Abstract
Chemotherapy eliminates most cancer cells except cancer stem cells. Chloroquine is a potential agent to target cancer stem cells. In this phase II trial for patients with breast cancer who were refractory to anthracycline-based chemotherapy, we combined chloroquine with taxane or taxane-like chemotherapy. The overall response rate of the combination was 45%, higher than the expected overall response rate of 30% with chemotherapy alone.
Introduction:
Chemotherapy eliminates most of the cancer cells except those with potential for self-renewal and tumor initiation, called cancer stem cells (CSCs). Chloroquine, through bioinformatics, was found to be a potential agent to target CSCs. We designed a phase II trial to test the efficacy and safety of chloroquine in combination with taxane or taxane-like chemotherapy agents in patients with advanced or metastatic breast cancer who are refractory to anthracycline-based chemotherapy.
Patients and Methods:
Female patients ≥ 18 years of age who had received prior anthracycline chemotherapy were enrolled in this study. Chloroquine 250 mg was given daily orally with either docetaxel or paclitaxel or nab-paclitaxel or ixabepilone every 3 weeks. The maximum number of 3-week cycles allowed was 6. The primary efficacy endpoint was the objective response rate (ORR). The secondary efficacy endpoints included progression-free survival (PFS) and safety analysis.
Results:
Thirty-eight patients were enrolled in the study, and 31 patients were evaluated for response. The median age was 54.1 years (range, 31.7–78.1 years). The ORR was 45.16% (95% confidence interval [CI], 29.2%–62.2%), which was higher than the expected ORR of 30% (P = .03). Patients were followed for a median of 25.4 months and experienced a median PFS of 12.4 months (95% CI, 4.9–24.6 months) and a median OS of 25.4 months (95% CI, 13.7–83.5 months). The combination was well-tolerated, with only 13.15% of patients experiencing grade ≥ 3 adverse events.
Conclusion:
A combination of chloroquine with taxane or taxane-like chemotherapy was efficacious in patients with locally advanced or metastatic breast cancer with prior anthracycline-based chemotherapy.
Keywords: Cancer stem cells, Choloroquine, Docetaxel, Ixabepilone, Triple negative breast cancer
Introduction
Conventional cytotoxic chemotherapy in breast cancer eliminates most of the cancer cells except cancer stem cells (CSCs), which have tumor-initiating potential and self-renewal properties.1 Targeting CSCs, therefore, is important to improve outcomes in breast cancer. Through systems biology analysis2 to facilitate drug repositioning for cancer therapy, chloroquine (a United States Food and Drug Administration-approved drug for treatment of malaria) has been identified as a potential agent to target breast CSCs.3 Chloroquine inhibits autophagy, which is critical in CSC self-renewal in breast cancer.4 Chloroquine also reduces CSCs through inhibition of Janus-activated kinase 2 (Jak2)-signal transducer and activator of transcription 3 (STAT3) signaling pathway by reducing the expression of Jak2 and DNA methyltransferase 1 (DNMT1).3 Chloroquine has been extensively studied over many decades and is well-tolerated with very few side effects. In combination with conventional chemotherapy, chloroquine improved survival when given in adjuvant setting post-surgery for glioblastoma multiforme.5 The risk of retinopathy with chloroquine is low, at 1% after 5 to 7 years of use or a cumulative dose of 460 grams of chloroquine. A baseline examination is advised for patients starting these drugs to serve as a reference point and to rule out maculopathy, which might be a contraindication to their use.6 In preclinical mouse models, we demonstrated that chloroquine enhanced the activity of paclitaxel, leading to a decrease in metastasis and tumor recurrence.3 With clear robust rationale for the combination of chloroquine with chemotherapy, we designed a phase II trial to assess the efficacy of chloroquine when used in combination with taxane or taxane-like chemotherapy agents (paclitaxel, docetaxel, nab-paclitaxel, and ixabepilone) in patients with advanced or metastatic breast cancer who previously did not benefit from anthracycline-based chemotherapy. Paclitaxel, one of the first taxanes to be isolated, is an anti-microtubule agent that promotes assembly and stabilization of microtubules from tubulin dimers. It inhibits the G2 mitotic phase, and thus cell replication is inhibited. The primary objective of the trial was to determine the anti-tumor activity of this combination as measured by objective response rate (ORR). The secondary objectives were (1) to assess the safety and tolerability of the combination and (2) to assess the progression-free survival (PFS) in patients receiving the combination.
