ABSTRACT
Antibody-drug conjugates (ADCs) are a rapidly expanding class of biotherapeutics that utilize antibodies to selectively deliver cytotoxic drugs to the tumor site. As of May 2021, the U.S. Food and Drug Administration (FDA) has approved ten ADCs, namely Adcetris®, Kadcyla®, Besponsa®, Mylotarg®, Polivy®, Padcev®, Enhertu®, Trodelvy®, Blenrep®, and Zynlonta™ as monotherapy or combinational therapy for breast cancer, urothelial cancer, myeloma, acute leukemia, and lymphoma. In addition, over 80 investigational ADCs are currently being evaluated in approximately 150 active clinical trials. Despite the growing interest in ADCs, challenges remain to expand their therapeutic index (with greater efficacy and less toxicity). Recent advances in the manufacturing technology for the antibody, payload, and linker combined with new bioconjugation platforms and state-of-the-art analytical techniques are helping to shape the future development of ADCs. This review highlights the current status of marketed ADCs and those under clinical investigation with a focus on translational strategies to improve product quality, safety, and efficacy.
KEYWORDS: Antibody–drug conjugate (adc), monoclonal antibody (mAb), cytotoxic payload, bioconjugation, cancer therapy, analytics, product quality
Introduction
Antibody-drug conjugates (ADCs) are a rapidly expanding class of anticancer therapeutics, consisting of an antibody attached, via a chemical linker, to a potent cytotoxic agent also named as “payload.” The antibody is designed to target a specific antigen (receptor) that is highly expressed in tumor cells. ADCs deliver a drug with high selectivity to tumors, thereby minimizing their systemic exposure, potentially leading to an improved therapeutic index (greater efficacy and less side effects). The majority of ADCs follow a similar mode of action that involves antibody mediated receptor binding, ADC internalization, and subsequent payload release and execution of cytotoxicity (Figure 1). The success of ADCs relies on several critical factors: 1) target antigens (e.g., CD30, HER2, CD22, CD33 CD79b, Nectin 4, trophoblast-cell surface antigen 2 (Trop2), B-cell maturation antigen (BCMA), CD19), 2) type of antibody (e.g., IgG1, IgG2, IgG4, nanobody, bispecific antibody), 3) type of payload (e.g., monomethyl auristatin E (MMAE), DM4, calicheamicin, DM1, monomethyl auristatin F (MMAF)), 4) type of linker (e.g., valine-citrulline, Sulfo-SPDB, hydrazone linker), 5) conjugation platform (e.g., lysine-, cysteine-, and site-specific conjugation), 6) target indications (e.g., breast cancer, lymphoma, leukemia, urothelial cancer, lung cancer, ovarian cancer). The complexity of ADCs requires state-of-the-art analytical techniques to adequately characterize and control product quality and manufacturing consistency. This review highlights the recent advances in the clinical development of ADCs and the translational strategies associated with ADC manufacture and quality assessment. Strategies to reduce toxicities of ADCs, including dosing regimens and payload-linker optimization, have been extensively discussed in previous reports,1, 2 and are not within the scope of this review.
Figure 1.
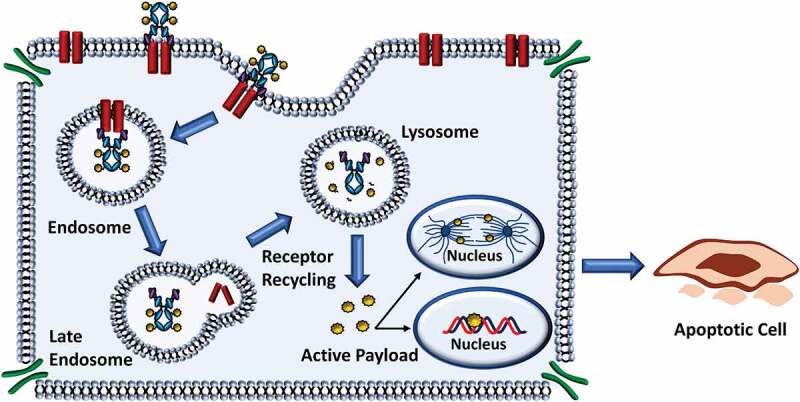
Cellular Processing of ADCs. Most ADCs undergo similar mechanisms to release the cytotoxic payload. In general, ADCs are designed for internalization and are processed via the endocytic pathway resulting in release of the payload and cytotoxic effect
The clinical pipeline for ADCs
To date, ten ADCs have been approved by the FDA, namely Adcetris®, Kadcyla®, Besponsa®, Mylotarg®, Polivy®, Padcev®, Enhertu®, Trodelvy®, Blenrep®, and Zynlonta™, with exclusively oncology indications (Table 1, Figure 2a). In addition, more than 80 ADCs are currently under active clinical development as monotherapy or combinational therapy for the treatment of various tumor types.
Table 1.
ADCs approved for clinical use
| ADC | Target | Antibody | Linker | Payload | Indication | Manufacturer | Approval Year |
|---|---|---|---|---|---|---|---|
| Adcetris® | CD30 | Chimeric IgG1 | Valine-citrulline | MMAE | Previously untreated stage III or stage IV classical Hodgkin’s Lymphoma (cHL); relapsed or refractory cHL; cHL after failure of auto-HSCT or failure of at least two prior multi-agent chemotherapy regimens; systemic anaplastic large cell lymphoma, primary cutaneous anaplastic large cell lymphoma other CD30-expressing peripheral T-cell lymphomas | Seattle Genetics (Seagen) | 2011 |
| Kadcyla® | HER2 | Humanized IgG1 | SMCC | DM1 | HER2-positive, metastatic breast cancer | Genentech | 2013 |
| Besponsa® | CD22 | Humanized IgG4 | ActBut | Calicheacmicin | Monotherapy in adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) | Pfizer | 2017 |
| Mylotarg® | CD33 | Humanized IgG4 | ActBut | Calicheacmicin | Single-agent and combinational therapy in newly-diagnosed CD33-positive acute myeloid leukemia (AML) in adults and relapsed or refractory CD33-positive AML in adults and pediatric patients (≥2 years). | Pfizer | 2000; 2017 |
| Polivy® | CD79b | Humanized IgG1 | Valine-citrulline | MMAE | Combinational use with bendamustine and a rituximab product in adult patients with relapsed or refractory diffuse B-cell lymphoma (DBCL) | Genentech | 2019 |
| Padcev® | Nectin-4 | Human IgG1 | Valine-citrulline | MMAE | Adult patients with locally advanced or metastatic urothelial cancer |
Astellas Pharma, inc. | 2019 |
| Enhertu® | Her2 | Humanized IgG1 | Tetrapeptide | exatecan-derivative topoisomerase I inhibitor (DXd) | Adult patients with unresectable or metastatic HER2-positive breast cancer |
Daiichi Sankyo | 2019 |
| Trodelvy® | Trop-2 | Humanized IgG1 | Hydrolysable CL2A | SN-38 Topo I inhibitor | Adult patients with metastatic triple-negative breast cancer who have received at least two prior therapies for metastatic disease. |
Immunomedics | 2020 |
| Blenrep® | BCMA | Humanized IgG1 | maleimidocaproyl | MMAF | Adult patients with relapsed or refractory multiple myeloma who have received at least 4 prior selected therapies |
GSK | 2020 |
| Zynlonta™ | CD19 | Humanized IgG1 | Valine-alanine | SG3249 PBD dimer | adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma | ADC Therapeutics | 2021 |
Each ADC listed has been approved for treatment of oncological indications in the clinical setting.
4-(4′-acetylphenoxy) butanoic acid (AcBut); 7-ethyl-10-hydroxycamptothecin (SN-38); Antibody–drug conjugate (ADC); B-cell maturation antigen (BCMA); classical Hodgkin’s lymphoma (cHL); GlaxoSmithKline (GSK); Mertansine (DM1); Monomethyl auristatin E (MMAE); Monomethyl auristatin F (MMAF); Pyrrolobenzodiazepine (PBD); Succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC); Trophoblast-cell surface antigen 2 (Trop2)
Figure 2.
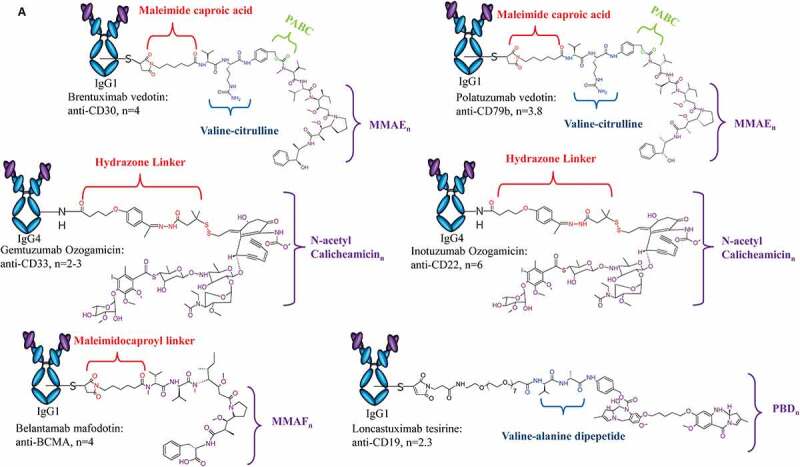
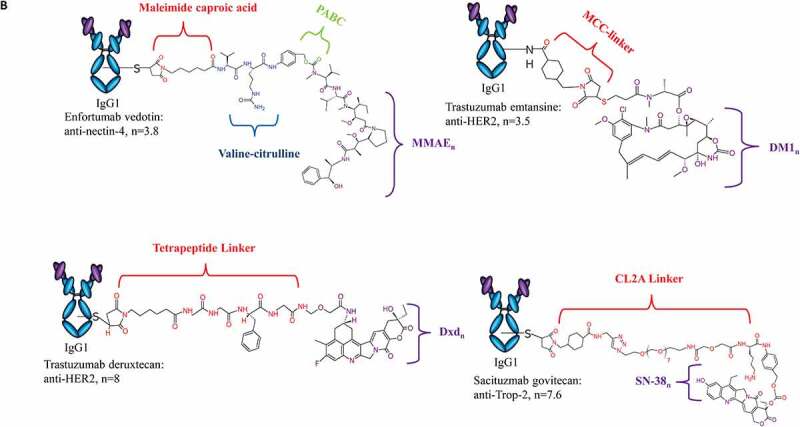
Structure of ADCs Approved for Clinical Use. Design of each approved ADC, highlighting antibody isotype, linker chemistry, payload class and DAR are provided. (A) ADCs approved for hematological malignancies include Adcetris®, Polivy®, Mylotarg®, Besponsa®, Blenrep®, and Zynlonta™. (B)ADCs approved for solid tumors include Kadcyla®, Padcev®, Enhertu® and Trodelvy®
Figure 2.
Continued
FDA Approved ADCs
Of the ten ADCs approved for clinical use (Table 1), six are indicated for treatment of hematological malignancies. Brentuximab vedotin (Adcetris®) is an ADC produced by Seattle Genetics (now known as Seagen). The anti-CD30 (cAC10) ADC consists of ~4 MMAE molecules conjugated through cysteines of reduced interchain disulfide bonds via a protease-cleavable linker (Figure 2a). 3 Brentuximab vedotin was granted accelerated approval in 2011 and full approval in 2015 for the treatment of classical Hodgkin’s lymphoma, systemic anaplastic large cell lymphoma, and peripheral T-cell lymphoma.
In 2017, inotuzumab ozogamicin (Besponsa®), a Pfizer product, was approved for treatment in adults with relapsed or refractory (R/R) B-cell precursor acute lymphoblastic leukemia. The ADC targets the CD22 surface marker using an IgG4 to deliver approximately 6 calicheamicin molecules. The payload is conjugated via surface-exposed lysines using an acid-labile linker.4 This design nearly mirrors that of gemtuzumab ozogamicin (Mylotarg®), an anti-CD33 ADC with an average of 2–3 calicheamicin payloads conjugated to the antibody. Gemtuzumab ozogamicin was the first ADC to receive accelerated approval in 2000, contingent on fulfilling the post-marketing requirement of a randomized trial to confirm clinical benefit (S0106; NCT00085709) (Figure 2a).5,6 However, the trial did not confirm the clinical benefit but instead raised safety concerns due to an increase in treatment-related fatalities compared to the control group receiving standard chemotherapy. The leading causes of fatality in the treatment arm were associated with infection and hemorrhage. Ultimately, the results of the trial led to the voluntary withdrawal of the application by Pfizer in 2010.7,8 Following modifications to the dosing schedule, which was associated with decreased incidence of hepatotoxicity and early mortality, and to address the critical unmet need for acute myeloid leukemia patients, gemtuzumab ozogamicin was granted approval again in 2017.9
Between 2019 and 2021, the FDA granted accelerated approval of polatuzumab vedotin (Polivy®) for R/R diffuse large B-cell lymphoma (DLBCL), belantamab mafodotin (Blenrep®) for R/R multiple myeloma, and loncastuximab tesirine (Zynlonta™) for R/R B-cell lymphoma, respectively. Polatuzumab vedotin is an ADC produced by Genentech with an average of 3.5 MMAE molecules conjugated to cysteines of reduced interchain disulfide bonds on an anti-CD79b antibody (Figure 2a).10 Belantamab mafodotin is a first-in-class anti-BCMA ADC produced by Astellas Pharma, Inc. The ADC carries approximately 4 MMAF molecules conjugated via a non-cleavable linker to the cysteines of the afucosylated anti-BCMA antibody (Figure 2b). Belantamab mafodotin exhibits versatile mechanisms of action (MOA), including inducing cell death by delivering the MMAF molecules to the target cell and inducing both antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).11 Loncastuximab tesirine, or Lonca-T, is an anti-CD19 ADC manufactured by ADC Therapeutics for R/R B-cell lymphomas, including DLBCL.12 The ADC is the latest to be approved as of May 2021 and is the first to carry the pyrrolobenzodiazepine (PBD) dimer toxin (indicated as SG3249). Approximately 2.3 SG3249 molecules are attached to the antibody via a cathepsin-cleavable valine-alanine linker, facilitating DNA minor groove interstrand crosslinking of target cells following payload release (Figure 2a).
