Abstract
Ex-vivo liver perfusion (EVLP) is an ideal platform to study liver disease, therapeutic interventions, and pharmacokinetic properties of drugs without any patient risk. Rat livers are an ideal model for EVLP due to less organ quality variability, ease of hepatectomy, well-defined molecular pathways, and relatively low costs compared to large animal or human perfusions. However, the major limitation with rat liver normothermic machine perfusion (NMP) is maintaining physiologic liver function on an ex-vivo machine perfusion system. To address this need, our research demonstrates 24-hour EVLP in rats under normothermic conditions. Early (6 hour) perfusate transaminase levels and oxygen consumption of the liver graft are shown to be good markers of perfusion success and correlate with viable 24-hour post-perfusion histology. Finally, we address overcoming challenges in long-term rat liver perfusions such as rising intrahepatic pressures and contamination, and offer future directions necessary to build upon our work.
Keywords: Machine Perfusion, Ex-Vivo Liver Perfusion, Normothermic Machine Perfusion
INNOVATION
Rat liver perfusion is an efficient and cost-effective method to study how various pharmacologic agents impact liver parenchyma. Normothermic machine perfusion (NMP) has the challenge of mirroring in-vivo settings as closely as possible for the liver allograft. This allows drugs, enzymatic reactions, repair processes, and metabolic pathways to affect liver function to their full capacity. However, under normothermic conditions (35°C–38°C), the perfusions become exponentially more complex when the perfusion duration is extended, severely limiting our ability to observe liver physiology and pharmacologic effects after 6 hours. Thus, our work builds on existing NMP systems with critical modifications in technique and design to extend the perfusion time of a rat liver allograft from 6 to 24 hours without complications such as contamination or ischemic events. Addressing these issues in NMP greatly expands the armamentarium of experiments that can be conducted to assess how livers responds to physiologic insults and pharmacologic agents ex vivo.
INTRODUCTION
Ex-vivo liver perfusion (EVLP) is an alternative to the current standard of static cold storage (SCS) and has a wide range of biological and clinical advantages. Machine perfusion allows us to investigate many aspects of liver disease such as nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, hepatitis, and ischemia-reperfusion injury prior to transplantation, in various animal models such as rats, pigs, and humans1. Furthermore, because treatment of the perfused organ on a pump places no risk to the host, EVLP is an ideal platform to study therapeutics2. RNA interference (RNAi) agents, defatting cocktails, vasodilators, anti-inflammatory agents, opioid agonists, and human liver stem cells extracellular vesicles (HLSC-EV) have all been incorporated in liver perfusion systems3–6. For example, studies have shown that using defatting cocktails during machine perfusion have reduced hepatic steatosis both at subnormothermic and normothermic temperatures7. Raigani et al. showed that 6 hours of NMP with a defatting cocktail containing visfatin, hypericin, forskolin, scoparone, L-carnitine, GW7647, and GW501516 reversed moderate-to-severe macrosteatosis in fatty rat livers in addition to improving lactate clearance and bile quality8. Finally, ex-vivo perfusion is useful for pharmaceutical development as it allows us to assess pharmacokinetic components (absorption, distribution, metabolism, and excretion) and toxicology to organ tissue without the systemic in-vivo risks9.
Compared to large animal and human models, EVLP of rat livers is particularly suitable for trialing different pharmacologic agents for therapeutic purposes. First, rat livers have less variation in organ quality due to the ability to control for age, disease, anatomic variation, procurement technique, or delivery time, compared to pig and human livers. Thus, the action of a drug on organ performance can be elucidated more easily with fewer confounding variables or off-target adverse effects10. Next, the rat hepatectomy is not associated with massive necrosis, resulting in a “clean” removal without necrosis or acute inflammation. Clean removal is significant because the response of the liver parenchyma to machine perfusion is due to intrinsic processes within the liver tissue and not secondary to the hepatectomy11. The molecular pathways of rat hepatic injury have also been well delineated, creating the opportunity to merge organ perfusion performance data with the underlying biology. Finally, multiple experiments can be run efficiently as a rat hepatectomy can be performed by a single individual in 1–2 hours on an animal bench12. Conversely, large animal hepatectomies are costly, require availability of a large animal room equipped with anesthesia capabilities, and a full surgical team13.
In EVLP, a liver graft’s vasculature is cannulated and a defined perfusate consisting of oxygen, nutrients, and metabolic substrates is circulated through the organ, washing out metabolic waste and supporting cellular function. EVLP can be performed at different temperatures, with normothermic perfusion at 35°C–38°C. NMP allows the liver graft to undergo full physiological function and recover from injury during procurement prior to pharmacologic intervention or transplantation14. The primary limitation with rat liver ex-vivo NMP is maintaining livers long enough on pump to see the effects of the intervention of interest. The vast majority of rat liver perfusions done under normothermic conditions are conducted for 6 hours or less15–18, a duration precluding assessment of the downstream effects of pharmacologic reconditioning. This work is the first study to our knowledge to demonstrate rat liver perfusion for 24 hours under normothermic conditions.
METHODS
Rat hepatectomy
We obtained adult, male Sprague Dawley rats (12 weeks old, weighing 250–300 g) from Charles River Laboratories in Boston. The animals were studied in strict accordance with the National Research Council guidelines and Institutional Animal Care and Use Committee (IACUC) protocols at Massachusetts General Hospital (MGH). Eight rats underwent total hepatectomy. Donor Sprague Dawley rats were anesthetized by inhalation of isoflurane in an induction chamber and during the procedure using a tabletop anesthesia apparatus connected to a standard rodent system. Anesthesia was deemed adequate when muscular contraction was absent following toe pinch.
The animal was placed on a surgical table in supine position and covered with sterile surgical drapes. The abdomen was opened with a transverse abdominal incision with scissors. Ligaments and adhesions at the superior and posterior aspect of the liver were divided and the liver was freed from its surroundings. Heparin (300 units) was injected into the intrahepatic inferior vena cava (IVC). The hepatic artery was dissected and ligated. The portal vein (PV) was cannulated with a 16-G angiocatheter and the infrahepatic IVC was transected immediately thereafter to exsanguinate the rat. The liver was then flushed with 50 mL of ice-cold lactated ringers (LR) with heparin through the PV. The PV cannula was secured in place and the liver was freed from its remaining attachments to the diaphragm, retroperitoneum, and intestine. The suprahepatic IVC was transected and the liver wasremoved from the body.
