatheter mitral valve chordal repair: current indications and future perspectives. Front Cardiovasc Med. 2019;6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colli A, Zucchetta F, Torregrossa G, Manzan E, Bizzotto E, Besola L, et al. Transapical off-pump mitral valve repair with neochord implantation (TOP-MINI): step-by-step guide. Ann Cardiothorac Surg. 2015;4:295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talukder S, Duncan A, Moat N. Harpoon repair for mitral regurgitation: a case report. CASE (Phila). 2019;3:22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha A, Roy S. Papillary muscles of left ventricle-morphological variations and its clinical relevance. Indian Heart J. 2018;70:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan MA, Hamdi M, Noma A. The nonlinear elastic and viscoelastic passive properties of left ventricular papillary muscle of a Guinea pig heart. J Mech Behav Biomed Mater. 2012;5:99–109. [DOI] [PubMed] [Google Scholar]
- 25.Carbon. SIL 30: technical data sheet. Carbon N. Y 2018. Available at: https://s3.amazonaws.com/carbon-static-assets/downloads/resin_data_sheets/SIL30_TDS.pdf. Accessed July 2, 2020. [Google Scholar]
- 26.Caimmi PP, Sabbatini M, Fusaro L, Borrone A, Cannas MA. A study of the mechanical properties of ePTFE suture used as artificial mitral chordae. J Card Surg. 2016;31:498–502. [DOI] [PubMed] [Google Scholar]
- 27.Imbrie-Moore AM, Paulsen MJ, Zhu Y, Wang H, Lucian HJ, Farry JM, et al. A novel cross-species model of Barlow’s disease to biomechanically analyze repair techniques in an ex vivo left heart simulator. J Thorac Cardiovasc Surg. February 19, 2020 [Epub ahead of print], [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Standard. ISO 5840–1:2015 (E): Cardiovascular Implants: Cardiac Valve Prostheses. Geneva: ISO Copyright Office; 2015:56. [Google Scholar]
- 29.Paulsen MJ, Imbrie-Moore AM, Wang H, Bae JH, Hironaka CE, Farry JM, et al. Mitral chordae tendineae force profile characterization using a posterior ventricular anchoring neochordal repair model for mitral regurgitation in a three-dimensional-printed ex vivo left heart simulator. Eur J Cardiothorac Surg. 2020;57:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulsen MJ, Hwa Bae J, Imbrie-Moore AM, Wang H, Hironaka CE, Farry JM, et al. Development and ex vivo validation of novel force-sensing neochordae for measuring chordae tendineae tension in the mitral valve apparatus using optical fibers with embedded Bragg gratings. J Biomech Eng. 2020; 142: 014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imbrie-Moore AM, Paullin CC, Paulsen MJ, Grady F, Wang H, Hironaka CE, et al. A novel 3D-printed preferential posterior mitral annular dilation device delineates regurgitation onset threshold in an ex vivo heart simulator. Med Eng Phys. 2020;77:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colli A, Bagozzi L, Banchelli F, Besola L, Bizzotto E, Pradegan N, et al. Learning curve analysis of transapical neochord mitral valve repair. Eur J Cardiothorac Surg. 2018;54:273–80. [DOI] [PubMed] [Google Scholar]
- 33.Colli A, Adams D, Fiocco A, Pradegan N, Longinotti L, Nadali M, et al. Transapical neochord mitral valve repair. Ann Cardiothorac Surg. 2018;7: 812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo YJ, Mac Arthur JW. Posterior ventricular anchoring neochordal repair of degenerative mitral regurgitation efficiently remodels and repositions posterior leaflet prolapse. Eur J Cardiothorac Surg. 2013;44:485–9; discussion 489. [DOI] [PMC free article] [PubMed] [Google Scholar]