The remaining four ADCs are approved for treatment of solid tumors, including three for breast cancers and one for urothelial cancer. Ado-trastuzumab emtansine (Kadcyla®), also notated as T-DM1, is a conjugation of ~3.5 maytansinoid DM1 molecules to the anti-HER2 antibody trastuzumab via surface-exposed lysines (Figure 2b).13 T-DM1, produced by Genentech, received FDA approval in 2013 for the treatment of HER-2 positive metastatic breast cancer (mBC), with additional approved uses including monotherapy and combinational administration as well as an adjuvant treatment for early breast cancer. Similar to belantamab mafodotin, T-DM1 induces cell death by release of the payload and ADCC activity retained in the parent mAb that inhibits HER2-signaling.14 Despite such anti-tumor activity, resistance to T-DM1 remains a challenge and will be discussed further in later sections.15
To address this barrier, trastuzumab deruxtecan (Enhertu®), an anti-HER2 ADC with several unique properties, was approved in 2019. Produced by Daiichi Sankyo for the treatment of HER2-positive metastatic breast cancer following a prior trastuzumab-based regimen, trastuzumab deruxtecan (T-Dxd) uses the same parent IgG1 antibody as T-DM1, but it is conjugated to approximately 8 molecules of an exatecan-derivative topoisomerase I inhibitor, Dxd, via a protease-cleavable tetrapeptide linker (Figure 2b).16 Release of the payload and influx into neighboring tumor cells exerts anti-tumor activity in heterogenous cell populations with varying levels of HER2 expression (high or low).17
Another ADC recently approved for breast cancer is sacituzumab govitecan (Trodelvy®), an anti-Trop2 ADC produced by Immunomedics.18 Sacituzumab govitecan is a first-in-class ADC for the treatment of metastatic triple-negative breast cancer (TNBC) in patients who have received two prior treatments for metastatic disease including chemotherapy, targeted, or immunotherapy. Sacituzumab govitecan is another example of an ADC product with a high drug-to-antibody ratio (DAR), consisting of ~7.6 SN-38 molecules, a moderately toxic topoisomerase I inhibitor, using a novel, hydrolysable linker called CL2A through cysteines (Figure 2b).18 SN-38 is the active drug form of the clinically used anticancer agent, CPT-11 or irinotecan. Interestingly, SN-38 was found to be more potent than CPT-11, but less potent than cytotoxic agents conventionally used in ADCs, including calicheamicin and MMAE derivatives.19 The use of moderately toxic payloads is being investigated as a method to increase payload concentration and overcome the challenges of stability and efficacy with higher DAR ADCs.
Of the four ADCs approved for solid tumors, enfortumab vedotin (Padcev®) is the only product approved for a solid tumor aside from breast cancer. Produced and marketed through a collaboration between Astellas Pharma Inc. and Seagen, enfortumab vedotin is a first-in-class therapeutic indicated for the treatment of Nectin-4 positive urothelial cancer.20 The ADC consists of a human IgG1 against nectin-4, a member of the nectin family of immunoglobulin-like adhesion molecules known to mediate Ca+-independent cell–cell adhesion through the recruitment of cadherins and modulation of cytoskeletal arrangements (Figure 2b).21 Approximately 3.8 MMAE molecules are conjugated through cysteines via the same cleavable linker technology previously used in other ADCs produced by Seagen.20
Novel ADCs in clinical trials
More than 80 ADCs are currently in active clinical trials, with a majority in phase I and I/II (Table 2, Figure 3a). Over 80% of the clinical trials are investigating ADC safety and efficacy in various solid tumors, while the remaining trials involve hematological malignancies (Figure 3b). This may suggest a shift in recent years toward investigational ADCs for solid tumors following the earlier success of T-DM1 and recent approvals of T-Dxd, sacituzumab govitecan, and enfortumab vedotin. Of this list, there are approximately 40 different targets with several ADCs against the same target (Table 2, Figure 3c).
Table 2.
ADCs currently under clinical investigation
| Cytotoxic Payload | ADC | Target | Conjugation | Phase | Conditions | Clinical Trial | Reference |
|---|---|---|---|---|---|---|---|
|
Tubulin disruptor/ anti-mitotic |
ASN004 | 5T4 | Cysteine | I | Advanced solid tumors | NCT04410224 | 22 |
| IMGC936 | ADAM9 | Site-specific | I | Advanced solid tumors | NCT04622774 | 23 | |
| ABGN-107 | AG7 | Undisclosed | I | Gastric, Colorectal, Pancreatic or Biliary Cancer | NCT02908451 | 24 | |
| AGS-16C3F | ENPP3 | Cysteine | II | Metastatic Renal Cell Carcinoma | NCT02639182 | 25 | |
| HUMAX-AXL-ADC | AXL | Cysteine | I/II | Ovarian Cancer, Cervical Cancer, Endometrial Cancer, Non-Small Cell Lung Cancer (NSCLC), Thyroid Cancer. and Melanoma Sarcoma | NCT02988817 | 26 | |
| BA3011 | AXL | Undisclosed | II I/II | NSCLC Solid tumors | NCT04681131 NCT03425279 | 27 | |
| CX-2009 | CD166 | Undisclosed | II | Advanced Breast Cancer |
NCT03149549 NCT04596150 |
||
| OBT076 | CD205 | Cysteine | I | Breast Cancer | NCT04064359 | 28 | |
| TRPH-222 | CD22 | Site-specific | I | R/R B-Cell Lymphoma | NCT03682796 | 29 | |
| SGN-CD228A | CD228 | Cysteine | I | Advanced solid tumors | NCT04042480 | 30 | |
| F0002-ADC | CD30 | Lysine | I | R/R hematologic malignancies | NCT03894150 | 31 | |
| Debio 1562 | CD37 | Lysine | II | R/R Diffused large B-cell lymphoma (DLBCL) and other forms of non-Hodgkin lymphoma | NCT02564744 | 32 | |
| STI-6129 | CD38 | Site-specific | I | R/R Systemic AL Amyloidosis | NCT04316442 | ||
| FOR46 | CD46 | Cysteine |
I I |
R/R Multiple myeloma (MM) Metastatic castration-resistant prostate cancer |
NCT03650491 NCT03575819 |
33 | |
| IMGN-901 | CD56 | Lysine | II | R/R Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, or synovial sarcoma | NCT02452554 | 34 | |
| CX-2029 | CD71 | Undisclosed | I/II | Solid tumors or DLBCL | NCT03543813 | 35 | |
| STRO-001 | CD74 | Site-specific | I | Advanced B-cell malignancies | NCT03424603 | 36 | |
|
SAR408701 |
CEACAM5 |
Lysine |
II II II I III I/II |
Advanced solid tumors Non-squamous NSCLC |
NCT04659603 NCT04524689 NCT04394624 NCT03324113 NCT04154956 NCT02187848 |
37 | |
|
ABBV-399 |
c-Met |
Cysteine |
II I II |
Advanced solid tumors Non-squamous NSCLC |
NCT03574753 NCT02099058 NCT03539536 |
38 | |
| RC108 | c-Met | Undisclosed | I | Advanced malignant solid tumors | NCT04617314 | ||
| ABT-414 | EGFR | Cysteine | II/III | Glioblastoma | NCT02573324 | 39 | |
| MRG003 | EGFR | Undisclosed |
II II II |
Recurrent or metastatic squamous cell carcinoma of head and neck, unresectable, locally advanced or metastatic biliary tract cancer, and advanced NSCLC |
NCT04838548 NCT04838964 NCT04868162 |
40 | |
| STRO-002 | FolRα | Site-specific | I | Ovarian and endometrial cancers | NCT03748186 | 41 | |
| MORAB-202 | FolRα | Cysteine |
I/II I |
Solid tumors |
NCT04300556 NCT03386942 |
42 | |
| IMGN853 | FolRα | Lysine |
II II III II I III I II I/II |
Endometrial, epithelial ovarian, fallopian tube, primary peritoneal, and triple negative breast cancers |
NCT03832361 NCT03835819 NCT04296890 NCT04274426 NCT03552471 NCT04209855 NCT02996825 NCT04606914 NCT02606305 |
43 | |
| CDX-011 | gpNMB | Cysteine | II | Recurrent or refractory osteosarcoma | NCT02487979 | 44 | |
| OBI-999 | Globo H | Site-specific | I/II | Advanced solid tumor | NCT04084366 | 45 | |
| PF-06804103 | HER2 | Site-specific | I | Solid tumors | NCT03284723 | 46 | |
| ZW49 | HER2 | Undisclosed | I | HER2-expressing tumors | NCT03821233 | 47 | |
| RC48 | HER2 | Cysteine |
II I I/II II II I/II II III II I/II III |
Metastatic breast, gastric, biliary tract, and urothelial cancers |
NCT04329429 NCT04280341 NCT04311034 NCT03809013 NCT04073602 NCT04264936 NCT03556345 NCT04400695 NCT03500380 NCT03052634 NCT04714190 |
48,49 | |
| ALT-P7 | HER2 | Site-specific | I | Breast cancer | NCT03281824 | 50 | |
| ARX788 | HER2 | Site-specific |
I II |
Breast and stomach neoplasms |
NCT03255070 NCT04829604 |
51 | |
| FS-1502 | HER2 | Undisclosed | I | Advanced solid tumors and metastatic breast cancer | NCT03944499 | ||
| A166 | HER2 | Site-specific | I/II | R/R HER2-expressing cancers | NCT03602079 | 50 | |
| MRG002 | HER2 | Undisclosed |
I/II II |
Advanced solid tumors, metastatic gastric/gastroesophageal junction cancer, and advanced metastatic breast cancer |
NCT04492488 NCT04742153 |
52 | |
| BAT8001 | HER2 | Undisclosed |
I/II I III |
Advanced breast cancer |
NCT04151329 NCT04189211 NCT04185649 |
53 | |
| W0101 | IGF-1 R | Cysteine | I/II | Advanced or metastatic solid tumors | NCT03316638 | 54 | |
| SGN-B6A | integrin-beta6 | Undisclosed | I | Advanced solid tumors | NCT04389632 | 55 | |
| SGN-LIV1A | LIV-1 | Cysteine |
I/II I II I I |
Advanced or metastatic triple negative breast cancer |
NCT03310957 NCT01969643 NCT04032704 NCT03424005 NCT01042379 |
56 | |
| BAY 94–9343 | Mesothelin | Lysine |
II I I/II I/II I/II |
R/R ovarian, fallopian tube, or primary peritoneal cancers, advanced pancreatic cancer, and pleural mesothelioma |
NCT03926143 NCT03102320 NCT03126630 NCT03587311 NCT03816358 |
57 | |
| BMS-986148 | Mesothelin | Undisclosed | I/II | Advanced solid tumors | NCT02341625 | 58 | |
| RC88 | Mesothelin | Undisclosed | I | Advanced solid tumors | NCT04175847 | ||
| XMT-1536 | NaPi2b | Cysteine |
I I/II |
Ovarian cancer and NSCLC |
NCT03319628 NCT04396340 |
59 | |
| XMT-1592 | NaPi2b | Site-specific | I/II | Ovarian cancer and NSCLC | NCT04396340 | 60 | |
| ARX517 | PSMA | Site-specific | I | Advanced solid tumors | NCT04662580 | ||
| VLS-101 | ROR1 | Lysine |
II I |
Solid tumors and hematological malignancies |
NCT04504916 NCT03833180 |
61 | |
| SGN-STV | STn | Undisclosed | I | Advanced solid tumors | NCT04665921 | ||
| HUMAX®-TF-ADC | TF | Cysteine |
II I/II |
Cervical cancer |
NCT03438396 NCT03786081 |
62 | |
| JS108 | Trop2 | Undisclosed | I | Advanced solid tumors | NCT04601285 | ||
| DNA Damaging | SYD1875 | 5T4 | Site-specific | I | Solid tumors | NCT04202705 | |
| MEDI2228 | BCMA | Site-specific | I | R/R MM | NCT03489525 | 63 | |
| IMGN632 | CD123 | Site-specific |
I/II I/II |
Acute lymphocytic leukemia, blastic plasmacytoid dendritic cell neoplasm, myeloproliferative neoplasm, and acute myeloid leukemia |
NCT03386513 NCT04086264 |
64 | |
| ADCT-602 | CD22 | Site-specific | I/II | R/R B-cell acute lymphoblastic lymphoma | NCT03698552 | 65 | |
| ADCT-301 | CD25 | Cysteine |
II I II II |
Acute myeloid lymphoma, myelodysplastic syndrome, myeloproliferative neoplasm, R/R Hodgkin lymphoma, and R/R DLBCL |
NCT04639024 NCT03621982 NCT04052997 NCT03589469 |
66 | |
| MGC018 | CD276 | Cysteine | I/II | Advanced solid tumors | NCT03729596 | 67 | |
| TR1801 | c-Met | Site-specific | I | Solid tumors | NCT03859752 | 68 | |
| ABBV-321 | EGFR | Site-specific | I | Advanced solid tumors | NCT03234712 | 69 | |
| SYD985 | HER2 | Cysteine |
I II I III I |
Metastatic breast cancer and endometrial carcinoma |
NCT04602117 NCT04205630 NCT04235101 NCT03262935 NCT01042379 |
70 | |
| NBE-002 | ROR1 | Site-specific | I/II | Advanced solid tumors | NCT04441099 | 71 | |
| Topo I | DS-7300a | B7-H3 | Cysteine | I/II | Advanced solid tumors | NCT04145622 | |
| DS-6157a | GPR20 | Cysteine | I | Gastrointestinal stromal tumors | NCT04276415 | 72 | |
| U3-1402 | HER3 | Cysteine |
II II I I/II II I |
Metastatic breast, colorectal, and non-small cell lung cancers |
NCT04699630 NCT04479436 NCT03260491 NCT02980341 NCT04619004 NCT04676477 |
73 | |
| DS-1062 | TROP2 | Cysteine |
II I I III I I/II |
NSCLC (advanced or metastatic) and triple negative breast cancer |
NCT04484142 NCT04612751 NCT04526691 NCT04656652 NCT03401385 NCT03742102 |
74 | |
| DS-6000 | CDH6 | Undisclosed | I/II | Renal cell carcinoma and ovarian cancers | NCT04707248 | ||
| SKB264 | TROP2 | Site-specific | I/II | Advanced or metastatic solid tumors | NCT04152499 | 75 | |
| RNA pol II | HDP-101 | BCMA | Site-specific | I/II | R/R MM | NCT04879043 | 76 |
| TLR agonists | BDC-1001 | HER2 | Undisclosed | I/II | HER-2 expressing advanced malignancies | NCT04278144 | |
| SBT6050 | HER2 | Undisclosed | I | Solid tumors | NCT04460456 | 77 | |
| BCL2 family protein inhibitor | ABBV-155 | CD276 | Cysteine | I | R/R solid tumors | NCT03595059 | 78 |
| Undisclosed | CC-99712 | BCMA | Undisclosed | I | R/R MM | NCT04036461 | |
| JBH492 | CCR7 | Undisclosed | I | Chronic lymphocytic leukemia and non-Hodgkin lymphoma | NCT04240704 | ||
| M1231 | EGFR/MUC1 | Undisclosed | I | Solid tumors, metastatic NSCLC, and esophageal squamous cell carcinoma | NCT04695847 | ||
| B003 | HER2 | Undisclosed | I | Metastatic breast cancers | NCT03953833 | ||
| BB-1701 | HER2 | Undisclosed | I | Locally advanced/metastatic solid tumors | NCT04257110 | ||
| DP303c | HER2 | Undisclosed |
II II I |
Advanced ovarian and gastric cancers and solid tumors |
NCT04828616 NCT04826107 NCT04146610 |
||
| GQ1001 | HER2 | Site-specific | I | Advanced solid tumors | NCT04450732 | ||
| SHR-A1811 | HER2 | Undisclosed |
I I/II I |
Advanced gastric or gastroesophageal junction adenocarcinoma, advanced NSCLC and colorectal cancer |
NCT04513223 NCT04818333 NCT04446260 |
||
| ARX517 | PSMA | Site-specific | I | Advanced solid tumors | NCT04662580 | ||
| BA3021 | ROR2 | Undisclosed | I/II | Solid tumors | NCT03504488 | ||
| MRG004A | Tissue factor | Undisclosed | I/II | Advanced or metastatic solid tumors | NCT04843709 | ||
| ABBV-011 | Undisclosed | Undisclosed | I | R/R Small cell lung cancer | NCT03639194 | ||
| SHR-A1904 | Undisclosed | Undisclosed | I | Advanced solid tumors | NCT04877717 |
Each ADC listed is currently under investigation in one or more active clinical trials as of 15 May 2021. The ADCs listed are all registered with clinicaltrials.gov with phase 1-3 trials of “Not yet recruiting”, “Recruiting”, “enrolling by invitation”, and “Active, not recruiting” status investigating use in cancer indications. ADCs marketed for clinical use and developmental ADCs with trials that have been terminated, withdrawn, completed, or are of unknown status were excluded from the table. Disclosed information regarding target, payload action, and conjugation technique are provided or otherwise noted as “Undisclosed”. Data shown was derived from the U.S. National Library of Medicine ClinicalTrials.gov (access date 15 May 2021, search terms of “antibody drug conjugate” and “cancer”)).