Normothermic machine perfusion
Following complete hepatectomy, the rat liver was placed on NMP. Ex-vivo NMP with Sprague Dawley rats was performed with a nonoxygen carrier, sterilized perfusate consisting of 950 mL of William’s E media (Sigma-Aldrich), 20 g of bovine serum albumin (Sigma-Aldrich), 20 g of polyethylene glycol 35,000(PEG) (Sigma-Aldrich), 20 mg of dexamethasone (Sigma-Aldrich), 2 mL of heparin, 1 mL of regular insulin, 10 mL penicillin-streptomycin (Thermo Fisher Scientific), 10 mL of antibiotic-antimycotic (anti-anti) (Sigma-Aldrich), and 2.2 g of sodium bicarbonate to obtain a pH of 7.4. 500 mL perfusate was circulated at 37°C at 25–30 mL/min through an oxygenator with 95% O2 and 5% CO2 for a PV intrahepatic inflow pressure of less than 12 mmHg. The perfusate was changed at 12 hours to limit bacterial growth due to potential contamination.
Perfusate entered the PV and exited freely from the supra-hepatic vena cava (SHVC) and IVC. The perfusion circuit consisted of a perfusion chamber, two Masterflex peristaltic pumps (Cole Parmer, Vernon Hill, IL), a membrane oxygenator (Radnoti), a heat exchanger (Radnoti), and a bubble trap (Radnoti). Liver temperature was regulated by a water bath (Lauda, Brinkmann, Westbury, NY) and constantly monitored (Fig. 1). During the 24-hour perfusion, perfusion pH was maintained at 7.35–7.45 with the supplementation of sodium bicarbonate as necessary19.
Figure 1.
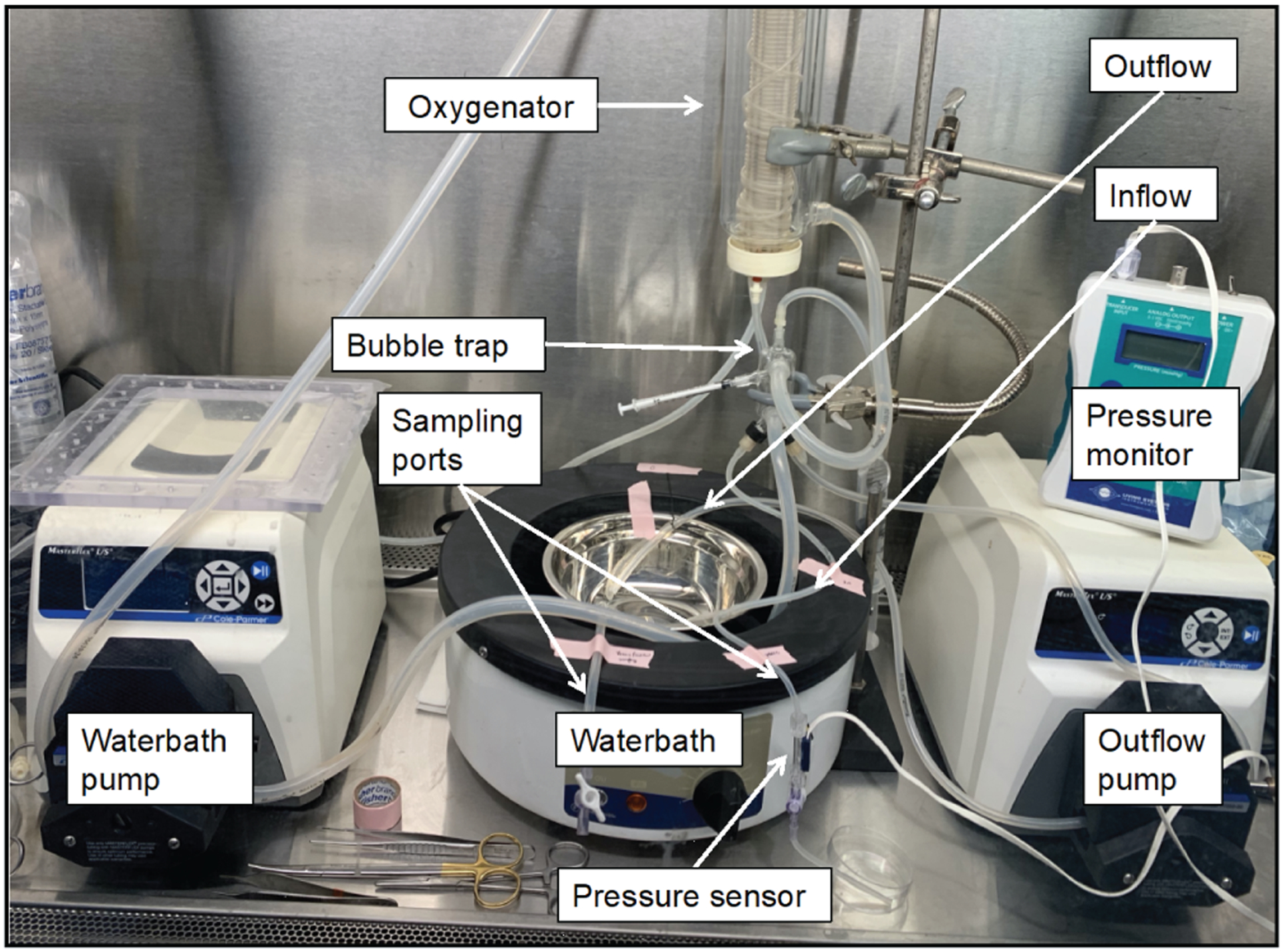
Normothermic machine perfusion system. The perfusion circuit consisted of a perfusion chamber, two Masterflex peristaltic pumps (Waterbath pump and outflow pump) (Cole Parmer, Vernon Hill, IL), a membrane oxygenator (Radnoti), a heat exchanger (Radnoti), and a bubble trap (Radnoti). Liver temperature was regulated by a water bath (Lauda, Brinkmann, Westbury, NY), intrahepatic pressure was monitored with a pressure sensor, and samples were collected without disturbing the liver via sampling ports.
Sample collection
Two sampling ports were placed proximal to and in the perfusion chamber to aseptically collect lab values for perfusion assessment of injury (aspartate aminotransferase [AST], alanine aminotransferase [ALT], resistance, and potassium) and metabolism (pH, lactate, oxygen consumption, and glucose). Resistance was defined as pressure divided by flow rate, and oxygen consumption was defined as inflow pO2 minus outflow pO2. Samples were collected at the following timepoints: 0, 2, 6, 12, 18, and 24 hours. Liver biopsies and perfusate samples were acquired before perfusion and after 24 hours of NMP. Rat liver tissue was fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
We hepatectomized eight rats for 24-hour NMP. Three of these liver allografts became contaminated with bacteria by 24 hours, which was confirmed visually by increased perfusate turbidity and with microscopic visualization (Supplementary Fig. 1). The remaining five uncontaminated liver allografts were labeled perfusion 1 through perfusion 5. They were further characterized as “viable” versus “nonviable” by blinded histology assessment at 24 hours. Figure 2 depicts the experimental outline.
Figure 2.