Patient and Methods
Patients
All female patients > 18 years of age with Eastern Cooperative Oncology Group performance status of ≤ 2 with pathologically determined advanced or metastatic breast cancer were eligible. Patients with advanced breast cancer must have progressed or have residual disease after treatment with a regimen that included at least 2 cycles of an anthracycline-containing regimen. Patients with metastatic breast cancer must have had at least 4 cycles of an anthracycline-containing regimen. The patient must have at least 1 measurable disease site of ≥ 1 cm. The patient should not have underlying ocular pathology, preexisting auditory damage, any cardiac conduction disturbances, and should not be taking any medication that might cause QT prolongation. Women of childbearing potential should have a negative serum pregnancy test and should be on adequate contraception throughout the study. The exclusion criteria included radiation therapy within 2 weeks, New York Heart Association class III or greater cardiac disease, history of myocardial infarction and stroke in the past 12 months, symptomatic central nervous system metastases, pregnant or nursing women, and concurrent use of potent CYP3A4 inhibitors or inducers.
Study Design
This study was conducted at a single center and affiliated sites. Prior to enrollment to the clinical study, all patients with advanced or metastatic breast cancer with a residual disease post anthracycline-based chemotherapy underwent baseline biopsy and a baseline ophthalmologic examination as a reference point and to rule out maculopathy. Baseline laboratory testing (urine or serum pregnancy test, complete blood count with differential, and comprehensive metabolic panel) and electrocardiogram (EKG) was obtained for all patients. Subjects eligible for treatment received 250 mg oral chloroquine daily together with paclitaxel 175 mg/m2 intravenously every 3 weeks (alternatively 80 mg/m2 weekly) or docetaxel 75 mg/m2 intravenously every 3 weeks (or 100 mg/m2 every 3 weeks in patients with advanced breast cancer) or nab-paclitaxel 260 mg/m2 intravenously every 3 weeks (alternatively 100 mg/m2 weekly) or ixabepilone 40 mg/m2 intravenously every 3 weeks. Study treatment was continued until disease progression, intolerable adverse events (AEs), or death. A study cycle was defined as a cycle length of 3 weeks. The maximum number of treatment cycles allowed was 6. Compliance with oral chloroquine was monitored through pill count at scheduled clinic visit. The response was measured using Response Evaluation Criteria In Solid Tumors (RECIST), v1.1. Imaging while on the study was obtained using computed tomography scan of chest and abdomen at baseline, every 8 weeks while on treatment and at the end of treatment. ORR and PFS were calculated for all evaluable patients in the clinical trial. The ORR was the proportion of patients having complete or partial response to therapy. PFS was defined as the time from initiation of chemotherapy to disease progression. Toxicity was assessed for all enrolled patients in the clinical study who received ≥ 1 dose of study drug combination by Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical Methods
This study was designed to compare the primary endpoint, ORR, with the published rate of 30% (docetaxel 100 mg/m2 every 3 weeks for a maximum of 10 cycles)7 using a 1-sided z-test for binomial proportion. Rates (eg, ORR) are presented along with their associated Wilson score 95% confidence intervals (CIs). Bivariate comparisons of proportions were conducted using the χ2 or Fisher exact tests, as appropriate. The median follow-up was calculated using the reverse Kaplan-Meier method. Survival was estimated using the method of Kaplan-Meier, and the method of Brookmeyer and Crowley was used to construct 95% CIs for median survival. All analyses were conducted using SAS 9.4.