Diffuse large B-cell lymphoma (DLBCL); Multiple myeloma (MM); Non-small cell lung cancer (NSCLC); Relapsed and/or refractory (R/R)
Figure 3.
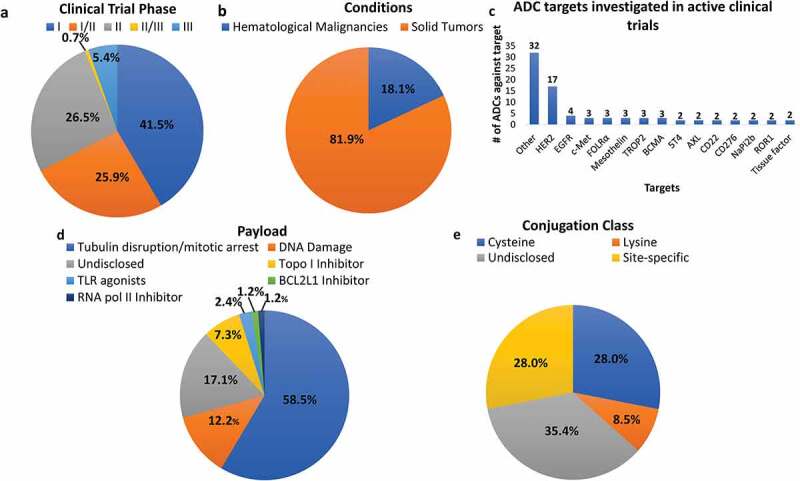
Novel ADCs in Clinical Trials for Oncology. There are currently 82 novel ADCs in 150 active clinical trials registered with clinicaltrials.gov for cancer patients. (a) Most of the ADCs are currently under investigation in phase 1 trials, while a small percentage has advanced to phase 3. (b) Of the 150 ongoing trials, more than 80% are evaluating ADC safety and efficacy in solid tumors whereas less than 20% are trials for hematological malignancies. (c) There are 43 disclosed targets organized here by the number of ADCs designed to recognize them. Most of these targets are under evaluation by a single ADC, while some are being investigated by several different ADCs. (d) Of the 82 novel ADCs, most employ tubulin disrupting payloads, followed by DNA-damaging molecules, topoisomerase I inhibitors, and finally unique payloads such as TLR agonists, a BCL2-xL inhibitor, and an RNA polymerase II inhibitor. Many payloads remain undisclosed. (e) Most ADCs under clinical investigation either utilize the conventional cysteine conjugation strategy or site-specific conjugation platforms while few conjugate to surface lysines. Many techniques remain undisclosed. Data shown was derived from the U.S. National Library of Medicine ClinicalTrials.gov (access date 15 May 2021, search terms of “antibody drug conjugate” and “cancer”)
HER2 is currently one of the most attractive targets for ADC development, with three anti-HER2 ADCs currently in phase III trials. One such anti-HER2 ADC is RC48, produced by RemeGen, joining an IgG1 anti-HER2 antibody, hertuzumab, to approximately four MMAE molecules via a protease-cleavable valine-citrulline linker through cysteine conjugation.48 In preclinical studies, RC48 demonstrated antitumor activity at lower doses in trastuzumab and lapatinib sensitive and resistant xenograft models. Superior inhibition was also observed when compared to T-DM1.48 Early clinical studies have shown a manageable safety profile in multiple phase I trials for HER2-positive malignancies. Notably, RC48 advanced as a potential therapeutic for the treatment of metastatic or unresectable urothelial carcinoma, demonstrating promising results in a phase II pivotal trial (NCT03507166), including an overall response rate (ORR) of 51.2% in pretreated HER-2 positive locally advanced or metastatic urothelial carcinoma.49
Other similarities among ADCs under clinical evaluation include the class of payload. Most use a payload to induce tubulin disruption and mitotic arrest, while a small number cause DNA damage (Figure 3d). Topoisomerase inhibitors, as seen in approved ADCs such as trastuzumab deruxtecan and sacituzumab govitecan, have also begun to appear more frequently in clinical trials, though they still represent a very small percentage. A number of novel payloads are emerging that target specific proteins or receptors. For example, several ADCs, such as the anti-HER2 immune-stimulating antibody conjugate BDC-1001, use a toll-like receptor 7/8 agonist as a payload to elicit immune-mediated tumor efficacy.79 BDC-1001 may activate human myeloid antigen-presenting cells within the tumor environment in addition to inducing ADCC and ADCP functions. Currently, BDC-1001 is being investigated in a phase I/II clinical trial for HER2-expressing solid tumors (NCT04278144). Another novel payload class targets the BCL-xL anti-apoptotic protein. ABBV-155 (mirzotamab clezutoclax) is the sole ADC under investigation that uses this class of payload and is designed to target tumors expressing CD276.78 ABBV-155 is being evaluated in a phase I trial for R/R solid tumors alone or in combination with taxane therapy (NCT03595059). RNA polymerase II inhibitors, such as amanitin derivatives, can halt cellular transcription processes and protein synthesis, resulting in apoptosis and cell death. HDP-101 is a BCMA-targeting ADC utilizing this derivative under clinical evaluation in a phase I/II trial in R/R multiple myeloma patients (NCT04879043).76
Conjugation methodology can directly affect the quality of the ADC, and subsequently the safety and efficacy profiles of the product. There are three main methods of conjugation, including through cysteines of reduced interchain disulfide bonds, surface-exposed lysines, and site-specific techniques. Of the investigational ADCs in active clinical trials, most are manufactured via conventional cysteine conjugation or proprietary site-specific technology licensed by the manufacturers. Only a small portion of ADCs in development use the conventional lysine conjugation methodology, likely due to the vast heterogeneity that can result, as will be discussed in later sections (Figure 3e).
As site-specific conjugation technology can vary among developers, it is worth mentioning several ADCs produced via unique platforms. TRPH-222 is an anti-CD22 ADC conjugated to a maytansinoid payload using the SMARTag™ (Specific Modifiable Aldehyde Recombinant Tag) technology. This platform uses the chemoenzymatic method to engineer a reactive aldehyde (formylglycine) into the mAb for aldehyde-specific conjugation, herein resulting in a controlled maximum DAR of 2.29 TRPH-222 is currently in a phase I trial for R/R B-cell lymphoma, though early results have demonstrated this ADC to be well tolerated (NCT03682796). XMT-1592 is an anti-NaPi2b ADC that is currently under investigation in a phase I/II study for NaPi2b-expressing tumors (NCT04396340). The ADC is produced by Mersana Therapeutics using the Dolasynthen platform that targets the glycan-remodeled Asn297 for site-specific conjugation. The payload auristatin F-hydroxypropylamide (AF-HPA) is membrane-permeable and capable of bystander killing, but it is further metabolized to the membrane-impermeable auristatin F (AF), locking the payload molecules within the cell to achieve “controlled bystander effect” (termed DolaLock).80 Preclinical data showed time-dependent accumulation of both AF-HPA and AF in cultured cancer cell lines and in xenograft tumors.81,82
Several novel antibody platforms are being applied to ADC development strategies. Variations in antibody size, such as the scFv-Fc format used in the ASN004 ADC, could demonstrate an advantage in permeability of solid tumors.22 Two PROBODY drug conjugates (PDCs) are under investigation for tumors expressing CD71 and CD166. Both surface markers are highly expressed in tumor tissues, while also ubiquitously expressed in normal tissues as well. PDCs are masked conjugates that restrict normal tissue recognition and are unmasked by tumor proteases, thereby restricting on-target toxicity outside the tumor site.83,84 CX-2029, an anti-CD71 PDC, is currently under evaluation in a phase I/II trial for solid tumors or DLBCL (NCT03543813). CX-2009 is an anti-CD166 PDC in a phase I/II trial for unresectable solid tumors (NCT03149549) and a phase II trial to assess activity as a monotherapy or combinational therapy in TNBC (NCT04596150).
Challenges in the development of ADCs
Despite the growing number of ADC approvals, challenges remain in the development of ADCs that demonstrate both superior safety and efficacy in the clinic. One unexpected challenge many developers face during clinical evaluation is the inability to demonstrate benefits over the control arm, such as occurred with MM-302. MM-302 was an anti-HER2 mAb conjugated to liposomal doxorubicin.85 The phase II HERMIONE trial (NCT02213744) was designed to determine the benefit of MM-302 treatment with trastuzumab compared to standard care chemotherapy as either gemcitabine, capecitabine, or vinorelbine in HER2-positive locally advanced mBC.86 However, the study was terminated due to lack of benefit over comparator treatments. Another ADC to report similar circumstances was AbbVie’s rovalpituzumab tesirine (Rova-T), which targeted cancer-stem cell-associated delta-like protein 3 (DLL3).87 Rova-T consisted of an IgG1 anti-DLL3 mAb conjugated to two PBD dimers via a valine-citrulline dipeptide linker. The ADC was intended to treat small cell lung cancer, which is known to overexpress DLL3 in 80% of small cell lung cancer patients with no expression on normal tissues.87 Encouraging results in the phase I trial reported 18% ORR in assessable patients and a 38% ORR in patients with high DLL3 expression (NCT01901653), but safety and efficacy concerns were raised due to the results of the phase II trial TRINITY (NCT02674568) in which the primary endpoint was not achieved and high toxicity rates were reported. The most frequent event among patients was pleural effusion, which is considered to be a toxicity associated with PBD dimers.88,89 Ultimately, the results of the phase III trials, TAHOE (NCT03061812) and MERU (NCT03033511), in which a lack of survival benefit over the control arm was observed, led to the complete discontinuation of the development of Rova-T by AbbVie.90
Further challenges in the development of ADCs as therapeutic agents involve toxicities that can be attributed to constituents of the ADC product. Such events have been investigated, mainly focusing on different adverse effects that can be attributed to specific payloads. For example, use of the calicheamicin payload has been associated with increased incidences of liver injury and hepatotoxicity.7,91 Specifically, increased incidences of veno-occlusive disease, also referred to as sinusoidal obstruction syndrome, and drug-induced liver injury were observed during clinical trials and post-approval use of gemtuzumab ozogamicin, despite dose reduction efforts that led to its re-approval in 2017. Similar occurrences have also been observed with the use of inotuzumab ozogamicin.92,93 A comprehensive review published in 2016 summarized key clinical toxicities of other approved and developmental ADCs.1 In general, their findings showed peripheral neuropathy and neutropenia induced by MMAE, which is consistent with adverse events listed for those approved ADCs carrying an MMAE payload. MMAF was associated with ocular toxicities, which is listed as a precaution for the administration of belantamab mafodotin. Differences in clinical toxicities observed between payloads of the same class, e.g., MMAE and MMAF, may indicate linker-associated contributions to these events. Incidences of neutropenia and gastrointestinal system effects have been observed with some ADCs carrying DM1, including T-DM1 and IMGN-901, with increased levels of liver enzymes occurring in some patients administered T-DM1.1,34 Neutropenia may be a common event among ADCs carrying a topoisomerase I inhibitor, as is observed with trastuzumab deruxtecan, sacituzumab govitecan, and even some developmental ADCs such as U3-1402.1,94 Myelosuppression, effusion, and inflammation were observed with ADCs carrying PBD dimers, such as Lonca-T and the previous Rova-T and may require concomitant meditation to reduce the incidence of side effects.69,95 As new payloads continue to emerge in clinical development, clinical data are awaited to understand safety profiles specific to those agents. Understanding events that may be associated with specific payloads can not only aid developers in ADC design but also spur the development of more novel payloads that are less likely to induce harmful events in patients.