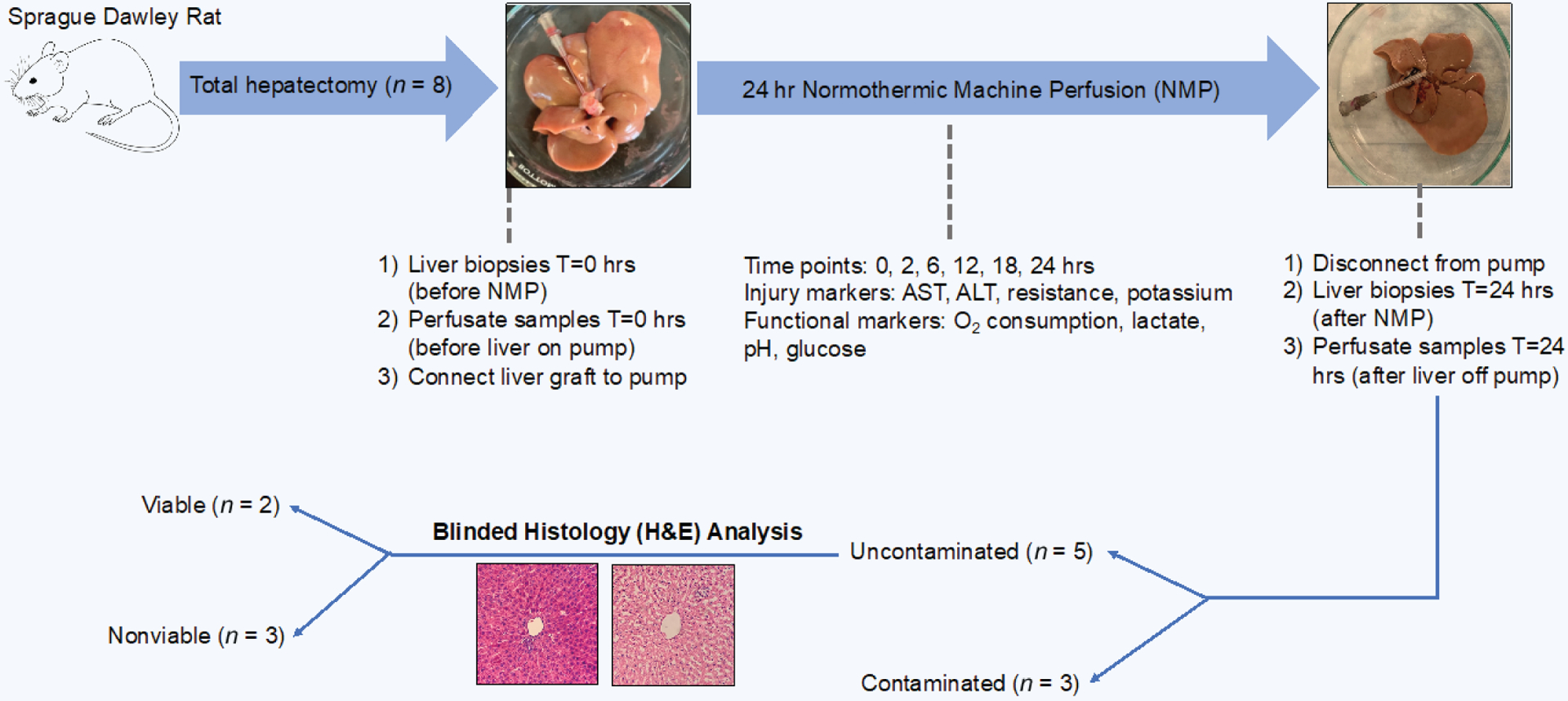
Experimental outline. Sprague Dawley rats underwent total hepatectomy followed by 24 hours of normothermic machine perfusion with time points at 0, 2, 6, 12, 18, and 24 hours. After perfusion, livers that were not contaminated underwent blinded histology analysis to determine viability.
Statistical analysis
Data analysis and graphic creation were done with Prism 8 software (GraphPad Software, San Diego, CA) software. Perfusion metrics were reported as means with standard deviations shown. Comparisons between contaminated (n = 3) and uncontaminated (n = 5) perfusions were made using two-tailed t-tests. Time statistics of perfusion injury and functional markers were conducted using paired, two-tailed t-tests and one-way analysis of variance (ANOVA) with a Greenhouse–Geisser correction adjusting for lack of sphericity. Statistical significance was defined as a p value < 0.05 (Table 1).
Table 1.
Time statistics of perfusion trends compared to baseline.
| Perfusion metric | Statistical test | Baseline timepoint | P value | Significance |
|---|---|---|---|---|
| Injury Markers | ||||
| *AST | Paired t-test | T = 6 hours | 0.029 | Yes |
| *ALT | Paired t-test | T = 6 hours | 0.050 | Yes |
| Resistance | One-way ANOVA | T = 6 hours | 0.098 | No |
| Perfusate potassium | One-way ANOVA | T = 6 hours | 0.434 | No |
| Functional Markers | ||||
| Oxygen consumption | One-way ANOVA | T = 2 hours | 0.609 | No |
| Arterial (inflow) lactate | One-way ANOVA | T = 2 hours | 0.053 | No |
| Arterial (inflow) pH | One-way ANOVA | T = 2 hours | 0.407 | No |
| *Glucose | One-way ANOVA | T = 2 hours | 0.014 | Yes |
(significant difference, p < 0.05)
With injury markers (AST, ALT, resistance, and potassium), T = 6 hours was used as the baseline since this was the endpoint of prior NMP rat perfusion studies19. Paired, two-tailed t-tests were used for AST and ALT comparing 6- and 12-hour timepoints, since the perfusate was refreshed after 12 hours. Thus, comparing 6 hours AST/ALT levels with 18- and 24-hour timepoints would be confounded by the addition of fresh perfusate, potentially diluting transaminase levels. Resistance and potassium were not dependent on the 12-hour perfusate change, so these markers were analyzed by ANOVA.
Functional markers (arterial pH, arterial lactate, oxygen consumption, and glucose) were compared to the T = 2 hour baseline, at which time the liver allograft had been stabilized on pump, flow had been increased to physiologic (30 mL/min), and intrahepatic pressure had reached its equilibrium (less than 12 mmHg). NMP attempted to mimic in-vivo conditions throughout the 24-hour perfusion; therefore, the T = 2 hour baseline was compared to the other timepoints with ANOVA.
RESULTS
Graft injury and viability
AST increased in all five un-contaminated perfusions (Supplementary Fig. 2a). Paired t-tests showed that AST levels were significantly increased at 12 hours (mean = 957.2 units/L, standard deviation [SD] 361 units/L) compared to the 6-hour baseline (mean = 348 units/L, SD 226 units/L), p value 0.029. AST level drops were seen at 18-hour timepoints due to the changing of perfusate at 12 hours. The sharpest increases in AST were seen between the 6- and 12-hour timepoints (Fig. 3a). Analogous to AST, ALT also increased in all five uncontaminated perfusions (Supplementary Fig. 2b) with level reductions seen after perfusate changes at 12 hours. The sharpest increases were also seen between the 6- and 12-hour timepoints (Fig. 3b). Paired t-tests showed that ALT levels were significantly increased at 12 hours (mean = 777 units/L, SD 333 units/L) compared to the 6-hour baseline (mean 302 units/L, SD 228 units/L), p value 0.050.