Results
Patient Characteristics
Thirty-eight patients with locally advanced or metastatic breast cancer with residual disease post-anthracycline–based chemotherapy were enrolled in the study from December 21, 2011 to August 17, 2017, after obtaining informed written consent (Figure 1). Locally advanced breast cancer was defined as any breast cancer without any distant metastasis that at least meets 1 of the criteria: (1) tumor more than 5 cm in size with regional lymphadenopathy (N1–3); (2) tumors of any size with direct extension to the chest wall or skin, or both, regardless of regional lymphadenopathy; or (3) presence of regional lymphadenopathy (clinically fixed or matted axillary lymph nodes, or any of infraclavicular, supraclavicular, or internal mammary lymphadenopathy) regardless of tumor stage. Metastatic breast cancer was defined as per American Joint Committee on Cancer (AJCC) seventh edition. The median age at enrollment of the whole cohort was 54.2 years (range, 31.7–78.1 years). Most patients in the study were non-Hispanic Caucasians (63.1%), followed by African-Americans (15.8%), Hispanic Caucasians (13.2%), and Asians (7.9%). Twenty-three (60.5%) patients had metastatic breast cancer, whereas fifteen (39.5%) had locally advanced breast cancer. Additionally, 20 (52.6%) patients had hormone receptor-positive (HR+)/human epithelial growth factor receptor 2-negative (HER2−) disease, and 18 (47.4%) had triple-negative breast cancer (TNBC) (HR−/HER2−). Most patients received ixabepilone (34.2%), followed by 26.3% each for docetaxel and nab-paclitaxel, and 13.1% received paclitaxel (Table 1).
Figure 1.
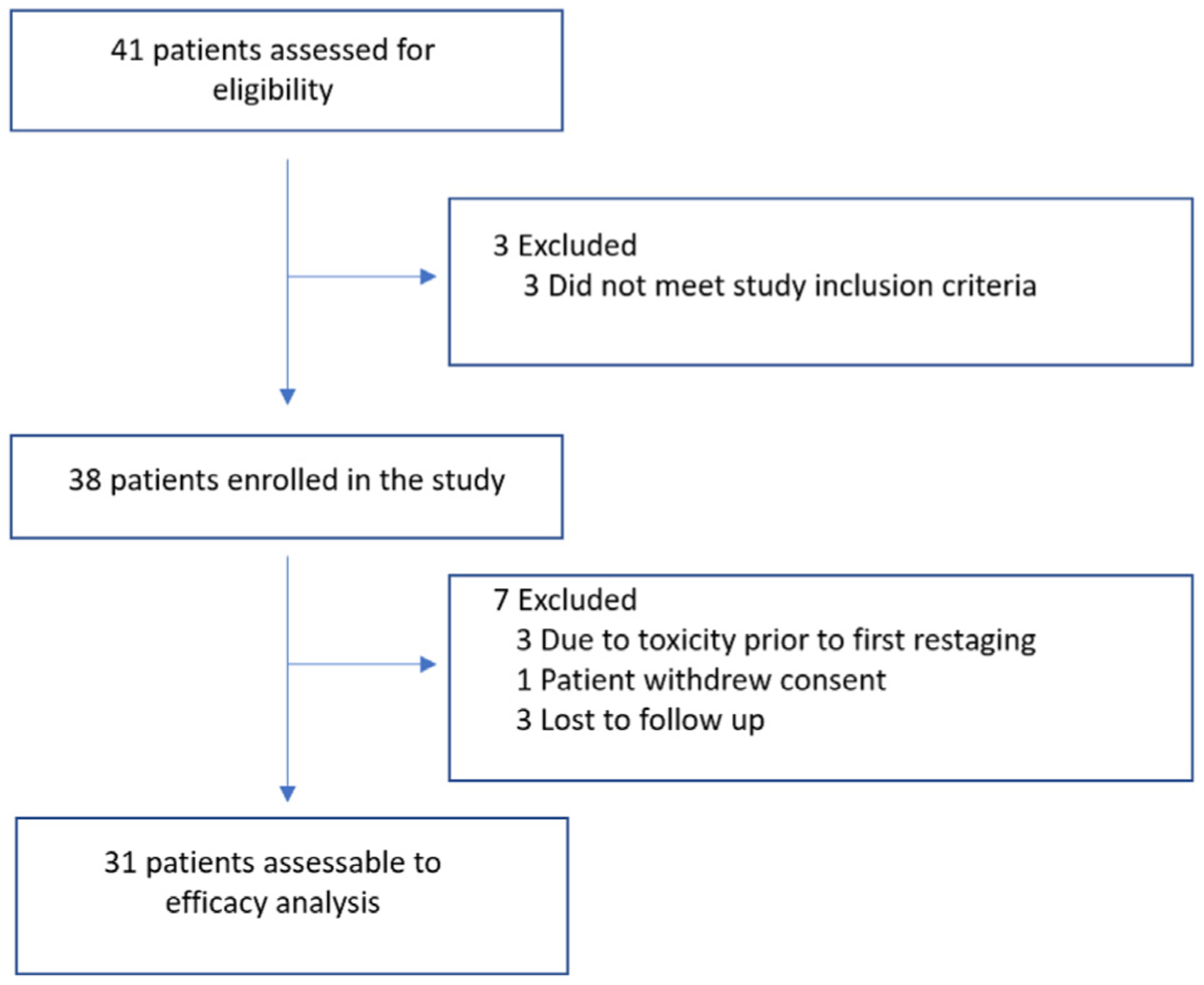
Consolidated Standards of Reporting Trials (CONSORT) Diagram
Table 1.