Over time, tumors can develop mechanisms to overcome drug efficacy, thereby limiting the success of the treatment. As ADCs are multifunctional therapeutics, some pathways of resistance can develop against individual components of the ADC (Figure 4). One mechanism of resistance could emerge from modulations in antigen recognition by the antibody. This could result from downregulation of the target from the cell surface, rendering the ADC relatively unable to exert their cytotoxic effect.96 Several preclinical studies have generated models of acquired resistance, in which cells consistently treated with the ADC over time eventually showed decreases in target antigen protein expression along with other effects.97,98 In this regard, novel formats of mAbs that can be incorporated into ADCs, such as bispecific or biparatopic mAbs that target two different antigens or nonoverlapping epitopes on the same target antigen, respectively, could aid in overcoming antigen-specific mechanisms of resistance. Li et al. synthesized a biparatopic anti-HER2 ADC conjugated to tubulysin. Preclinical data indicated its ability to restrict tumor growth in four T-DM1-resistant cell lines, though it is not clear whether this is entirely due to the antibody format, or if the novel linker and payloads included in the ADC design contributed as well.99 ZW49, a new biparatopic anti-HER2 ADC, is currently undergoing evaluation in a phase I clinical study (NCT03821233). M1231, a bispecific anti-EGFR/MUC1 ADC is also in a phase I trial (NCT04695847).
Figure 4.
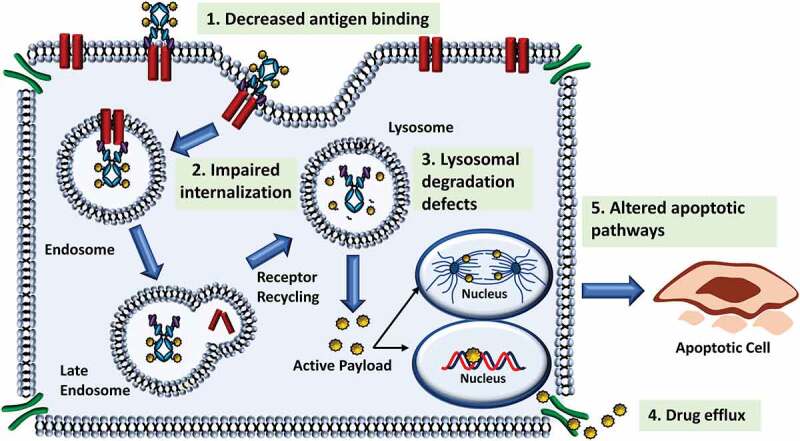
Mechanisms of ADC Resistance. Like other therapeutics, tumor cells may develop resistance against ADCs. (1) One mechanism, common to ADCs and monoclonal antibody therapeutics is a reduction in antigen binding, most notably by decreased antigen expression. (2) Most ADCs are internalized following antigen binding, however if internalization of the antigen-ADC complex is impaired, efficacy of the ADC can be reduced. (3) Following internalization, the antibody of the ADC is degraded leaving only the payload (cleavable linkers) or a linker-payload complex (non-cleavable linkers). Defects in the lysosomal degradation process can prevent release of the payload. (4) A common mechanism of resistance to ADCs is the elimination of the payload via drug transporters prior to payload-induced cytotoxic effect. Many traditional payloads of ADCs are substrates of these transporters. (5) Alterations to payload-specific cytotoxicity or cell death pathways can prevent eradication of the tumor cell
Another common mechanism of drug resistance is the removal of the payload via ATP-binding cassette transporters.100 Many of the cytotoxic warheads used in ADCs may be substrates for these pumps, which can cause drug efflux out of the target cell and a reduction in drug efficacy.101 Clinical data have demonstrated that efflux pumps contribute to the reduced efficacy of gemtuzumab ozogamicin.102,103 For instance, calicheamicin has been shown to be a substrate of multi-drug resistance mutation 1 (MDR1), and MDR1 expression and activity has been associated with response to gemtuzumab ozogamicin with similar preclinical results observed for inotuzumab ozogamicin.102–104 Increased drug transporter protein expression has also been observed in T-DM1 resistant cells in addition to decreases in surface antigen expression.105 Thus, the ability of the small molecule to bypass efflux pump-mediated drug resistance should be considered during the selection of the cytotoxic payload. Other mechanisms of drug resistance may be influenced by any of the several steps involved in the ADC MOA: 1) defects in internalization, trafficking, and recycling, 2) lysosomal degradation leading to impairment of drug release, or 3) alterations in cell death pathways (Figure 4).100 Preclinical evaluation of these and other potential mechanisms is critical to optimizing ADC development and improving clinical benefit. More attention should be paid to the technical considerations involving ADC design that ultimately influence cellular uptake and processing of the ADC.
Key considerations for ADC design
ADCs use three components to achieve greater clinical benefit, i.e., the mAb, the cytotoxic payload, and a chemical linker. By combining a targeting molecule with a cytotoxic payload, conceivably, the therapeutic window of ADCs is wider than treatment with small-molecule drugs alone. Several reviews have highlighted the complexity of ADCs and the challenges in developing products with improved therapeutic index.106–109 Optimization of ADC design is critical and requires a more mechanistic understanding of the ADC and its components to heighten the clinical benefit of ADCs.
1. Target antigen
Improvement of ADC safety and efficacy profiles relies significantly on selection of the target antigen and its interaction with the mAb of the ADC. Two critical parameters involved in the selection of the target antigen are tumor specificity and expression level.110,111 Ideally, the chosen target will exhibit a high level of tumor-specific or disease-specific expression and be minimal to absent in normal tissues. Specificity of the target is critical to reducing toxicity of ADCs, and thus plays a substantial role in their overall success. For oncological indications, the antigen can be expressed as a surface receptor on tumor cells, tumor stem cells, or within the tumor vasculature and microenvironment.112,113 In best cases, the antigen will also be expressed homogenously across tumor cells at similar levels.111 ADCs with sufficient control of bystander effect may overcome the challenge of heterogenous cell populations within a tumor.
2. Monoclonal antibody
After selecting a target, mAbs are produced and screened based on selectivity, tumor penetrating ability, and isotype.111 ADCs, both in development and approved, belong to the IgG1, IgG2, or IgG4 subclasses. These subclasses differ in cross-linking capabilities and biological activity, including ADCC and complement-dependent cytotoxicity (CDC) effector functions.114 IgG1 is commonly used due to its enhanced delivery capabilities and additional effector functions compared to IgG2 and IgG4.115 However, when considering the target characteristics and the proposed MOA, effector function may not, in some cases, be desirable, and IgG2 and IgG4 antibodies may be preferred. Isotype selection can also play a role in drug-linker conjugation, particularly when conjugating via cysteine residues.
2.1. Size of the monoclonal antibody
After selection of target antigen and antibody isotype, it is pertinent to consider the size constraints of the antibody for targeting tumors. Classical ADCs include a full-length antibody molecule, which may present challenges for the uptake and permeability of some solid tumors.116 To generate ADCs with improved uptake and penetration, several strategies in novel ADC design have pivoted toward the use of smaller formats of the antibody, including Fab-drug conjugates, scFv-drug conjugates, and diabody-drug conjugates.117–119 However, these smaller formats may be associated with faster clearance compared to the full-length IgG.116 Although the theoretical potential for these smaller formats exists, much work is still needed to prove a clear benefit.
2.2. Antibody modifications
Another important factor to address when considering ADC design is the posttranslational modifications (PTMs) of the antibody. Like most proteins, antibodies are subject to modifications both during antibody production and storage. Modifications can affect the stability, structure, and biological activity of the antibody, and consequently the ADC.120 PTMs, such as deamidation, sialylation, and c-terminal lysine cleavage, can affect the net charge of the mAbs.121 These changes can lead to the production of charge variants and heterogeneity of the ADC with wider consequences for antibody structure and biologic activity. With regard to the ADC, these changes can interfere with target-ADC binding and ADC entry into the tumor cells, resulting in lower efficacy of the ADC molecule.122,123 ADCC or CDC functions may be inhibited through PTMs, further hindering the ADC efficacy. To ensure batch-to-batch consistency, it is critical that the ADC charge profile and other modifications that may have been introduced are thoroughly characterized during development.
2.3. ADC internalization
Most ADCs are designed against a target antigen that displays efficient internalization via receptor-mediated endocytosis to facilitate ADC entry upon recognition.124 Receptor internalization has long stood as a requirement for effective ADC design to enable release of the cytotoxic payload with limited effects on healthy cells.125 To design a successful internalizing ADC, target accessibility, density, internalization rate, and intracellular trafficking of the ADC must be assessed. In general, ADCs against targets expressed on solid tumors have more physical barriers to overcome to reach the antigen following administration compared to hematological malignancies in which the targets are readily exposed to circulating ADCs.126 Further, targets can sometimes “shed” from the surface and be released into the blood, posing challenges against loss of ADC in circulation, clearance by the liver, and overall lower efficacy.127 Determination of the receptor expression (receptor copies/cell), internalization rate, and rate of recycling can all directly affect ADC entry into target cells and can be difficult to address.
While it is a known fact that the targeting mAb should exhibit high affinity toward the antigen, establishing a minimum threshold for target binding can be variable.109 As stated earlier, target density and internalization rates are key to ADC entry, metabolism, and payload accumulation within the tumor cell, but these may also be challenging to optimize. Efforts have been made to explore the potential of non-internalizing ADCs that target structural components of the environment surrounding the tumor cell.128,129 Such an approach may help overcome the penetration barriers of solid tumors by targeting an antigen highly expressed within the tumor stroma.113 In such cases, proteases shed from nearby apoptotic cells allow for the release of the payload which, due to its smaller size, can cross the membrane of tumor cells.130 A recent study showed anti-tumor activity in vivo of a non-internalizing ADC toward Gal-3BP protein that is secreted by cancer cells and localized to the cell surface. Due to accumulation at the surface of cancer cells, toxicity to normal tissues was limited, suggesting that non-internalizing ADCs can exhibit both potency and safety.131 Similarly, ABBV-085, produced by AbbVie, is an anti-LRRC15 ADC that was recently evaluated in clinical trials.132 Leucine-rich repeat containing 15 (LRRC15) is a member of the LRR superfamily with expression primarily on the surface of cancer-associated fibroblasts and stromal cells.133 In preclinical studies, ABBV-085 demonstrated anti-tumor activity in several LRRC15-positive cancer models, as well as LRRC15 stromal fibroblast-positive/cancer-negative models.133 The cell permeable properties of the two MMAE molecules conjugated to the antibody allowed for bystander activity, while an increase in immune infiltrate was also observed in the tumor microenvironment, both contributing to the efficacy of the ADC. Despite such promising preclinical data, only 14.8% of sarcoma patients treated with ABBV-085 at the recommended phase I b dose demonstrated a confirmed partial response, while 29.6% maintained stable disease, and progressive disease was observed in 40.7%.132 As of May 2021, no clinical trials that include ABBV-085 are ongoing. More studies are needed to look into the effectiveness of non-internalizing and tumor microenvironment-directed ADCs compared to those that are classically internalized by tumor cells in the clinical setting.
3. Cytotoxic payload
While the mAb is arguably the most important component in ensuring ADC efficiency, the cytotoxic payload is responsible for the execution of tumor cell killing.112 The cytotoxic payload (sometimes referred to as the “warhead”) is typically a small-molecule drug with the purpose of eliciting cell killing of the targeted tumor cells/tissues. The first generations of ADCs used drugs approved for clinical use, including doxorubicin, and resulted in low clinical activity.134 The next wave of ADCs adopted the use of more potent small-molecule drugs that were too toxic as a stand-alone treatment, but showed promise in efficacy when selectively delivered to target cells with IC50s in the 0.01–0.1 nM range.134 Even so, due to biodistribution, uptake, and loss of conjugation in circulation, it is estimated that only 1–2% of ADC payload will reach the intracellular target.108 Thus, the potency of the payload must be high (ideally in the subnanomolar range) so that even at a lower accumulated concentration, the ADC can still eradicate the target cells. To achieve this goal, current ADCs mostly incorporate potent molecules that either disrupt tubulin polymerization or induce DNA-damage (Figure 3d).24 Understanding the MOA of the ADC payloads and its applicability to the target is critical. While many ADCs in development currently use anti-mitotic tubulin disruptors for their selective eradication of rapidly proliferating cells, these payloads may not be effective toward targets that are not highly proliferative. It is worth noting the emergence of various toxic molecules conjugated to antibodies that are currently being investigated in clinical trials. Payloads such as topoisomerase inhibitors are gaining interest as cytotoxic agents that may be less toxic, allowing for higher DAR ADCs. This effect can be observed in the recently approved HER2-targeting ADC, trastuzumab deruxtecan, in which both a high DAR and reduced toxicity of the payload were used to produce a molecule with increased stability, efficient cytotoxic effect, and improved safety profile compared to T-DM1. Other payloads such as PBD dimers that exhibit high potency are also emerging for ADC design. These agents, as seen in the recently approved Lonca-T, can exert cytotoxicity at low concentrations with other advantages including efficient bystander cell killing and the potential for low systemic toxicity due to such short half-lives, dependent on several factors, such as conjugation strategies. This often results in low DAR species (e.g., DAR2) and lower dosing compared to ADCs carrying less potent payloads to balance the anti-tumor activity and safety profile of PBD-ADCs. Novel payloads such as immunostimulatory agents, RNA polymerase II inhibitors, and pro-apoptotic BCL-xL inhibitors are also emerging. Further, the choice of payload should also consider potential drug resistance mechanisms. Although payloads such as MMAE and calicheamicin have been shown to be good substrates of P-glycoprotein, others such as PBD dimers and some topoisomerase I inhibitors have been shown to exhibit anti-tumor activity in multi-drug resistant cancer cells.135,136
While aggregation due to unfolding and exposure of certain hydrophobic residues are concerns that exist for the parent mAb, this challenge is heightened with regard to ADCs due to conjugation methods and linker-payload additions.137 Research groups have demonstrated the effects of small-molecule drugs on the hydrophobicity of the ADCs, making the drug more prone to aggregation, particularly under thermal stress.138,139 As with unconjugated mAbs, aggregation decreases the activity of the ADC and can render the molecule less effective. Apart from aggregation due to linkage, hydrophobic drugs that are conjugated to the mAb can, if exhibiting efficient hydrophobicity, enter neighboring cells upon release from specific chemical linkers and induce killing of non-target cells. For ADCs carrying PBD or MMAE such as the vedotin ADCs, the payload’s cell permeability allows for a bystander killing effect within a heterogenous population. T-Dxd has also been reported to cause bystander killing via drug efflux into neighboring antigen-negative tumor cells.17
Several conjugation methods address the issue of hydrophobicity by using hydrophilic spacers, linkers, or payloads.140,141 In a recent study, Satomaa et al. demonstrated the enhanced stability of a novel hydrophilic payload that allowed for higher DAR achievement and low toxicity as a free drug while maintaining high cytotoxicity in target cells.142 This auristatin glycoside, β-D-glucuronyl-monomethylauristatin E also showed efficient internalization, metabolic processing, and bystander killing effect following conversion to MMAE through cellular metabolism.142 Further study into a hydrophobicity balance is needed to promote the efficacy of ADCs, hinging on both linker-payload choice and conjugation characteristics.