Figure 3.
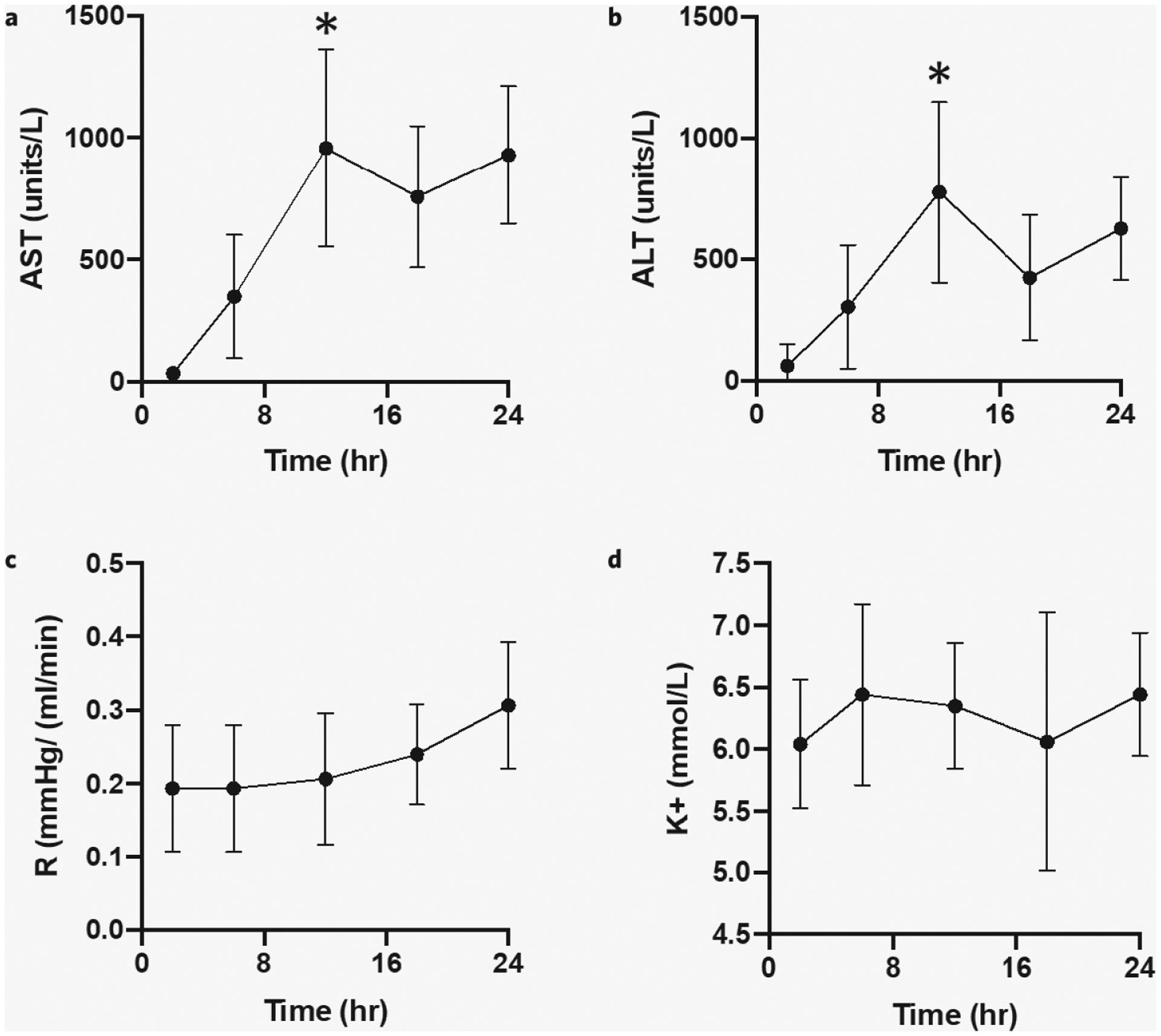
24-hour rat liver NMP injury indicators. Compared to the 6-hour baseline (a) AST levels were significantly increased at 12 hours (p value 0.029); (b) ALT levels were significantly increased at 12 hours (p value 0.050); (c) resistance (pressure over flow rate) increase over 24 hours but did not reach statistical significance at 12, 18, and 24 hours (p value 0.098); and (d) potassium levels were stable over 24 hours (p value 0.434). Error bars represent standard deviations. *significant difference, p < 0.05.
Next, resistance remained stable through 12 hours. After 12 hours, we detected an overall rise in resistance (Fig. 3c), specifically in perfusions 2, 3, and 4 (Supplementary Fig. 2c). However, this increase did not meet statistical significance on ANOVA testing comparing baseline resistance at 6 hours with resistance at 12, 18, and 24 hours (p value 0.098).
Finally, perfusate potassium was measured as our primary intracellular cation. Overall, potassium levels remained constant between 5.5 and 6.5 over 24 hours (Fig. 3d), with no statistically significant increase at 12, 18, or 24 hours compared to the 6-hour baseline level on ANOVA testing (p value 0.434). Potassium levels did not significantly drop after the 12 hour perfusate changes as our perfusate was originally prepared with physiologic levels of potassium ( mmol/L).
Graft function
Oxygen levels remained relatively stable between 300 and 400 mmHg over 24 hours (Fig. 4a) with no statistically significant change at 12, 18, or 24 hours compared to the 2-hour baseline on ANOVA testing (p value 0.609). Only perfusion 1 showed a steady decrease in O2 consumption with respect to time after 12 hours (Supplementary Fig. 3a).
Figure 4.