Baseline Patient Characteristics (n = 38)
| N (%) | |
|---|---|
| Median age, y (range) | 54.2 (31.7–78.1) |
| Race | |
| Non-Hispanic Caucasian | 24 (63.1) |
| African-American | 6 (15.8) |
| Hispanic Caucasian | 5 (13.2) |
| Asian | 3 (7.9) |
| Stage prior to treatment | |
| Locally advanced | 15 (39.5) |
| Metastatic | 23 (60.5) |
| Chemotherapy used in combination with chloroquine | |
| Ixabepilone | 13 (34.2) |
| Docetaxel | 10 (26.3) |
| Nab-paclitaxel | 10 (26.3) |
| Paclitaxel | 5 (13.1) |
| Type of breast cancer | |
| HR+/HER2− | 20 (52.6) |
| TNBC | 18 (47.4) |
Abbreviations: HER2− = human epidermal growth factor receptor 2-negative; HR+ = hormone receptor-positive; TNBC = triple negative breast cancer.
Treatment Response
Of 38 patients enrolled, 3 were taken off trial owing to toxicity prior to the first restaging, 1 withdrew consent, and 3 patients did not follow-up; thus, 7 patients were not evaluated for treatment response. Thirty-one patients were assessed for treatment response; 14 were HR+/HER2−, and 17 were TNBC. Of 31 patients, 3 (9.68%) patients had a complete response (CR) to therapy, and 11 (35.48%) patients had a partial response (PR). The ORR for a combination of chloroquine plus taxane or taxane-like chemotherapy was 45.16% (95% CI, 29.2%–62.2%), based on 1-sided binominal proportion; the ORR of the evaluable cohort is significantly greater than the 30% expected (P = .03). The ORR for patients with HR+/HER2− disease was higher at 52.94% versus 35.91% for patients with TNBC, although the difference was not statistically significant (P = .47). Of 3 patients who had CR, 1 (7.14%) had TNBC and 2 (11.76%) had HR+/HER2− disease; the rate of CR was not statistically different (P = 1). We also calculated the clinical benefit rate (CBR) of the whole cohort, which is the percentage of patients who had CR plus PR and stable disease (SD) with combination therapy; the CBR was 74.2% (23/31). The rate of CBR was higher for patients with HR+/HER2− disease at 88.24% (15/31) versus 57.14% (8/31) for TNBC, although this difference was not statistically significant (P = .09) (Table 2).
Table 2.
Treatment Response for 31 Evaluable Patients
| % | |
|---|---|
| ORR (CR + PR) | |
| Whole cohort (14/31) | 45.16 (95% CI, 29.2–62.2) |
| HR+/HER2− (9/17) | 52.94 |
| TNBC (5/14) | 35.71 |
| CR rate | |
| Whole cohort (3/31) | 9.68 (95% CI, 3.4–24.9) |
| HR+/HER2− (2/17) | 11.76 |
| TNBC (1/14) | 7.14 |
| CBR (CR + PR + SD) | |
| Whole cohort (23/31) | 74.19 (95% CI, 56.8–86.3) |
| HR+/HER2− (15/17) | 88.24 |
| TNBC (8/14) | 57.14 |
Abbreviations: CBR = clinical benefit rate; CI = confidence interval; CR = complete response; HER2− = Human epidermal growth factor receptor 2-negative; HR+ = hormone receptor-positive; ORR = overall response rate; PR = partial response; SD = stable disease; TNBC = triple negative breast cancer.