4. Linker chemistry and conjugation methods
The chemical linker is a critical component of the ADC that joins the mAb and the cytotoxic payload. The linker facilitates ADC stability in circulation until the ADC reaches the target cell and the payload is released.143 There are two classes of linkers: cleavable and non-cleavable.144 Cleavable linkers can be cleaved in response to certain environment factors to release the free drug into the cytosol.145 This includes hydrazine linkers that are cleaved in response to the acidic environment of the endosome and lysosome, exhibited in gemtuzumab ozogamicin. Cleavable linkers can also be cleaved in the presence of proteases or reducing agents, such as cathepsin B or high levels of glutathione.145 For non-internalizing ADCs, drug release relies on extracellular cleavage by glutathione and proteases that have been shed as a result of tumor cell death.146 Non-cleavable linkers are resistant to proteolytic degradation and rely on the full degradation of the antibody to release the attached linker-payload complex. This requires the payload to remain active, while linker bound.144 Because of this, non-cleavable linkers have been proposed as a strategy to overcome drug resistance as the linker-payload complex is no longer a substrate for MDR1.147 Therefore, the proposed MOA of the ADC can be a determinant for linker choice.
For some ADCs, the chemical linker may also serve to balance the hydrophobicity between the mAb and payload, therefore reducing potential aggregation. In this regard, analyzing the bioanalytical significance of all components of an ADC is important for evaluating the safety and efficacy of the drug. Hydrophilic linkers and spacers, including cyclodextrins, polyethylene glycol, and other polymers, may play a role in improving the stability of circulation, potency toward the target cells, and overall pharmacokinetics of the conjugate.148–150
In addition to selectively choosing a chemical linker, the method by which the payloads are conjugated to the antibody is essential in modulating the homogeneity and potency of the ADC.151 Until recently, conventional methods relied upon lysine and interchain cysteines to conjugate cytotoxic molecules to the antibody. In the case of lysine conjugation, heterogeneity was unavoidable due to the large number of lysines available for conjugation compared to cysteine conjugation.144 Due to the lack of control of conjugation site and quantity, lysines proximal to Fc binding can be affected by drug conjugation, resulting in lower efficiency of binding and cytotoxicity of the ADC.152
Currently, most ADCs in use or under development rely on interchain disulfide cysteines for conjugation, in which the 4 (IgG1 and IgG4) or 6 (IgG2) interchain disulfide bonds are reduced by an excess reducing agent, namely tris(2-carboxyethyl)phosphine or dithiothreitol.153 This spares disruption of intrachain disulfide bonds while freeing sulfhydryl groups from cysteine residues participating in interchain disulfide bonds (Figure 3e). The resulting product is a mixture of ADCs containing 0–8 drugs per parent IgG1 or IgG4 and 0–12 per IgG2, with predominantly even numbered DAR (0, 2, 4, 6, 8, 10, 12) species within the ADC mixture. Homogeneity of ADCs has improved because substantially fewer cysteines are available for conjugation following reduction compared to lysines. However, even with more homogenous methods, control over DAR and drug-load distribution (DLD) can still be enhanced. Optimizing the DAR and DLD is critical for the pharmacokinetics of the ADC and eradication of target cells.154 Ensuring homogeneity across all ADCs produced is a key aspect of quality control for developers and manufacturers to advocate for the safety of the product. Early studies initially indicated that DAR of 2–4 drug molecules per antibody is ideal for ensuring stability in circulation and efficacy.155 ADCs with too few conjugated payloads may exhibit low potency, while increased off-target toxicity and rapid clearance were previously observed in ADCs with higher DAR.155 However, the recent approvals of trastuzumab deruxtecan and sacituzumab govitecan have challenged this previously defined limit of 4, as both carry nearly eight payloads per antibody. Further, there is a broad range of average DAR in ADCs under clinical evaluation, with as low as 1 payload per antibody such as BDC-1001 and as many as 15 such as ASN004. The DAR may also influence dosing, antibody concentration to be administered, and subsequent tumor uptake of the ADC. ADCs of low DAR may be administered at higher doses depending on payload potency, which delivers a higher antibody concentration to facilitate ADC penetration into solid tumors. ADCs of high DAR may be administered at lower doses, which may lead to a lower antibody concentration and poorer tumor uptake. This notion was supported by in vitro studies involving co-administration of DAR0 or the naked antibody.156,157
Novel site-specific conjugation methods using unique linker chemistries that yield homogenous ADCs of desired DARs have emerged.107 One technique involves installing natural or unnatural amino acids into the antibody sequence for strict control over DAR and DLD. The most notable approach to engineering natural amino acids is THIOMAB™, which inserts cysteines at specific sites to allow for thiol conjugation.158 The resulting ADCs, referred to as THIOMAB™ -drug conjugates or TDCs, have shown improved homogeneity compared to conventionally conjugated ADCs.
Engineering of unnatural amino acids has included examples such as p-acetylphenylalanine and p-azidomethyl-L-phenylalanine, yielding ADCs in which DAR and DLD could be regulated.159,160 Another strategy is the SMARTag™ technology, which uses chemoenzymatic reactions to install an aldehyde tag for site-specific conjugation,144 as mentioned above. Here, the conjugation site is a formylglycine (aldehyde) residue produced through enzymatic oxidation of a cysteine in a specific pentapeptide consensus sequence in the mAb.29,161 A similar engineering method installs natural or synthetic carbohydrate moieties onto the glycan as points of target for drug conjugation.162 Not only does this technique address homogeneity concerns, but it also provides consistency in loading despite the heterogeneity of N-glycan forms of immunoglobulins. Thompson et al. conjugated PBD with DARs of 4 to azide-modified GalNAc to demonstrate the utility of glycoengineering in ADC design.163 The glycoengineered ADCs exhibited potent killing both in vitro and in vivo. With this method, developers enzymatically alter the glycan profile by introducing particular carbohydrate moieties for drug conjugation, yielding evidence of increased homogeneity and potency over conventional conjugation methods. Using enzymes that recognize specific engineered amino acid sequences to cleave and covalently attach drug molecules can also improve control over ADC homogeneity.
Another site-specific conjugation method that has gained attention both among researchers and biotechnology companies is disulfide rebridging.164 This method is attractive due to its ability to control DAR and DLD without the need of re-engineering the mAb. The technique takes advantage of the conventional cysteine coupling method to conjugate a bifunctional payload.165 As a result, one drug molecule is coupled per interchain disulfide bond. Using this method, developers achieve consistent DARs of 4 and 6, depending on the immunoglobulin isotype, with expected DLDs. This technique has shown promise to address homogeneity concerns as demonstrated by Bryant and colleagues.166 One drawback to this method is the use of additional chemicals, requiring additional purification methods and analytical characterization of the final product. Further, as with other described methodologies, the success of each technique in producing a homogenous product is dependent on other factors, such as the nature of the antibody and payload.
Quality assessment
ADCs are complex molecules with unique critical quality attributes (CQAs), including DAR, DLD, the amount of unconjugated payload or unconjugated antibody, antigen binding, and cellular activity, in addition to the quality requirements for naked mAbs. To ensure product quality and manufacturing consistency, each CQA must be adequately evaluated. Several analytical platforms are adopted from mAb analysis, with state-of-the-art analytical techniques being developed to assess ADC-specific CQAs, such as high-resolution native mass spectrometry (MS), native ion-mobility (IM) MS, and two-dimensional high performance liquid chromatography (2D-HPLC) (Table 3).167,168
Table 3.
Analytical characterization of ADC CQAs
| Quality Attributes | Analytical Methods |
||
|---|---|---|---|
| Cysteine Conjugates | Lysine Conjugates | Site-Specific Conjugates | |
| DAR, DLD, and unconjugated species (DAR-0) |
|
|
|
| Conjugation sites |
|
|
|
| Posttranslational modifications (PTMs) |
|
|
|
| Free drug species |
|
|
|
| Size variants |
|
|
|
| Charge variants |
|
|
|
The listed techniques have been used to characterize the CQAs of cysteine, lysine, and site-specific conjugates.
Antibody–drug conjugate (ADC); Analytical ultracentrifugation (AUC); Capillary isoelectric focusing (CIEF); Capillary electrophoresis-sodium dodecyl sulfate (CE-SDS); Cation exchange chromatography (CEX); Hydrophobic interaction chromatography (HIC); Hydrophilic interaction liquid chromatography (HILIC); Ion mobility mass spectrometry (IM-MS); Microfluidic capillary electrophoresis (mCE); Mass spectrometry (MS); Reverse phase liquid chromatography (RPLC); Size exclusion chromatography (SEC); Solid phase extraction (SPE); Ultraviolet-visible spectroscopy (UV/Vis)
1. DAR and DLD
For cysteine-linked ADCs, hydrophobic interaction chromatography is commonly used to determine the average DAR, DLD, and unconjugated mAb species (DAR-0).139,169,170 Emerging techniques include high-resolution MS and native IM-MS, operated under native conditions using MS-compatible ammonium acetate buffer at neutral pH.171 Similar analytical approaches can also be applied to other site-specific ADCs.172,173 Lysine-linked ADCs are inherently associated with a high heterogeneity, posing an analytical challenge. For those ADCs, DAR is usually determined by measuring both the drug-specific absorbance and the mAb absorbance at 280 nm.174,175 The conjugation sites and PTMs of mAbs can be determined through peptide mapping, producing a single tryptic peptide map for cysteine-linked ADCs, or a combination of tryptic, Asp-N, and Glu-C maps for lysine-linked ADCs;176,177 alternatively, sheathless capillary electrophoresis (CE) coupled with MS/MS can also be used to assess these attributes.178
2. Process- and product-related impurities
ADCs are associated with specific process-related impurities, such as unconjugated payload, free linker, or other chemicals used in the manufacturing process. Reverse-phase (RP)-HPLC provides a platform for assessing these potential impurities in the final products.179 Removal of protein-containing species (e.g., intact ADC, unconjugated antibody) can help improve assay performance, using protein precipitation, size-exclusion chromatography (SEC), or SEC×RP 2D-LC.180,181 2D-LC-MS can increase assay sensitivity, enabling detection of trace amount of free payload.182 Product-related impurities such as aggregates, fragments, charge variants, and other PTMs on the antibody can be assessed by a combination of SEC, analytical ultracentrifugation, CE, capillary isoelectric focusing, ionic exchange chromatography, and peptide mapping.183,184,185
3. Potency assays
Potency assays are a critical component of quality control strategies for complex drug products,together with physicochemical tests to ensure manufacturing consistency in the product lifecycle. In general, potency assays should reflect the product’s MOAs. For multifunctional products, more than one potency assay will be needed to fully capture the biological activities. An ADC may retain its mAb-associated MOAs, such as signaling blockade, ADCC, or CDC. An ADC may also elicit a bystander effect, thereby affecting both antigen-positive and antigen-negative cells upon release of the cytotoxic payload into the surrounding tumor microenvironment. Therefore, ADCs should be evaluated using both antigen-binding assays and cell-based functional assays as appropriate.
Future perspectives
The availability of ADCs offers a promising therapeutic option for numerous cancer types. With more ADCs entering clinical trials, the industry is gradually shifting from conventional technologies to newer and more robust approaches to develop such complex products. This includes strategies for exploring novel tumor antigens, antibody formats, payloads, linkers, and advanced conjugation technologies, each with the aim of improving the therapeutic window of ADCs. Among the emerging antibody formats, scFv may have better solid tumor penetration and uptake. Bispecific and biparatopic ADCs may overcome the barrier of tumor heterogeneity. Probodies and other conditionally active biologics (CABs) may reduce off-target effects. Multiple payload classes besides microtubule-disrupting agents, including PBD dimers, topoisomerase inhibitors, anthracyclines, and protein-specific modulators, are being introduced into a new generation of ADCs. Furthermore, several site-specific conjugation platforms are now used to enhance ADC stability in circulation while maintaining efficient release of the payload (Figure 5). The complexity of ADCs poses daunting analytical challenges, especially when hydrophobic payloads are incorporated. State-of-the-art analytical techniques are required and continue to evolve in alignment with the rapid growth of ADC development. Applying the appropriate sets of analytical techniques is crucial for adequately characterizing product attributes, thereby ensuring manufacturing consistency during development and throughout the product lifecycle.