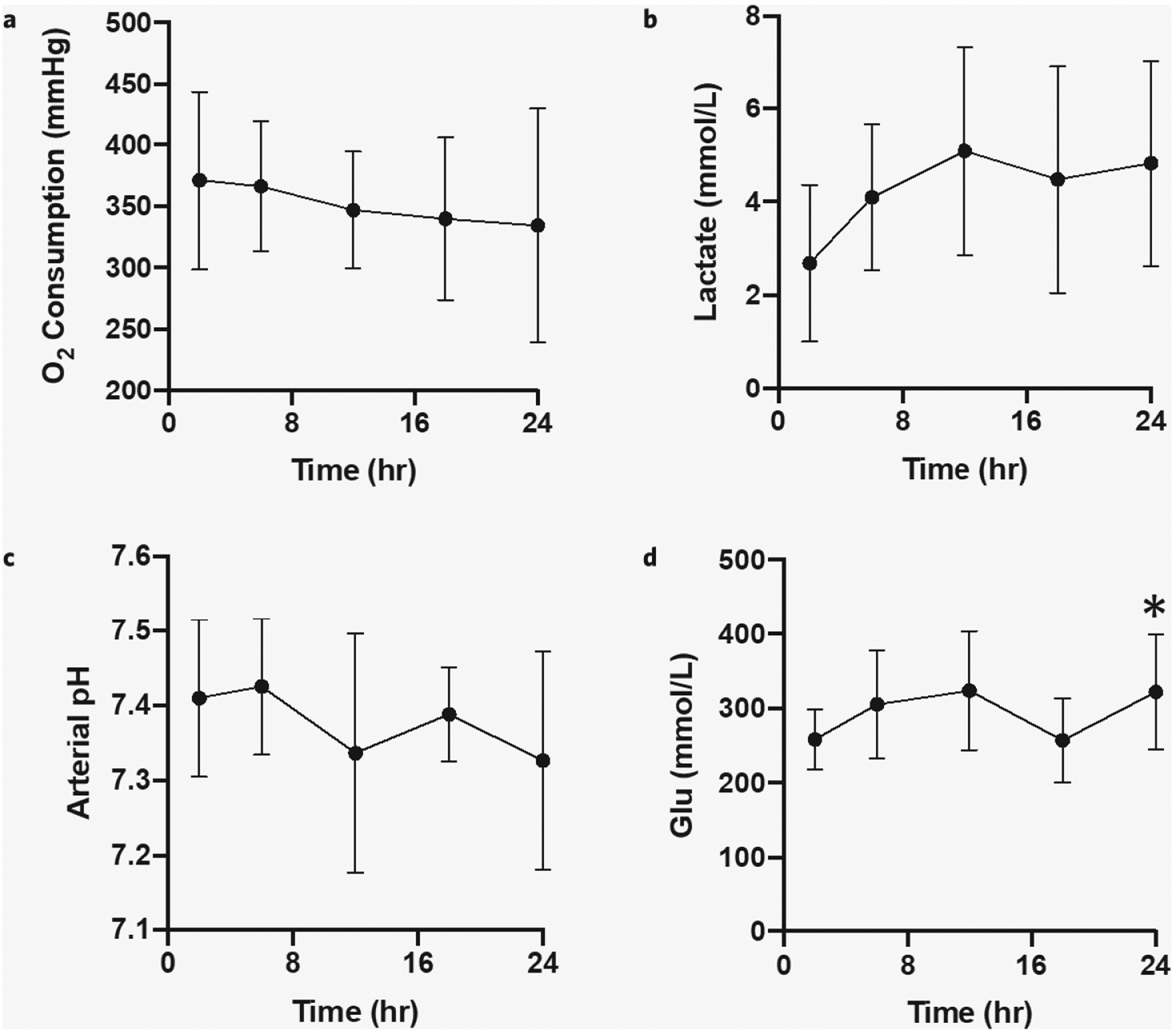
Function during 24-hour rat liver NMP. Compared to the 2-hour baseline (a) O2 consumption (inflow pO2 minus outflow pO2) (p value 0.609), (b) arterial (inflow) lactate (p value 0.053), and (c) arterial (inflow) pH (p value 0.407) were stable over 24 hours. (d) Glucose levels were significantly higher at 24 hours compared to the 2-hour baseline (p value 0.014). Error bars represent standard deviations. *significant difference, p < 0.05.
Inflow lactate increased over the first 12 hours of perfusion but leveled off with time (Fig. 4b), not reaching a statistically significant increase over 24 hours compared to the 2-hour baseline on ANOVA (p value 0.053). Perfusions 1 and 5 had liver grafts that cleared lactate at 12 hours, perfusion 3 had a stable lactate at 3 mmol/L over 24 hours, and perfusions 2 and 4 had consistently rising lactates through 24 hours (Supplementary Fig. 3b).
Next, arterial (inflow) perfusate pH was measured as a confirmatory check for lactate levels as well as to allow appropriate titration of bicarbonate. The trend was a decrease in arterial pH over 24 hours (Fig. 4c) but did not reach statistical significance on ANOVA (p value 0.407). In general, the rat livers were maintained with an arterial pH between 7.3 and 7.5 over the full 24-hour perfusion.
Finally, glucose levels rose over the first 12 hours but then stabilized between 200 and 400 mmol/L (Fig. 4d). ANOVA testing showed a significant increase in glucose levels over 24 hours, compared to the 2-hour baseline (p value 0.014). Glucose levels did not significantly drop after the 12-hour perfusate changes as our perfusate was originally prepared with physiologic levels of glucose (mean = 202 mmol/L, SD 10 mmol/L).
Histological evaluation
Following total hepatectomy at T = 0 hours, we demonstrated normal liver architecture across all five uncontaminated perfusions (Fig. 5a, c, e, g, and i). H&E stains at this time showed normal PV structure, with a thin wall, large lumen, and irregular shape. Hepatocytes were well organized into lobules with clear nuclear envelopes and a distinctive nucleolus. Sinusoids were well demarcated by intact endothelial cells and properly interposed between hepatic lobules.
Figure 5.