Survival Analysis
At a median follow-up of 25.36 months (95% CI, 13.70–58.35 months) from study enrollment, 32.25% (10/31) patients were still alive. The median PFS from the date of study enrollment was 12.4 months (95% CI, 4.86–24.64 months) (Figure 2). The median OS from the date of study enrollment of the whole cohort was 25.4 months (95% CI, 13.70–83.48 months). The estimated 1-year survival from study enrollment was 80.64% (95% CI, 61.90–90.80 months) (Figure 3).
Figure 2.
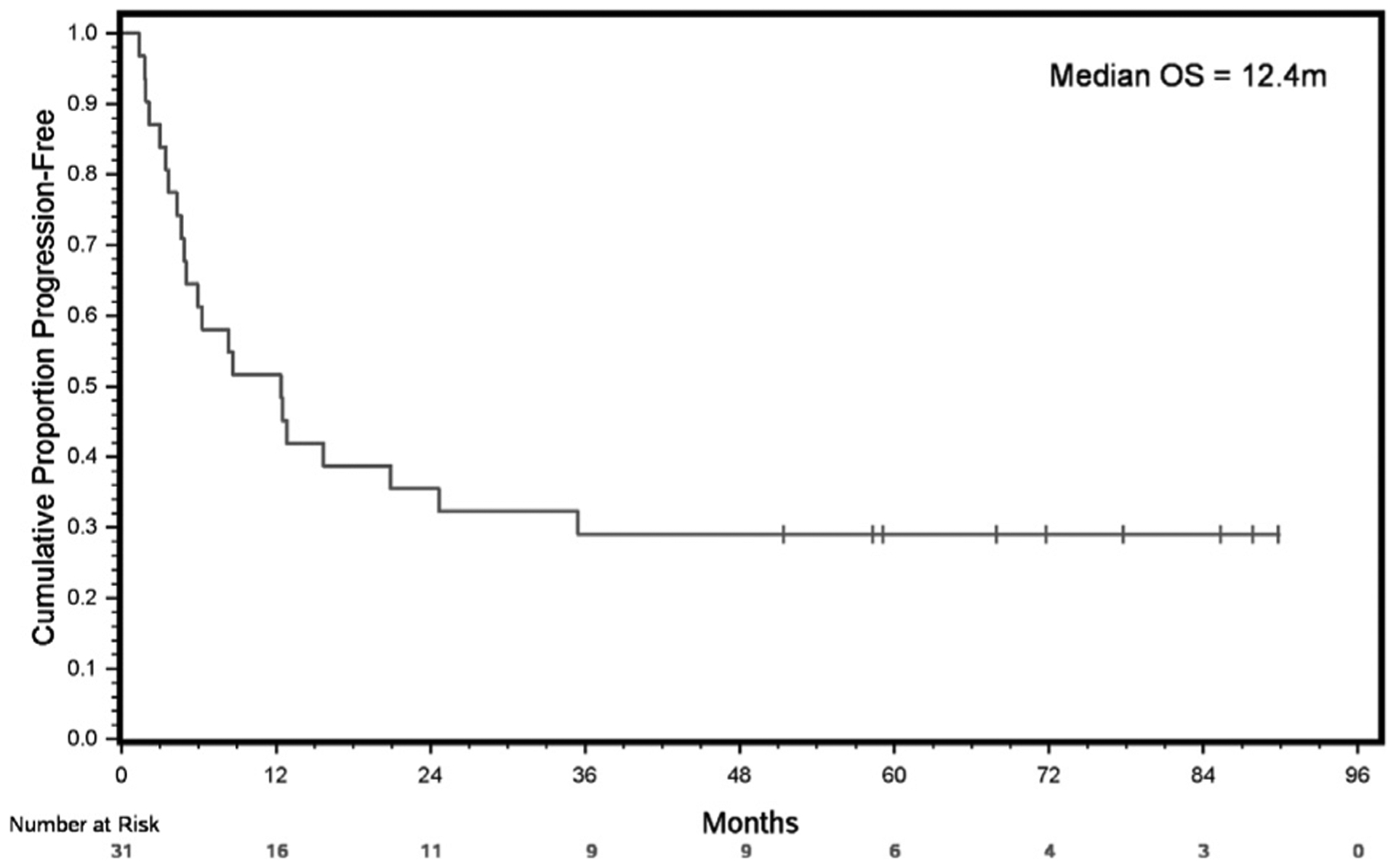
PFS From Date of Study Enrollment
Abbreviations: OS = overall survival; PFS = progression-free survival.