Figure 5.
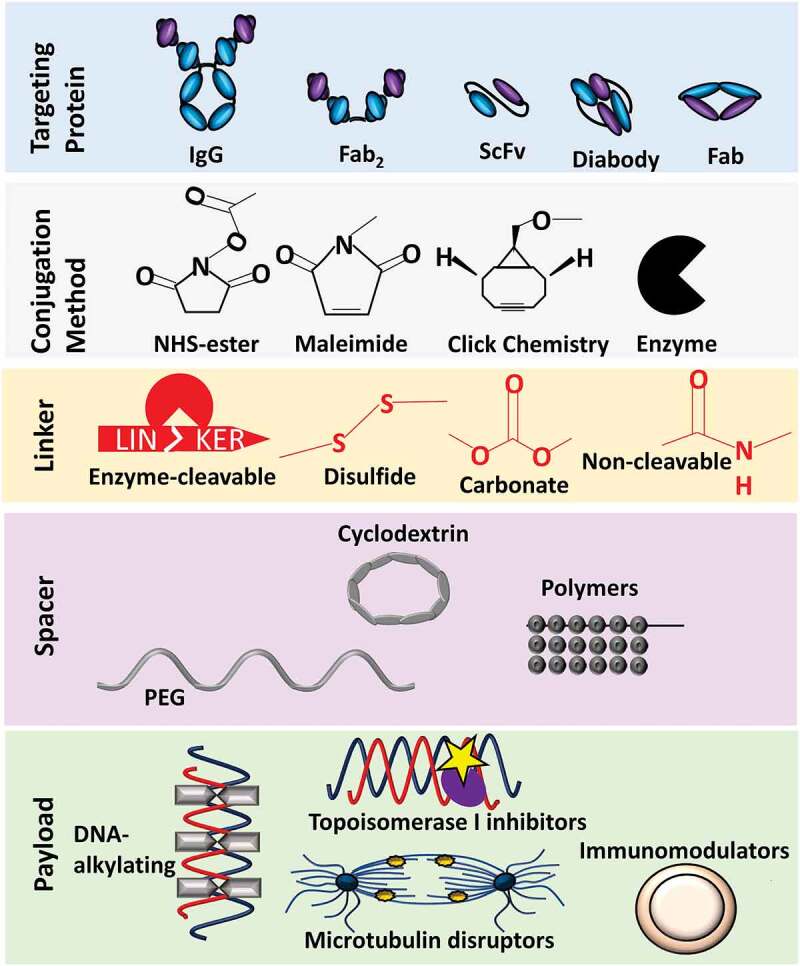
Expanding the ADC Framework. New monoclonal antibody formats, conjugation methods, linker and spacer techniques are emerging to optimize safety and efficacy profiles for oncological indications
The therapeutic potential of ADCs is also highlighted by the expansion of clinical indications, shifting from hematological malignancies (lymphoma and leukemia) to an increase in solid tumors (e.g., breast cancer, urothelial cancer, lung cancer, and ovarian cancer). Many ADCs within the clinical pipeline are being evaluated in combination with other established therapeutic classes, such as immune checkpoint inhibitors and mAbs targeting different antigens. The cumulative clinical data, combined with the product quality information described here, are helping to shape the future development of ADCs. As more data becomes publicly available, a comprehensive analysis of potential correlations between specific product quality attributes and the safety and efficacy profiles of individual products will certainly inform optimization of ADC design and manufacturing toward next-generation innovative cancer medicines.
Acknowledgments
The authors would like to thank Drs. Jacek Cieslak [Office of Biotechnology Products (OBP), CDER, FDA], Ancy Nalli [OBP, CDER, FDA], and Serge Beaucage [OBP, CDER, FDA] for their critical review on the manuscript.
Funding Statement
This work was funded by the U.S. Food and Drug Administration. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
| 2D-HPLC | Two-dimensional high performance liquid chromatography |
| ADC | Antibody-drug conjugate |
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
| AF | Auristatin F |
| AF-HPA | Auristatin F-hydroxypropylamide |
| BCMA | B-cell maturation antigen |
| CAB | Conditionally active biologic |
| CDC | Complement-dependent cytotoxicity |
| CE | Capillary electrophoresis |
| cIEF | Capillary isoelectric focusing |
| CQA | Critical quality attribute |
| DAR | Drug-to-antibody ratio |
| DLBCL | Diffuse large B-cell lymphoma |
| DLD | Drug-load distribution |
| DLL3 | Delta-like protein 3 |
| DNA | Deoxyribonucleic acid |
| FDA | U.S. Food and Drug Administration |
| Fv | Variable fragment |
| IM | Ion mobility |
| Lonca-T | Loncastuximab tesirine |
| LRRC15 | Leucine-rich repeat containing 15 |
| mAb | Monoclonal antibody |
| mBC | Metastatic breast cancer |
| MDR | Multi-drug resistance |
| MMAE | Monomethyl auristatin E |
| MMAF | Monomethyl auristatin F |
| MMAU | Monomethyl auristatin derivative |
| MOA | Mechanism of action |
| MS | Mass spectrometry |
| ORR | Overall response rates |
| PBD | Pyrrolobenzodiazepine |
| PDC | PROBODY-drug conjugates |
| PTM | Posttranslational modifications |
| Rova-T | Rovalpituzumab tesirine |
| R/R | Relapsed or refractory |
| RP | Reverse-phase |
| ScFv | Single-chain variable fragment |
| SEC | Size exclusion chromatography |
| SMARTag™ | Specific Modifiable Aldehyde Recombinant Tag |
| TDC | THIOMAB™-drug conjugates |
| T-DM1 | Trastuzumab emtansine |
| T-Dxd | Trastuzumab deruxtecan |
| TNBC | Triple-negative breast cancer |
| Trop2 | Trophoblast-cell surface antigen 2 |
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs. 2016;8(4):659–23. doi: 10.1080/19420862.2016.1156829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J Ocul Pharmacol Ther. 2015;31(10):589–604. doi: 10.1089/jop.2015.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, DeBlanc R, Toki BE, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–65. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 4.DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, Kunz A, Hamann PR, Gorovits B, Udata C, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103(5):1807–14. doi: 10.1182/blood-2003-07-2466. [DOI] [PubMed] [Google Scholar]
- 5.Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, Shinjo K, Yoshida H, Ohnishi K, Mori M, Terakawa S, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14(8):1436–43. doi: 10.1038/sj.leu.2401851. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration Center for Drug and Research Evaluation . Division Director Summary Review for Regulatory Action and Cross-Discipline Team Leader Review - Application Number(s) 761060Orig1s000 and 761060Orig2s000. Maryland): FDA; 2017. [Google Scholar]
- 7.Neumeister P, Eibl M, Zinke-Cerwenka W, Scarpatetti M, Sill H, Linkesch W. Hepatic veno-occlusive disease in two patients with relapsed acute myeloid leukemia treated with anti-CD33 calicheamicin (CMA-676) immunoconjugate. Ann Hematol. 2001;80(2):119–20. doi: 10.1007/s002770000239. [DOI] [PubMed] [Google Scholar]
- 8.Norsworthy KJ, Ko CW, Lee JE, Liu J, John CS, Przepiorka D, Farrell AT, Pazdur R. FDA Approval Summary: mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncologist. 2018;23(9):1103–08. doi: 10.1634/theoncologist.2017-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron J, Wang ES. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev Clin Pharmacol. 2018;11(6):549–59. doi: 10.1080/17512433.2018.1478725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornan D, Bennett F, Chen Y, Dennis M, Eaton D, Elkins K, French D, Go MA, Jack A, Junutula JR, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood. 2009;114(13):2721–29. doi: 10.1182/blood-2009-02-205500. [DOI] [PubMed] [Google Scholar]
- 11.Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, Craigen J, Yates J, Gliddon L, Fieles W, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123(20):3128–38. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zammarchi F, Corbett S, Adams L, Tyrer PC, Kiakos K, Janghra N, Marafioti T, Britten CE, Havenith CEG, Chivers S, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131(10):1094–105. doi: 10.1182/blood-2017-10-813493. [DOI] [PubMed] [Google Scholar]
- 13.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti R, Lopez S, Bellone S, Cocco E, Schwab CL, Black JD, Centritto F, Zhu L, Bonazzoli E, Buza N, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against uterine and ovarian carcinosarcomas overexpressing HER2. Clin Exp Metastasis. 2015;32(1):29–38. doi: 10.1007/s10585-014-9688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 17.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–91. [PubMed] [Google Scholar]
- 20.Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, Raitano A, Nadell R, Liu W, Lortie DR, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016;76(10):3003–13. doi: 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]
- 21.Ogita H, Rikitake Y, Miyoshi J, Takai Y. Cell adhesion molecules nectins and associating proteins: implications for physiology and pathology. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(6):621–29. doi: 10.2183/pjab.86.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frigerio M, Kyle AF. The Chemical Design and Synthesis of Linkers Used in Antibody Drug Conjugates. Curr Top Med Chem. 2017;17(32):3393–424. doi: 10.2174/1568026618666180118155847. [DOI] [PubMed] [Google Scholar]
- 23.Hicks S, Loo D, Sinkevicius K, Scribner J, Barat B, Yoder N, Espelin C, Themeles M, Chen F, Lucas J, et al. Abstract 1533: IMGC936, a first-in-class ADAM9-targeting antibody-drug conjugate, demonstrates promising anti-tumor activity. Cancer Res. 2019;79(13 Supplement). doi: 10.1158/1538-7445.Am2019-1533 [DOI] [Google Scholar]
- 24.Yaghoubi S, Karimi MH, Lotfinia M, Gharibi T, Mahi-Birjand M, Kavi E, Hosseini F, Sineh Sepehr K, Khatami M, Bagheri N, et al. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol. 2020;235(1):31–64. doi: 10.1002/jcp.28967. [DOI] [PubMed] [Google Scholar]
- 25.Kollmannsberger C, Choueiri TK, Heng DYC, George S, Jie F, Croitoru R, Poondru S, Thompson JA. A Randomized PhaseII Study of AGS-16C3F Versus Axitinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma. Oncologist. 2021;26(3):182–e361. doi: 10.1002/onco.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman LA, Terp MG, Zom GG, Janmaat ML, Jacobsen K, Gresnigt-van den Heuvel E, Brandhorst M, Forssmann U, De Bree F, Pencheva N, et al. Enapotamab vedotin, an AXL-specific antibody-drug conjugate, shows preclinical antitumor activity in non-small cell lung cancer. JCI Insight. 2019;4(21):21. doi: 10.1172/jci.insight.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahnert JR, Taylor MH, O’Reilly EM, Zhang J, Doebele RC, Ben Y, Sharp LL, Boyle WJ, Chang C, Frey G, et al. A phase 1/2 dose-escalation and expansion study of a conditionally active anti-AXL humanized monoclonal antibody (BA3011) in patients with advanced solid tumors. J Clin Oncol. 2018;36(no. 15_suppl). doi: 10.1200/JCO.2018.36.15_suppl.TPS12126 [DOI] [Google Scholar]
- 28.Gaudio E, Tarantelli C, Spriano F, Guidetti F, Sartori G, Bordone R, Arribas AJ, Cascione L, Bigioni M, Merlino G, et al. Targeting CD205 with the antibody drug conjugate MEN1309/OBT076 is an active new therapeutic strategy in lymphoma models. Haematologica. 2020;105(11):2584–91. doi: 10.3324/haematol.2019.227215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drake PM, Carlson A, McFarland JM, Banas S, Barfield RM, Zmolek W, Kim YC, Huang BCB, Kudirka R, Rabuka D. CAT-02-106, a Site-Specifically Conjugated Anti-CD22 Antibody Bearing an MDR1-Resistant Maytansine Payload Yields Excellent Efficacy and Safety in Preclinical Models. Mol Cancer Ther. 2018;17(1):161–68. doi: 10.1158/1535-7163.MCT-17-0776. [DOI] [PubMed] [Google Scholar]
- 30.Patnaik A, Meric-Bernstam F, Lima CMSPR, Robert F, Dowlati A, Kindler HL, Davar D, Powell SF, Garfin PM. SGN228-001: a phase I open-label dose-escalation, and expansion study of SGN-CD228A in select advanced solid tumors. J Clin Oncol. 2020;38(no. 15_suppl):3652–3652. doi: 10.1200/JCO.2020.38.15_suppl.TPS3652.32886536 [DOI] [Google Scholar]
- 31.Shen Y, Yang T, Cao X, Zhang Y, Zhao L, Li H, Zhao T, Xu J, Zhang H, Guo Q, et al. Conjugation of DM1 to anti-CD30 antibody has potential antitumor activity in CD30-positive hematological malignancies with lower systemic toxicity. MAbs. 2019;11(6):1149–61. doi: 10.1080/19420862.2019.1618674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks SW, Lai KC, Gavrilescu LC, Yi Y, Sikka S, Shah P, Kelly ME, Lee J, Lanieri L, Ponte JF, et al. The Antitumor Activity of IMGN529, a CD37-Targeting Antibody-Drug Conjugate, Is Potentiated by Rituximab in Non-Hodgkin Lymphoma Models. Neoplasia. 2017;19(9):661–71. doi: 10.1016/j.neo.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherbenou DW, Aftab BT, Su Y, Behrens CR, Wiita A, Logan AC, Acosta-Alvear D, Hann BC, Walter P, Shuman MA, et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J Clin Invest. 2016;126(12):4640–53. doi: 10.1172/JCI85856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Socinski MA, Kaye FJ, Spigel DR, Kudrik FJ, Ponce S, Ellis PM, Majem M, Lorigan P, Gandhi L, Gutierrez ME, et al. Phase 1/2 Study of the CD56-Targeting Antibody-Drug Conjugate Lorvotuzumab Mertansine (IMGN901) in Combination With Carboplatin/Etoposide in Small-Cell Lung Cancer Patients With Extensive-Stage Disease. Clin Lung Cancer. 2017;18(1):68–76 e2. doi: 10.1016/j.cllc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Newman DJ. The “Utility” of Highly Toxic Marine-Sourced Compounds. Mar Drugs. 2019;17(6):324. doi: 10.3390/md17060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahams CL, Li X, Embry M, Yu A, Krimm S, Krueger S, Greenland NY, Wen KW, Jones C, DeAlmeida V, et al. Targeting CD74 in multiple myeloma with the novel, site-specific antibody-drug conjugate STRO-001. Oncotarget. 2018;9(102):37700–14. doi: 10.18632/oncotarget.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decary S, Berne PF, Nicolazzi C, Lefebvre AM, Dabdoubi T, Cameron B, Rival P, Devaud C, Prades C, Bouchard H, et al. Preclinical Activity of SAR408701: a Novel Anti-CEACAM5-maytansinoid Antibody-drug Conjugate for the Treatment of CEACAM5-positive Epithelial Tumors. Clin Cancer Res. 2020;26(24):6589–99. doi: 10.1158/1078-0432.CCR-19-4051. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Anderson MG, Oleksijew A, Vaidya KS, Boghaert ER, Tucker L, Zhang Q, Han EK, Palma JP, Naumovski L, et al. ABBV-399, a c-Met Antibody-Drug Conjugate that Targets Both MET-Amplified and c-Met-Overexpressing Tumors, Irrespective of MET Pathway Dependence. Clin Cancer Res. 2017;23(4):992–1000. doi: 10.1158/1078-0432.CCR-16-1568. [DOI] [PubMed] [Google Scholar]