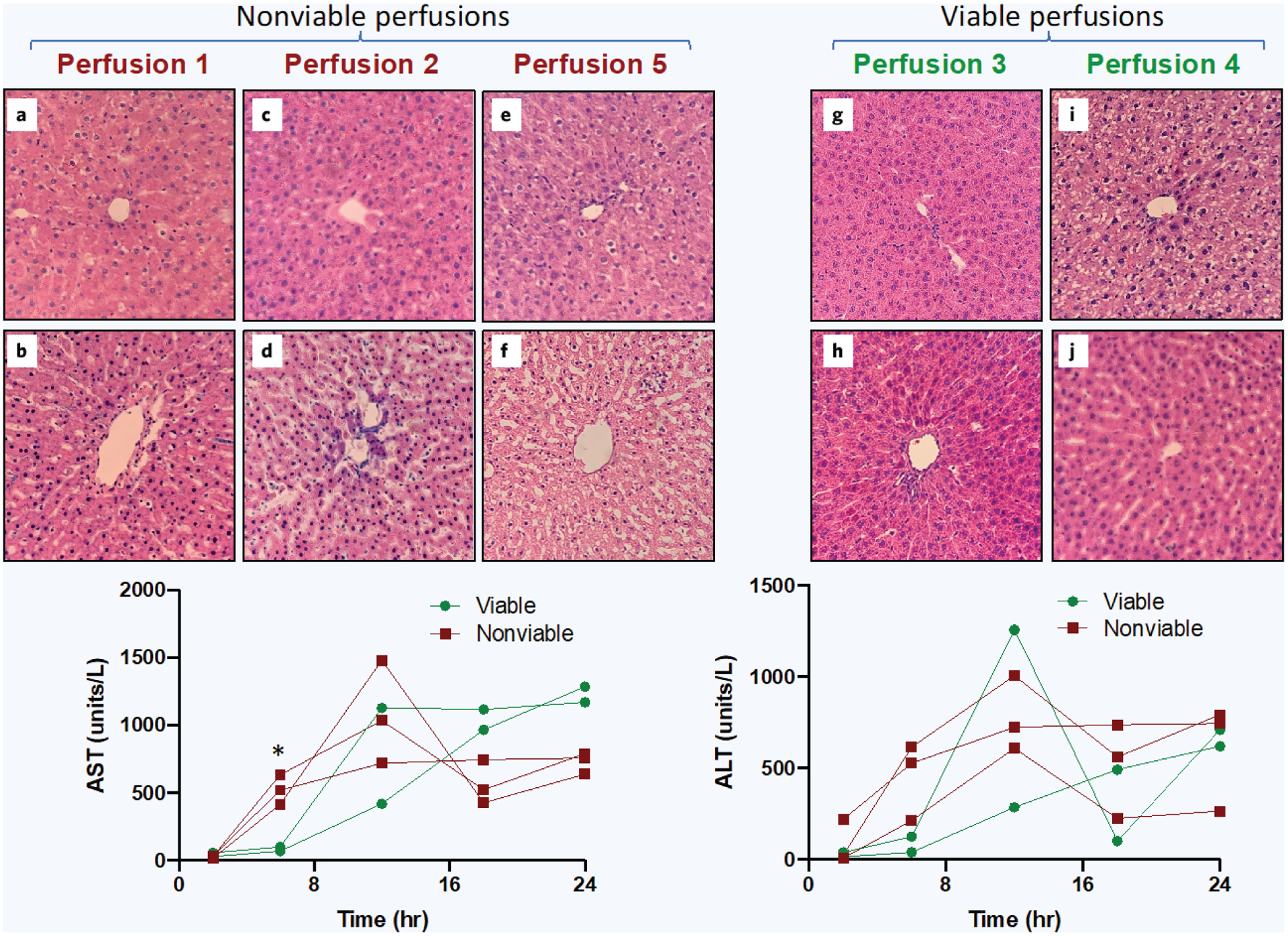
20× H&E staining of rat liver parenchyma before and after 24-hour normothermic machine perfusion with corresponding AST/ALT levels between viable and nonviable perfusions. a, c, e, g, and i show H&E at T = 0 hours (before perfusion). b, d, f, h, and j show the corresponding liver grafts H&E at T = 24 hours (after perfusion). Blinded histology revealed perfusions 1, 2, and 5 were nonviable and perfusions 3 and 4 were viable. Perfusion metrics revealed a statistically significant difference in 6-hour AST levels between viable and nonviable groups (p value 0.013), *significant difference, p < 0.05.
However, after 24 hours of perfusion, there was a large variation in histology among the five uncontaminated perfusion liver grafts. Perfusions 1, 2, and 5 showed structural damage to hepatocytes with no clear demarcation between nuclear envelope and the nucleolus Fig. 5b). As hepatocytes and endothelial cells underwent cell death, the sinusoids expanded (Fig. 5d). We also observed swelling of hepatocytes from the constant perfusion pressure (Fig. 5f). Perfusions 3 and 4 on the other hand exhibited normal post-perfusion histology (Fig. 5h, j). Based on blinded histology assessment, uncontaminated perfusions 1, 2, and 5 were labeled nonviable and perfusions 3 and 4 were labeled viable.
The major early difference in perfusion injury indicators between nonviable and viable liver grafts based on histology was AST and ALT levels at the 6-hour timepoint. The average AST level at 6 hours in the nonviable group was 523.3 u/L versus only 85.0 u/L in the viable group (p value 0.013). Regarding ALT, the average 6-hour level in the nonviable group was 452.3 u/L versus only 82.5 u/L in the viable group. However, due to the small sample size, the unpaired, two-tailed t-test did not show a statistically significant difference (p value 0.104). The remaining early perfusion metrics (2 and 6 hours) also did not show any significant differences between viable and nonviable groups (Fig. 5).
Comparison of uncontaminated and contaminated 24-hour perfusions
Out of eight total experiments, five liver grafts reached 24 hours without contamination (labeled perfusions 1 through 5) and three were compromised by bacterial contamination. These five uncontaminated 24-hour normothermic perfusions were compared to the three contaminated (failed) attempts. From this analysis, there were significant differences in both resistance and oxygen consumption. Resistance was comparable between uncontaminated and contaminated attempts at 0 to 12 hours. However, we found a statistically significant difference between resistance at 18 hours (p value 0.0148) and at 24 hours (p value 0.003) (Fig. 6a). Oxygen consumption was significantly higher at every timepoint for uncontaminated perfusions compared to contaminated attempts (Fig. 6b).
Figure 6.
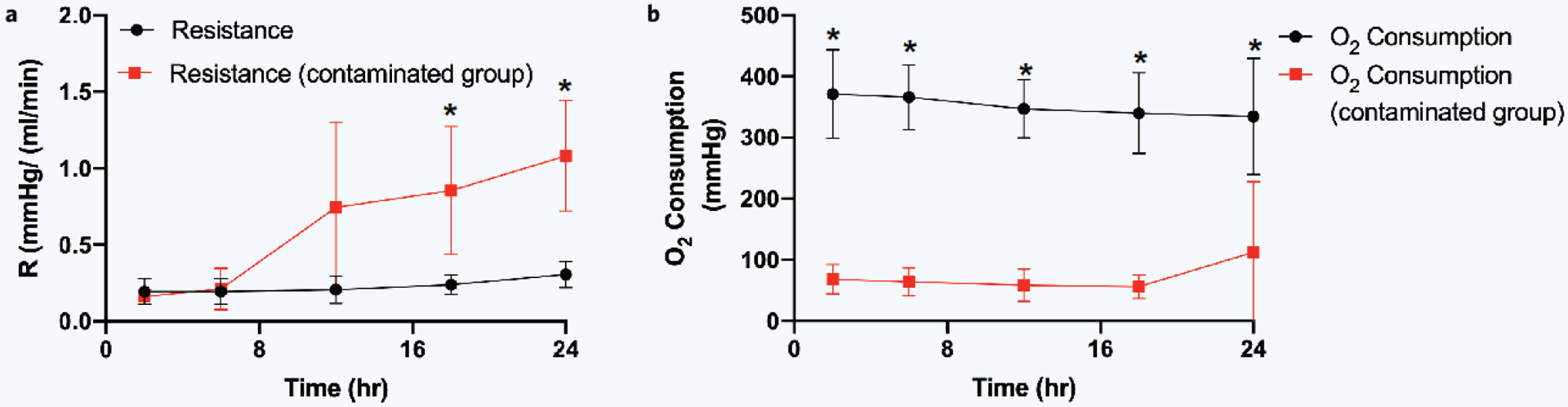
Differences between contaminated and uncontaminated perfusions. (a) Resistance (pressure over flow rate) comparison, (b) oxygen consumption (inflow pO2 minus outflow pO2) comparison (*significant difference, p < 0.05). Error bars represent standard deviations.