Figure 3.
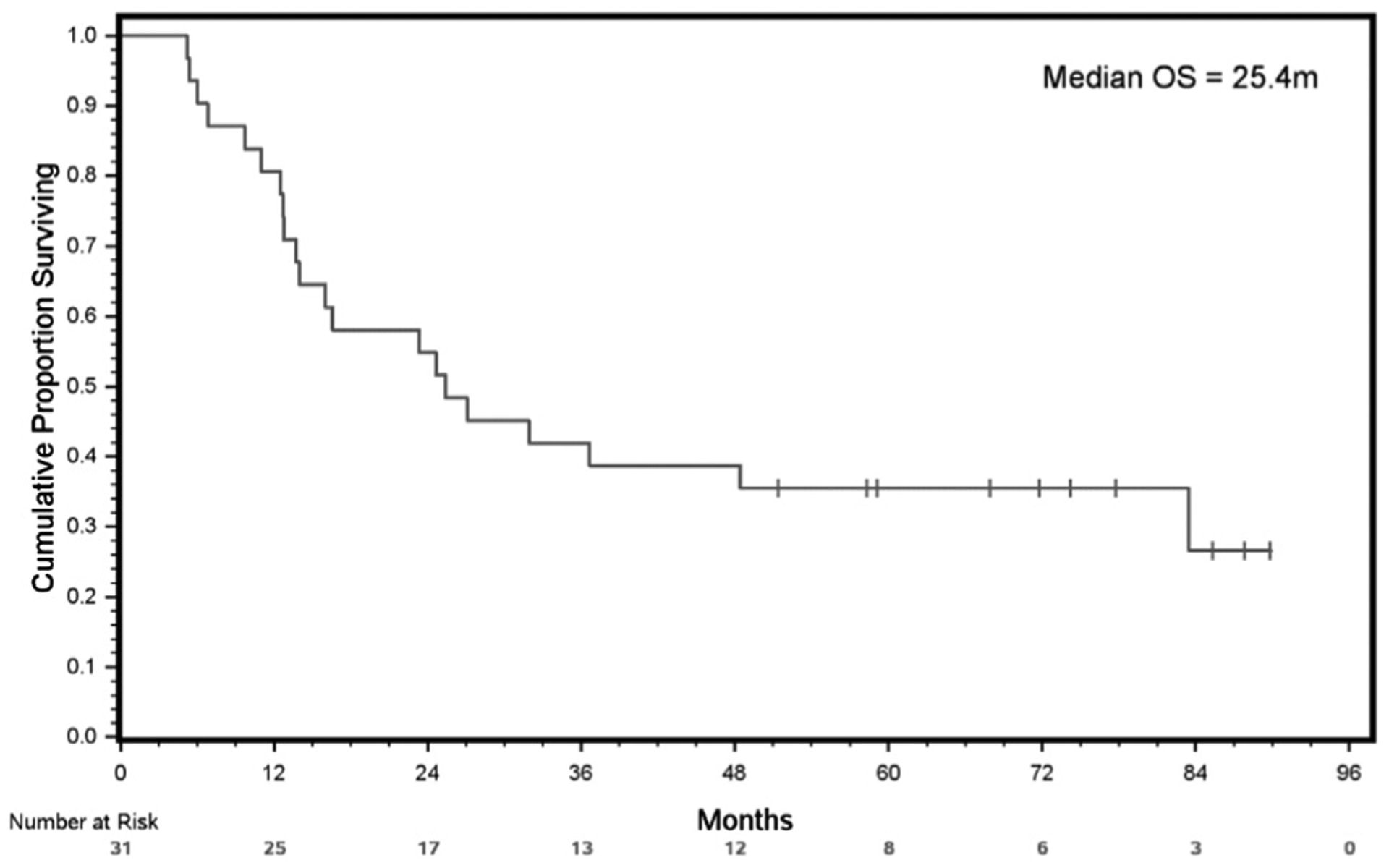
OS From Date of Study Enrollment
Abbreviation: OS = overall survival.