- 39.van den Bent M, Gan HK, Lassman AB, Kumthekar P, Merrell R, Butowski N, Lwin Z, Mikkelsen T, Nabors LB, Papadopoulos KP, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80(6):1209–17. doi: 10.1007/s00280-017-3451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu R-H, Qiu M-Z, Zhang Y, Wei X-L, Hu C. First-in-human dose-escalation study of anti-EGFR ADC MRG003 in patients with relapsed/refractory solid tumors. J Clin Oncol. 2020;38(15_suppl):3550. doi: 10.1200/JCO.2020.38.15_suppl.3550. [DOI] [Google Scholar]
- 41.Li X, Abrahams C, Zhou S, Krimm S, Henningsen R, Stephenson H, Hanson J, Masikat MR, Bajjuri K, Heibeck T, et al. Abstract 1782: discovery and activity of STRO-002, a novel ADC targeting folate receptor alpha for ovarian and endometrial cancer. Cancer Res. 2018;78(13 Supplement):1782. doi: 10.1158/1538-7445.Am2018-1782. [DOI] [Google Scholar]
- 42.Cheng X, Li J, Tanaka K, Majumder U, Milinichik AZ, Verdi AC, Maddage CJ, Rybinski KA, Fernando S, Fernando D, et al. MORAb-202, an Antibody-Drug Conjugate Utilizing Humanized Anti-human FRalpha Farletuzumab and the Microtubule-targeting Agent Eribulin, has Potent Antitumor Activity. Mol Cancer Ther. 2018;17(12):2665–75. doi: 10.1158/1535-7163.MCT-17-1215. [DOI] [PubMed] [Google Scholar]
- 43.Moore KN, Martin LP, O’Malley DM, Matulonis UA, Konner JA, Perez RP, Bauer TM, Ruiz-Soto R, Birrer MJ. Safety and Activity of Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, in Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer: a Phase I Expansion Study. J Clin Oncol. 2017;35(10):1112–18. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keir CH, Vahdat LT. The use of an antibody drug conjugate, glembatumumab vedotin (CDX-011), for the treatment of breast cancer. Expert Opin Biol Ther. 2012;12(2):259–63. doi: 10.1517/14712598.2012.642357. [DOI] [PubMed] [Google Scholar]
- 45.Walsh SJ, Bargh JD, Dannheim FM, Hanby AR, Seki H, Counsell AJ, Ou X, Fowler E, Ashman N, Takada Y, et al. Site-selective modification strategies in antibody-drug conjugates. Chem Soc Rev. 2020. doi: 10.1039/d0cs00310g. [DOI] [PubMed] [Google Scholar]
- 46.Betts A, Clark T, Jasper P, Tolsma J, van der Graaf PH, Graziani EI, Rosfjord E, Sung M, Ma D, Barletta F. Use of translational modeling and simulation for quantitative comparison of PF-06804103, a new generation HER2 ADC, with Trastuzumab-DM1. J Pharmacokinet Pharmacodyn. 2020;47(5):513–26. doi: 10.1007/s10928-020-09702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126(19):4278–88. doi: 10.1002/cncr.33102. [DOI] [PubMed] [Google Scholar]
- 48.Yao X, Jiang J, Wang X, Huang C, Li D, Xie K, Xu Q, Li H, Li Z, Lou L, et al. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res Treat. 2015;153(1):123–33. doi: 10.1007/s10549-015-3503-3. [DOI] [PubMed] [Google Scholar]
- 49.Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, Shi B, Liu J, He Z, Yu G, et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res. 2021;27(1):43–51. doi: 10.1158/1078-0432.CCR-20-2488. [DOI] [PubMed] [Google Scholar]
- 50.Rinnerthaler G, Gampenrieder SP, Greil R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int J Mol Sci. 2019;20(5):5. doi: 10.3390/ijms20051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skidmore L, Sakamuri S, Knudsen NA, Hewet AG, Milutinovic S, Barkho W, Biroc SL, Kirtley J, Marsden R, Storey K, et al. ARX788, a Site-specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-low and T-DM1-resistant Breast and Gastric Cancers. Mol Cancer Ther. 2020;19(9):1833–43. doi: 10.1158/1535-7163.MCT-19-1004. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Guo Y, Xue J, Peng W, Ge X, Zhao W, Dai C, Xue L, Tang W, Hu C. First-in-human phase I study of anti-HER2 ADC MRG002 in patients with relapsed/refractory solid tumors. J Clin Oncol. 2020;38(15_suppl):1101. doi: 10.1200/JCO.2020.38.15_suppl.TPS1101. [DOI] [Google Scholar]
- 53.Hong R, Xia W, Wang L, Lee K, Lu Q, Jiang K, Li S, Yu J, Wei J, Tang W, et al. Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: an open-label, dose-escalation, phase I study. Cancer Commun (Lond). 2021;41(2):171–82. doi: 10.1002/cac2.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akla B, Broussas M, Loukili N, Robert A, Beau-Larvor C, Malissard M, Boute N, Champion T, Haeuw JF, Beck A, et al. Efficacy of the Antibody-Drug Conjugate W0101 in Preclinical Models of IGF-1 Receptor Overexpressing Solid Tumors. Mol Cancer Ther. 2020;19(1):168–77. doi: 10.1158/1535-7163.MCT-19-0219. [DOI] [PubMed] [Google Scholar]
- 55.Lyon RP, Schmitt MW, Jonas M, Franz CE, Trueblood ES, Yumul R, Westendorf L, Ryan MC. Abstract 2906: SGN-B6A: a new MMAE ADC targeting integrin beta-6 in multiple carcinoma indications. Cancer Res. 2020;80(16 Supplement). doi: 10.1158/1538-7445.Am2020-2906. [DOI] [Google Scholar]
- 56.Sussman D, Smith LM, Anderson ME, Duniho S, Hunter JH, Kostner H, Miyamoto JB, Nesterova A, Westendorf L, Van Epps HA, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13(12):2991–3000. doi: 10.1158/1535-7163.MCT-13-0896. [DOI] [PubMed] [Google Scholar]
- 57.Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, Mayer-Bartschmid A, Unterschemmann K, Bruder S, Linden L, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13(6):1537–48. doi: 10.1158/1535-7163.MCT-13-0926. [DOI] [PubMed] [Google Scholar]
- 58.Clarke J, Chu S-C, Siu LL, Machiels J-P, Markman B, Heinhuis K, Millward M, Lolkema M, Patel SP, Souza P, et al. Abstract B057: BMS-986148, an anti-mesothelin antibody-drug conjugate (ADC), alone or in combination with nivolumab demonstrates clinical activity in patients with select advanced solid tumors. Mol Cancer Ther. 2019;18(12 Supplement). doi: 10.1158/1535-7163.Targ-19-b057 [DOI] [Google Scholar]
- 59.Tolcher AW, Ulahannan SV, Papadopoulos KP, Edenfield WJ, Matulonis UA, Burns TF, Mosher R, Fielman B, Hailman E, Burris HA, et al. Phase 1 dose escalation study of XMT-1536, a novel NaPi2b-targeting antibody-drug conjugate (ADC), in patients (pts) with solid tumors likely to express NaPi2b. J Clin Oncol. 2019;37(15_suppl):3010–3010. doi: 10.1200/JCO.2019.37.15_suppl.3010. [DOI] [Google Scholar]
- 60.Fessler S, Dirksen A, Collins SD, Xu L, Lee W, Wang J, Eydelloth R, Ter-Ovanesyen E, Zurita J, Ditty E, et al. Abstract 2894: XMT-1592, a site-specific Dolasynthen-based NaPi2b-targeted antibody-drug conjugate for the treatment of ovarian cancer and lung adenocarcinoma. Cancer Res. 2020;80(16 Supplement). doi: 10.1158/1538-7445.Am2020-2894 [DOI] [Google Scholar]
- 61.Vaisitti T, Jessen K, Vo -T-T, Ko M, Arruga F, Vitale N, Braggio E, Di Napoli A, Chadburn A, Allan JN, et al. Vls-101 Is a Novel Therapeutic Antibody-Drug Conjugate (ADC) Targeting Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1) in Richter’s Syndrome (RS). Blood. 2019;134(Supplement_1):2856–2856. doi: 10.1182/blood-2019-126827. [DOI] [Google Scholar]
- 62.De Bono JS, Concin N, Hong DS, Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH, Nielsen D, Windfeld K, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(3):383–93. doi: 10.1016/S1470-2045(18)30859-3. [DOI] [PubMed] [Google Scholar]
- 63.Kinneer K, Flynn M, Thomas SB, Meekin J, Varkey R, Xiao X, Zhong H, Breen S, Hynes PG, Fleming R, et al. Preclinical assessment of an antibody-PBD conjugate that targets BCMA on multiple myeloma and myeloma progenitor cells. Leukemia. 2019;33(3):766–71. doi: 10.1038/s41375-018-0278-7. [DOI] [PubMed] [Google Scholar]
- 64.Angelova E, Audette C, Kovtun Y, Daver N, Wang SA, Pierce S, Konoplev SN, Khogeer H, Jorgensen JL, Konopleva M, et al. CD123 expression patterns and selective targeting with a CD123-targeted antibody-drug conjugate (IMGN632) in acute lymphoblastic leukemia. Haematologica. 2019;104(4):749–55. doi: 10.3324/haematol.2018.205252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zammarchi F, Corbett S, Adams L, Mellinas-Gomez M, Tyrer P, Dissanayake S, Sims S, Havenith K, Chivers S, Willimas DG, et al. hLL2-Cys-PBD, a New Site-Specifically Conjugated, Pyrrolobenzodiazepine (PBD) Dimer-Based Antibody Drug Conjugate (ADC) Targeting CD22-Expressing B-Cell Malignancies. Blood. 2016;128(22):22. doi: 10.1182/blood.V128.22.4176.4176. [DOI] [Google Scholar]
- 66.Flynn MJ, Zammarchi F, Tyrer PC, Akarca AU, Janghra N, Britten CE, Havenith CE, Levy JN, Tiberghien A, Masterson LA, et al. ADCT-301, a Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody-Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Mol Cancer Ther. 2016;15(11):2709–21. doi: 10.1158/1535-7163.MCT-16-0233. [DOI] [PubMed] [Google Scholar]
- 67.Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, Li H, De Costa A, Li Y, Chen Y, et al. Preclinical Development of MGC018, a Duocarmycin-based Antibody-drug Conjugate Targeting B7-H3 for Solid Cancer. Mol Cancer Ther. 2020;19(11):2235–44. doi: 10.1158/1535-7163.MCT-20-0116. [DOI] [PubMed] [Google Scholar]
- 68.Gymnopoulos M, Betancourt O, Blot V, Fujita R, Galvan D, Lieuw V, Nguyen S, Snedden J, Stewart C, Villicana J, et al. TR1801-ADC: a highly potent cMet antibody-drug conjugate with high activity in patient-derived xenograft models of solid tumors. Mol Oncol. 2020;14(1):54–68. doi: 10.1002/1878-0261.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartley JA. Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Expert Opin Biol Ther. 2020:1–13. doi: 10.1080/14712598.2020.1776255. [DOI] [PubMed] [Google Scholar]
- 70.Nadal-Serrano M, Morancho B, Escriva-de-Romani S, Morales CB, Luque A, Escorihuela M, Espinosa Bravo M, Peg V, Dijcks FA, Dokter WHA, et al. The Second Generation Antibody-Drug Conjugate SYD985 Overcomes Resistances to T-DM1. Cancers (Basel). 2020;12(3):3. doi: 10.3390/cancers12030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beerli RR, Waldmeier L, Gébleux R, Pretto F, Abstract GU. LB-197: NBE-002, an anthracycline-based immune-stimulatory antibody drug conjugate (iADC) targeting ROR1 for the treatment of triple-negative breast cancer. Cancer Res. 2019;79(13 Supplement). doi: 10.1158/1538-7445.Am2019-lb-197. [DOI] [Google Scholar]
- 72.Iida K, Abdelhamid AH, Nagatsuma AK, Shibutani T, Yasuda S, Kitamura M, Hattori C, Abe M, Hasegawa J, Iguchi T, et al. Abstract 5181: therapeutic targeting of GPR20, selectively expressed in gastrointestinal stromal tumor (GIST), with DS-6157a, an antibody-drug conjugate (ADC). Cancer Res. 2020;80(16 Supplement). doi: 10.1158/1538-7445.Am2020-5181 [DOI] [PubMed] [Google Scholar]
- 73.Yonesaka K, Takegawa N, Watanabe S, Haratani K, Kawakami H, Sakai K, Chiba Y, Maeda N, Kagari T, Hirotani K, et al. An HER3-targeting antibody-drug conjugate incorporating a DNA topoisomerase I inhibitor U3-1402 conquers EGFR tyrosine kinase inhibitor-resistant NSCLC. Oncogene. 2019;38(9):1398–409. doi: 10.1038/s41388-018-0517-4. [DOI] [PubMed] [Google Scholar]
- 74.Lisberg AE, Sands J, Shimizu T, Greenberg J, Phillips P, Guevara FM, Noguchi Y, Toki T, Spira AI, Yamamoto N, et al. Dose escalation and expansion from the phase I study of DS-1062, a trophoblast cell-surface antigen 2 (TROP2) antibody drug conjugate (ADC), in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(15_suppl):9619. doi: 10.1200/JCO.2020.38.15_suppl.9619. [DOI] [Google Scholar]