DISCUSSION
This research was the first study to our knowledge that maintained a rat liver allograft on NMP for 24 hours. The purpose was to create a platform to trial long-term pharmacologic interventions and study liver disease without the cost and manpower required in lengthy large animal or human perfusions. Out of eight total normothermic perfusions, three were contaminated and five were carried to 24 hours with intrahepatic pressures less than 12 mmHg, perfusate flow rates greater than 25 mL/min, and no signs of contamination. While this study represents a large increase in rat liver normothermic perfusion time compared to prior studies20,21, there are aspects of our perfusion system that require improvement.
Significant perfusion metrics
First, intrahepatic resistance remained stable, but only through 12 hours. We suspect that after 12 hours, the rise in resistance seen in uncontaminated perfusions 2, 3, and 4 was due to the perfusion pressure shearing endothelial cells, causing obstructions from cellular debris in the small branches of the PV through which the perfusate was being pumped. Further cell release perfusion studies can verify this hypothesis22. In contaminated perfusions, resistance would spike between 12 to 18 hours, and histology would reveal bacteria in the vasculature, further obstructing perfusate flow.
Next, oxygen consumption was significantly different at all timepoints between our uncontaminated versus contaminated perfusions. This finding suggests that the contaminated liver allografts were not being adequately oxygenated from the beginning of perfusion, either due to surgical injury, liver graft quality, or perfusate flow obstruction. This data suggests that oxygen consumption levels less than 100 mmHg can be utilized as an early sign of error within the perfusion circuit. However, once all contaminated issues were addressed, oxygen consumption did not differentiate between viable and nonviable livers (Supplementary Fig. 3a).
Finally, arterial (inflow) lactate was utilized as a measure of liver allograft hypoperfusion Prior studies have shown that lactate rises steadily over 6 hours of rat liver NMP15,23, which our work mirrored as well. A novel finding was that some rat liver grafts were able to clear lactate for the entire 24 hours (uncontaminated perfusions 1 and 5), analogous to human livers on NMP. However, this finding was not consistent, as perfusions 2 and 4 had rising arterial lactates across all timepoints.
Histology
H&E staining of our rat livers before perfusions revealed normal architecture following total hepatectomy and initial flush in all samples, confirming the effectiveness of our surgical technique. There were also no erythrocytes seen on the T = 0 hour histology (before perfusion), indicating the 50 cc LR flush with heparin cleared the vasculature before NMP.
The H&E results after 24-hour perfusions were much more variable. The link between high 6-hour AST and ALT levels and nonviable 24-hour histology (Fig. 5) has several implications. First, it suggests that the first few hours on NMP are the most critical for long-term viability. It is during this time that high flow rates can shear cells, large temperature fluctuations can alter enzymatic pathways, and poor oxygenation can lead to ischemic areas of the liver21. Liver allografts exhibiting rapid increases in transaminases are in shock and should have their perfusion systems carefully assessed for technical errors. These errors can include inflow decannulation, a disruption in oxygenation, kinking of the PV, or torsion of liver lobes. Second, rising transaminases upon starting perfusion underscore the importance of slowly increasing flow rates when placing the allograft on pump to avoid damaging the intrahepatic vasculature.
Unsurprisingly, livers that had high transaminases during the first few hours of machine perfusion also had more apoptosis, necrosis, and distorted architecture on 24-hour H&E staining (nonviable perfusions 1, 2, and 5). Our research suggests that early AST and ALT levels (less than 6 hours) can be utilized as a marker for liver viability on a cellular level in long-term perfusions. This is significant because other injury markers such as resistance do not change until 12–24 hours into machine perfusion, at which point there is little that can be done to reverse allograft damage. The loss of structural architecture in some H&E samples at 24 hours but not in others suggests that hepatocytes and endothelial cells from the wedge biopsies in perfusions 1, 2, and 5 were in the process of dying, whereas those in perfusions 3 and 4 were not. Cell-release assays can be conducted to further characterize these perfusion phenotypes. Finally, the entirety of the liver cannot be assessed through wedge biopsies. Thus, there was some intrinsic variation in histology taken near the PV cannulation site versus where ours were taken at the periphery. Imaging the vascular anatomy following perfusion would be a way to complement our H&E wedge biopsy results.
Contamination
A major limitation in long normothermic perfusions is bacterial contamination. A rat liver bathing in an oxygen- and nutrient-rich perfusate media at 37°C for 24 hours is primed to become contaminated. Bacteria can also be introduced to the liver during procurement, transportation, and handling during the connection to the perfusion pump. In our perfusions, contamination effects manifested between 18 and 24 hours, due to three likely sources: (i) bacterial contamination of the perfusion system, (ii) bacterial growth in the perfusate, and (iii) obstruction of perfusate flow from cellular debris leading to ischemia. Detailed below is how these sources of contamination can be identified and corrected.
i) Perfusion system contamination
Perfusion systems have several sources of potential bacterial contamination, both on glassware and perfusate tubing. Thus, long-term perfusions should be conducted as sterile as possible. All surgical instruments, glassware, and tubing should be autoclaved after every 24-hour perfusion. Although this change reduces the efficiency perfusions can be run, it helps prevent contamination from prior hepatectomies and experiments. The entire perfusion system should be contained in a sterile hood to minimize disruptions and perfusion errors. Finally, any material that enters the hood should first be cleaned with Isopropanol.
ii) Perfusate contamination
Next, Williams E based perfusates contain amino acids, high levels of glucose, and are heated to 37°C in NMP, ideal conditions for bacteria to proliferate (Supplementary Fig. 1a). Perfusate contamination can be evaluated by centrifuging 100 mL of 24-hour perfusate, collecting the pellet, and using Hoechst DNA staining to image the sample with a cyto-chip disposable. This process allows for visualization of bacterial contamination (Supplementary Fig. 1b,c). Adding anti-anti in addition to penicillin-streptomycin can address perfusate contamination by increasing antibacterial coverage and adding antifungal coverage to the perfusate medium. Another strategy is changing the perfusate every 12 hours, which compromises perfusate metabolomic trends, but provides the liver allograft with fresh nutrients devoid of bacteria and cellular debris. A third measure to prevent contamination of the perfusate is changing perfusate sampling methods. Instead of placing syringes into the perfusate bowl at every timepoint, installing inflow and outflow sampling ports allows for perfusate collection while leaving the liver allograft covered and uninterrupted during the entire perfusion (Fig. 1).
iii) Flow obstruction
Obstruction of perfusate flow secondary to cellular debris makes ischemic liver parenchyma more susceptible to bacterial contamination. Using larger Sprague Dawley adult rats with larger hepatic vasculature can address this issue and makes technical PV cannulation easier.