Toxicity
The regimen was well-tolerated, and only 5 (13.15%) of 38 patients experienced grade ≥ 3 toxicities. Only 1 patient had a grade 4 AE (neutropenia); the rest had grade 3 AEs. One patient experienced 6 grade ≥ 3 toxicities. No patient had grade ≥ 3 EKG abnormality (Table 3).
Table 3.
Adverse Events Grade ≥ 3 Observed in 5 of 38 Patients
| Adverse Event | Grade, n | |
|---|---|---|
| 4 | 3 | |
| Syncope | 0 | 1 |
| Back pain | 0 | 1 |
| Fatigue | 0 | 2 |
| Dehydration | 0 | 1 |
| Thrombocytopenia | 0 | 1 |
| Neutropenia | 1 | 0 |
| Diarrhea | 0 | 1 |
| Hand-foot syndrome | 0 | 1 |
| Infusion reaction | 0 | 1 |
Discussion
In this study, we demonstrated that a combination of chloroquine with taxane or taxane-like chemotherapy produced an ORR of 45%, and the combination was well-tolerated, with only 13% of the patients experiencing grade ≥ 3 or more AEs. Docetaxel is the only agent that has shown a survival benefit in anthracycline-refractory patients with metastatic breast cancer. The ORR for docetaxel was significantly higher at 30% when compared with 11.6% in the comparator arm of mitomycin plus vinblastine in a phase III trial.7 The ORR for paclitaxel for anthracycline-refractory metastatic breast cancer is only 25%.8 Another agent that has shown benefit in the anthracycline chemotherapy-refractory setting is ixabepilone, an epothilone B analog whose mechanism of action, which is microtubule stabilization, is similar to paclitaxel.9 The ORR for ixabepilone, when given in the first-line setting in metastatic breast cancer in those patients who had anthracycline chemotherapy in the adjuvant setting, was 40%.10 In this study, the observed ORR was superior to docetaxel7 and paclitaxel8 and improved compared with ixabepilone alone.10 We observed a higher rate of ORR in this clinical trial for patients with HR+/HER2− disease, although the difference was not statistically significant compared with patients with TNBC. The explanation of this observation needs more studies; however, a preclinical mouse model showed that tamoxifen resistance of HR+ breast cancer was overcome by treatment with chloroquine.11 The CBR rate in this study, which also included patients who had SD while on treatment, was 74.2%. In patients with TNBC with anthracycline-resistant disease, the CBR rate was 57.14%, which is clinically meaningful. A preclinical TNBC model suggests that chloroquine causes mitochondrial damage and hinders homologous recombination necessary for DNA repair in CSCs.12
In this study, the PFS for anthracycline refractory breast cancer was 12.4 months. The median OS from study enrollment was > 2 years, which is also beneficial for this patient population, which is anthracycline-resistant and usually has a higher tumor burden. In terms of AEs, none of the patients had to stop chloroquine early; no grade ≥ 3 EKG abnormality or retinopathy was noted.