If larger rats are used, it is important to increase the amount of post-hepatectomy flush (50 cc LR with heparin) to ensure debris and clot are removed before starting NMP. Next, it is important to slowly increase the flow rates once on NMP. Increasing the flow rates too quickly may shear off endothelial cells, and the resulting cellular debris can obstruct perfusate flow. The tradeoff of lower flow rates in the critical first hour of perfusion is failure to adequately perfuse the liver periphery. However, contamination is often a greater threat to long-term perfusion. In addition, once flow rates reach the goal of 30 mL/min with normal intrahepatic pressure (less than 12 mmHg), very little flow manipulation is required for the remainder of the perfusion. The combination of all these interventions can help overcome contamination issues through 24 hours (Supplementary Fig. 1d).
Hepatic artery cannulation
Finally, a technical limitation of this model that might contribute to poor perfusion is lack of hepatic artery cannulation. The hepatic artery contributes 1/6 of the blood flow to the rat liver24, which could become significant over 24 hours. The lack of hepatic artery cannulation, coupled with imperfect perfusion of the PV through variations in surgical cannulation can result in lobes that become ischemic over a 24-hour perfusion. Once a liver is on pump, there is little that can be done to revive the dying lobe, but that segment releases dying cells, potassium, and lactate into the perfusion system, which affects surrounding tissue. A possible method to perfuse the hepatic artery for long-term NMP could be to cannulate the aorta and ligate all other arterial branches.
Future directions
Incorporating advanced technologies such as the parallel dialysis machine and standardizing perfusion circuits are the next steps required to prime the rat liver perfusion field to study liver disease and trial pharmacologic agents across multiple labs. Nosser et al. developed a highly specialized rat liver NMP system using 50 mL of perfusate, a high erythrocyte concentration, and a novel parallel dialysis machine in the circuit. Their perfusion metrics showed steadily increasing liver enzymes, rising perfusion pressures after 6 hours, and increased potassium concentrations in their perfusate over time20. Tolboom et al. also showed effective use of a secondary dialysis circuit with a 2200-cm2 membrane area and a 30-kDa nominal molecular weight cutoff. The dialysis medium consisted of phenol red-free Williams E, insulin, penicillin, streptomycin, L-glutamine, hydrocortisone, and heparin. Even with this secondary circuit in place, AST and ALT increased throughout the 6-hour perfusion, analogous to our findings19. Furthermore, adding these devices to a perfusion circuit increases the cost and complexity of each perfusion.
CONCLUSIONS
Perfusion technology is rapidly expanding, and with it comes the potential for a new era in understanding liver physiology. Our 24-hour rat liver normothermic perfusion system was able to overcome issues with high resistance, low oxygen consumption, and contamination. Specifically, contamination prevention strategies need to consider the perfusate, the perfusion system, and preserving vascular integrity to maximize success in long-term rat liver perfusions. However, there is still need for improvement. Insights from these experiments reveal perfusate transaminase levels and liver allograft oxygen consumption are early predictive markers for long-term perfusion success. Our hope is this work will enable researchers to efficiently assess pharmacologic interventions and subsequent liver physiology on normothermic machine perfusion for longer periods of time in the rat model before having to spend costly resources on large animal or human perfusions.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded from the US National Institutes of Health (NSF ATP-Bio ERC grant (NSF 1941543), R01DK096075, R01DK114506, R01DK107875). Further, we gratefully acknowledge research support to Omar Haque by the American Liver Foundation (2019 Hans Popper Memorial Postdoctoral Research Fellowship) and the American College of Surgeons (Grant number 1123–39991 scholarship endowment fund).
Footnotes
CONFLICT OF INTEREST
The authors declare competing financial interests. Drs. Reinier de Vries and Korkut Uygun have provisional patent applications relevant to this study. Dr. Korkut Uygun has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Dr. Korkut Uygun’s interests are managed by the Mass General Brigham in accordance with their conflict of interest policies.
REFERENCES
- 1.Detelich D & Markmann JF The dawn of liver perfusion machines. Curr. Opin. Organ. Transplant 2, 151–161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchwald JE, Xu J, Bozorgzadeh A & Martins PN Therapeutics administered during ex vivo liver machine perfusion: An overview. World J. Transplant 1, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakai T, Tanimura H, Hirokawa F & Tamaki T Altered hepatic hemodynamics and improved liver function following intrahepatic vascular infusion of prostaglandin E1. J. Gastroenterol 3, 362–367 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Imber CJ, St. Peter, S.D., Handa, A. & Friend, P.J. Hepatic steatosis and its relationship to transplantation. Liver Transplant. 5, 415–23 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Winbladh A et al. N-acetyl cysteine improves glycogenesis after segmental liver ischemia and reperfusion injury in pigs. Scand J. Gastroenterol 2, 225–236 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Herrera MB et al. Human liver stem cells improve liver injury in a model of fulminant liver failure. Hepatology. 1, 311–319 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Liu Q et al. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant. Proc 9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raigani S et al. Improvement of steatotic rat liver function with a defatting cocktail during ex situ normothermic machine perfusion is not directly related to liver fat content. PLOS ONE 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissenbacher A, Vrakas G, Nasralla D & Ceresa CDL The future of organ perfusion and re-conditioning. Transpl. Int 6, 586–597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nevzorova Y, Tolba R, Trautwein C & Liedtke C Partial hepatectomy in mice. Lab. Anim 1, 81–88 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Michalopoulos G Liver regeneration. J. Cell Physiol 276, 60–66 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madrahimov N, Dirsch O, Broelsch C & Dahmen U Marginal Hepatectomy in the Rat. Ann. Surg 244, 89089 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filipponi F et al. A new technique for total hepatectomy in the pig for testing liver support devices. Surgery 125, 448–455 (1999). [PubMed] [Google Scholar]
- 14.Nasralla D et al. A randomized trial of normothermic preservation in liver transplantation. Nature 577, 50–56 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Izamis ML et al. Resuscitation of ischemic donor livers with normothermic machine perfusion: A metabolic flux analysis of treatment in rats. PLOS ONE 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.op den Dries S et al. Normothermic machine perfusion reduces bile duct injury and improves biliary epithelial function in rat donor livers. Liver Transplant. 22, 944–1005 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Tolboom H et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplant. 87, 170–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolboom H et al. Sequential cold storage and normothermic perfusion of the ischemic rat liver. Transplant. Proc 40, 1306–1309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolboom H et al. A model for normothermic preservation of the rat liver. Tissue Eng. 13, 2143–2151 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Nösser M et al. Development of a rat liver machine perfusion system for normothermic and subnormothermic conditions. Tissue Eng. - Part A 26, 57–65 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Raschzok N et al. Evaluation of a system for normothermic and subnormothermic machine perfusion of the rat liver. J. Am. Coll. Surg 225 (2017). [Google Scholar]
- 22.de Vries RJ et al. Cell release during perfusion reflects cold ischemic injury in rat livers. Sci. Rep 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izamis ML et al. , Machine perfusion enhances hepatocyte isolation yields from ischemic livers. Cryobiology. 71, 244–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vdoviaková K et al. Importance rat liver morphology and vasculature in surgical research. Med. Sci. Monit 22, 4716–4728 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