Anthracycline resistance in breast cancer can be attributed to different mechanisms, including patients who have primary resistance to anthracycline owing to increased expression of P-glycoprotein,13 whereas secondary resistance can develop owing to CSCs, which have the potential for tumor initiation and self-renewal. Therefore, targeting CSCs may be important for effective treatment of breast cancer. In this study, we used chloroquine to address targeting CSCs in a population with breast cancer that had been previously treated with anthracycline. Chloroquine has the potential to be used in an earlier line of therapy and in a population of patients who cannot receive anthracycline owing to poor cardiac function or who have had a maximum lifetime cumulative dose. The addition of autophagy inhibition to chemotherapy has shown benefit in pancreatic cancer as a part of preoperative chemotherapy leading to increased pathologic tumor response.14 Also, preclinical data suggests a potential benefit in osteosarcoma.15
This trial had limitations owing to a small number of patients and it being a single-arm study with no randomization. However, the trial was able to achieve its primary endpoint, which was a statistically significant improvement in ORR of 45% compared with the expected 30%. Also, the combination of chloroquine was well-tolerated with taxane or taxane-like chemotherapy, which warrants further prospective studies looking at this combination for breast cancer.
Conclusion
The combination of chloroquine in combination with taxane or taxane-like chemotherapy was active in patients with locally advanced or metastatic breast cancer who had prior anthracycline-based chemotherapy, with an ORR of 45%. The combination was well-tolerated without significant toxicity. This is the first study that used chloroquine to target CSCs, which leads to chemotherapy resistance in breast cancer. Further studies are warranted to evaluate this combination.
Clinical Practice Points.
The combination of chloroquine plus taxane or taxane-like chemotherapy was active in patients with locally advanced or metastatic breast cancer who were refractory to prior anthracycline-based chemotherapy.
The combination was well-tolerated with an expected toxicity profile.
Further prospective trials are need to evaluate this regimen in patients with chemotherapy-refractory breast cancer.
Acknowledgment
This study was partially supported by the Breast Cancer Research Foundation, CREDO, the T.T. & W.F. Chao Foundation, the John S Dunn. Research Foundation, and the National Institutes of Health (grant numbers U54 CA210181 and U54 CA149196). The funders for this study were not involved with the implementation of the protocol, analysis of the data, or preparation of the manuscript.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 2009; 106:13820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin G, Fu C, Zhao H, Cui K, Chang J, Wong ST. A novel method of transcriptional response analysis to facilitate drug repositioning for cancer therapy. Cancer Res 2012; 72:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi DS, Blanco E, Kim YS, et al. Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells 2014; 32:2309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Vellon L, Menendez JA. Autophagy positively regulates the CD44+ CD24−/low breast cancer stem-like phenotype. Cell Cycle 2011; 10:3871–85. [DOI] [PubMed] [Google Scholar]
- 5.Briceño E, Reyes S, Sotelo J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus 2003; 14:e3. [DOI] [PubMed] [Google Scholar]
- 6.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmol 2011; 118:415–22. [DOI] [PubMed] [Google Scholar]
- 7.Nabholtz JM, Senn HJ, Bezwoda WR, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. J Clin Oncol 1999;17:1413–24. [DOI] [PubMed] [Google Scholar]
- 8.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 2005; 23:5542–51. [DOI] [PubMed] [Google Scholar]
- 9.Lee FY, Borzilleri R, Fairchild CR, et al. BMS247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 2001; 7:1429–37. [PubMed] [Google Scholar]
- 10.Roché H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol 2007; 25:3415–20. [DOI] [PubMed] [Google Scholar]
- 11.Cook KL, Wärri A, Soto-Pantoja DR, et al. Hydroxychloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer. Clin Cancer Res 2014; 20:3222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang DH, Choi DS, Ensor JE, Kaipparettu BA, Bass BL, Chang JC. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett 2016; 376:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JD, Brinkhuis RF, van Deemter L, Wijnholds J, Schinkel AH. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res 2000; 60:5761–6. [PubMed] [Google Scholar]
- 14.Zeh HJ, Bahary N, Boone BA, et al. A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/nab-paclitaxel in pancreatic cancer patients. Clin Cancer Res 2020; 26:3126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Lee SG, Kim YJ, et al. Cytoprotective role of autophagy during paclitaxel-induced apoptosis in Saos-2 osteosarcoma cells. Int J Oncol 2013; 42: 1985–92. [DOI] [PubMed] [Google Scholar]