- 75.Liu Y, Lian W, Zhao X, Diao Y, Xu J, Xiao L, Qing Y, Xue T, Wang J. SKB264 ADC: a first-in-human study of SKB264 in patients with locally advanced unresectable/metastatic solid tumors who are refractory to available standard therapies. J Clin Oncol. 2020;38(15_suppl). doi: 10.1200/JCO.2020.38.15_suppl.TPS3659. [DOI] [Google Scholar]
- 76.Figueroa-Vazquez V, Ko J, Breunig C, Baumann A, Giesen N, Palfi A, Muller C, Lutz C, Hechler T, Kulke M, et al. HDP-101, an Anti-BCMA Antibody-Drug Conjugate, Safely Delivers Amanitin to Induce Cell Death in Proliferating and Resting Multiple Myeloma Cells. Mol Cancer Ther. 2021;20(2):367–78. doi: 10.1158/1535-7163.MCT-20-0287. [DOI] [PubMed] [Google Scholar]
- 77.Comeau MR, Brender T, Childs M, Brevik J, Winship D, Metz H, Chang J, Adamo J, Setter B, Xu H, et al. Abstract 4537: SBT6050, a HER2-directed TLR8 ImmunoTAC™therapeutic, is a potent human myeloid cell agonist that provides opportunity for single agent clinical activity. Cancer Res. 2020;80(16 Supplement). doi: 10.1158/1538-7445.Am2020-4537 [DOI] [Google Scholar]
- 78.Zhang X, Liu X, Zhou D, Zheng G. Targeting anti-apoptotic BCL-2 family proteins for cancer treatment. Future Med Chem. 2020;12(7):563–65. doi: 10.4155/fmc-2020-0004. [DOI] [PubMed] [Google Scholar]
- 79.Frega G, Wu Q, Le Naour J, Vacchelli E, Galluzzi L, Kroemer G, Kepp O. Trial Watch: experimental TLR7/TLR8 agonists for oncological indications. Oncoimmunology. 2020;9(1):1796002. doi: 10.1080/2162402X.2020.1796002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yurkovetskiy AV, Bodyak ND, Yin M, Thomas JD, Clardy SM, Conlon PR, Stevenson CA, Uttard A, Qin L, Gumerov DR, et al. Dolaflexin: a Novel Antibody-Drug Conjugate Platform Featuring High Drug Loading and a Controlled Bystander Effect. Mol Cancer Ther. 2021;20(5):885–95. doi: 10.1158/1535-7163.MCT-20-0166. [DOI] [PubMed] [Google Scholar]
- 81.Mersana Therapeutics . XMT-1592. Cambridge (MA): Mersana Therapeutics, 2021; [accessed: 2021 01 12]. https://www.mersana.com/pipeline/xmt-1592/ [Google Scholar]
- 82.Clardy SM, Yurkovetskiy A, Yin M, Gumerov D, Xu L, Ter-Ovanesyan E, Bu C, Johnson A, Protopopova M, Zhang Q, et al. Abstract 754: unique pharmacologic properties of Dolaflexin-based ADCs—a controlled bystander effect. Cancer Res. 2018;78(13 Supplement):754. doi: 10.1158/1538-7445.Am2018-754. [DOI] [Google Scholar]
- 83.Kavanaugh WM. Antibody prodrugs for cancer. Expert Opin Biol Ther. 2020;20(2):163–71. doi: 10.1080/14712598.2020.1699053. [DOI] [PubMed] [Google Scholar]
- 84.Chomet M, Schreurs M, Nguyen M, Howng B, Villanueva R, Krimm M, Vasiljeva O, van Dongen G, Vugts DJ. The tumor targeting performance of anti-CD166 Probody drug conjugate CX-2009 and its parental derivatives as monitored by (89) Zr-immuno-PETin xenograft bearing mice. Theranostics. 2020;10(13):5815–28. doi: 10.7150/thno.44334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Espelin CW, Leonard SC, Geretti E, Wickham TJ, Hendriks BS. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res. 2016;76(6):1517–27. doi: 10.1158/0008-5472.CAN-15-1518. [DOI] [PubMed] [Google Scholar]
- 86.Miller K, Cortes J, Hurvitz SA, Krop IE, Tripathy D, Verma S, Riahi K, Reynolds JG, Wickham TJ, Molnar I, et al. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naive, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16(1):352. doi: 10.1186/s12885-016-2385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang A, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7(302):302ra136. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA 3rd, Robert F, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, Glisson BS, Farago AF, Dowlati A, Rudin CM, et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: results From the Phase II TRINITY Study. Clin Cancer Res. 2019;25(23):6958–66. doi: 10.1158/1078-0432.CCR-19-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, Majem M, Nackaerts K, Syrigos K, Hansen K, et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: results From the Phase 3 TAHOE Study. J Thorac Oncol. 2021. doi: 10.1016/j.jtho.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 91.McDonald GB, Freston JW, Boyer JL, DeLeve LD. Liver Complications Following Treatment of Hematologic Malignancy With Anti-CD22-Calicheamicin (Inotuzumab Ozogamicin). Hepatology. 2019;69(2):831–44. doi: 10.1002/hep.30222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kantarjian HM, DeAngelo DJ, Advani AS, Stelljes M, Kebriaei P, Cassaday RD, Merchant AA, Fujishima N, Uchida T, Calbacho M, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4(8):e387–e98. doi: 10.1016/S2352-3026(17)30103-5. [DOI] [PubMed] [Google Scholar]
- 93.Ladha A, Mannis G, Muffly L. Hepatic veno-occlusive disease in allogeneic stem cell transplant recipients with prior exposure to gemtuzumab ozogamicin or inotuzumab ozogamicin. Leuk Lymphoma. 2020:1–7. doi: 10.1080/10428194.2020.1827247. [DOI] [PubMed] [Google Scholar]
- 94.Kogawa T, Yonemori K, Norikazu M, Takahashi S, Takahashi M, Iwase H, Nakayama T, Saeki T, Toyama T, Takano T, et al. Single agent activity of U3-1402, a HER3-targeting antibody-drug conjugate, in breast cancer patients: phase 1 dose escalation study. J Clin Oncol. 2018;36(15_suppl):2512–2512. doi: 10.1200/JCO.2018.36.15_suppl.2512. [DOI] [Google Scholar]
- 95.Saber H, Simpson N, Ricks TK, Leighton JK. An FDA oncology analysis of toxicities associated with PBD-containing antibody-drug conjugates. Regul Toxicol Pharmacol. 2019;107:104429. doi: 10.1016/j.yrtph.2019.104429. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Alonso S, Ocana A, Pandiella A. Resistance to Antibody-Drug Conjugates. Cancer Res. 2018;78(9):2159–65. doi: 10.1158/0008-5472.CAN-17-3671. [DOI] [PubMed] [Google Scholar]
- 97.Chen R, Hou J, Newman E, Kim Y, Donohue C, Liu X, Thomas SH, Forman SJ, Kane SE. CD30 Downregulation, MMAE Resistance, and MDR1 Upregulation Are All Associated with Resistance to Brentuximab Vedotin. Mol Cancer Ther. 2015;14(6):1376–84. doi: 10.1158/1535-7163.MCT-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Endo Y, Shen Y, Youssef LA, Mohan N. Wu WJ. T-DM1-resistant cells gain high invasive activity via EGFR and integrin cooperated pathways. MAbs. 2018;10(7):1003–17. doi: 10.1080/19420862.2018.1503904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, Hinrichs MJ, Bezabeh BZ, Fleming RL, Dimasi N, et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell. 2016;29(1):117–29. doi: 10.1016/j.ccell.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 100.Loganzo F, Sung M, Gerber HP. Mechanisms of Resistance to Antibody-Drug Conjugates. Mol Cancer Ther. 2016;15(12):2825–34. doi: 10.1158/1535-7163.MCT-16-0408. [DOI] [PubMed] [Google Scholar]
- 101.Liu-Kreyche P, Shen H, Marino AM, Iyer RA, Humphreys WG. Lysosomal P-gp-MDR1 LY Confers Drug Resistance of Brentuximab Vedotin and Its Cytotoxic Payload Monomethyl Auristatin E in Tumor Cells. Front Pharmacol. 2019;10:749. doi: 10.3389/fphar.2019.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyer T, Gonzales F, Barthelemy A, Marceau-Renaut A, Peyrouze P, Guihard S, Lepelley P, Plesa A, Nibourel O, Delattre C, et al. Clinical Significance of ABCB1 in Acute Myeloid Leukemia: a Comprehensive Study. Cancers (Basel). 2019;11(9):9. doi: 10.3390/cancers11091323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Linenberger ML, Hong T, Flowers D, Sievers EL, Gooley TA, Bennett JM, Berger MS, Leopold LH, Appelbaum FR, Bernstein ID. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001;98(4):988–94. doi: 10.1182/blood.v98.4.988. [DOI] [PubMed] [Google Scholar]
- 104.Takeshita A, Shinjo K, Yamakage N, Ono T, Hirano I, Matsui H, Shigeno K, Nakamura S, Tobita T, Maekawa M, et al. CMC-544 (inotuzumab ozogamicin) shows less effect on multidrug resistant cells: analyses in cell lines and cells from patients with B-cell chronic lymphocytic leukaemia and lymphoma. Br J Haematol. 2009;146(1):34–43. doi: 10.1111/j.1365-2141.2009.07701.x. [DOI] [PubMed] [Google Scholar]
- 105.Loganzo F, Tan X, Sung M, Jin G, Myers JS, Melamud E, Wang F, Diesl V, Follettie MT, Musto S, et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther. 2015;14(4):952–63. doi: 10.1158/1535-7163.MCT-14-0862. [DOI] [PubMed] [Google Scholar]
- 106.Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–37. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 107.McCombs JR, Owen SC. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015;17(2):339–51. doi: 10.1208/s12248-014-9710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17(20):6389–97. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 109.Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani AH. Antibody-Drug Conjugates: possibilities and Challenges. Avicenna J Med Biotechnol. 2019;11:3–23. [PMC free article] [PubMed] [Google Scholar]
- 110.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64(1):15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 111.Damelin M, Zhong W, Myers J, Sapra P. Evolving Strategies for Target Selection for Antibody-Drug Conjugates. Pharm Res. 2015;32(11):3494–507. doi: 10.1007/s11095-015-1624-3. [DOI] [PubMed] [Google Scholar]
- 112.Tang H, Liu Y, Yu Z, Sun M, Lin L, Liu W, Han Q, Wei M, Jin Y. The Analysis of Key Factors Related to ADCs Structural Design. Front Pharmacol. 2019;10:373. doi: 10.3389/fphar.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Staudacher AH, Li Y, Liapis V, Hou JJC, Chin D, Dolezal O, Adams TE, van Berkel PH, Brown MP. APOMAB Antibody–Drug Conjugates Targeting Dead Tumor Cells are Effective In Vivo. Mol Cancer Ther. 2019;18(2):335–45. doi: 10.1158/1535-7163.MCT-18-0842. [DOI] [PubMed] [Google Scholar]
- 114.Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67(2 Pt A):171–82. doi: 10.1016/j.molimm.2015.03.255. [DOI] [PubMed] [Google Scholar]
- 115.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10(2):101–11. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 116.Li Z, Li Y, Chang HP, Chang HY, Guo L, Shah DK. Effect of Size on Solid Tumor Disposition of Protein Therapeutics. Drug Metab Dispos. 2019;47(10):1136–45. doi: 10.1124/dmd.119.087809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruddle BT, Fleming R, Wu H, Gao C, Dimasi N. Characterization of Disulfide Bond Rebridged Fab-Drug Conjugates Prepared Using a Dual Maleimide Pyrrolobenzodiazepine Cytotoxic Payload. ChemMedChem. 2019;14(12):1185–95. doi: 10.1002/cmdc.201900077. [DOI] [PubMed] [Google Scholar]
- 118.Deonarain MP, Xue Q. Tackling solid tumour therapy with small-format drug conjugates. Antib Ther. 2020;3(4):237–45. doi: 10.1093/abt/tbaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Q, Barrett A, Vijayakrishnan B, Tiberghien A, Beard R, Rickert KW, Allen KL, Christie RJ, Marelli M, Harper J, et al. Improved Inhibition of Tumor Growth by Diabody-Drug Conjugates via Half-Life Extension. Bioconjug Chem. 2019;30(4):1232–43. doi: 10.1021/acs.bioconjchem.9b00170. [DOI] [PubMed] [Google Scholar]
- 120.Jefferis R. Posttranslational Modifications and the Immunogenicity of Biotherapeutics. J Immunol Res. 2016;2016:5358272. doi: 10.1155/2016/5358272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leblanc Y, Ramon C, Bihoreau N, Chevreux G. Charge variants characterization of a monoclonal antibody by ion exchange chromatography coupled on-line to native mass spectrometry: case study after a long-term storage at +5 degrees C. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1048:130–39. doi: 10.1016/j.jchromb.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 122.Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010;9(8):1716–28. doi: 10.1074/mcp.M900540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu X, Machiesky LA, De Mel N, Du Q, Xu W, Washabaugh M, Jiang XR, Wang J. Characterization of IgG1 Fc Deamidation at Asparagine 325 and Its Impact on Antibody-dependent Cell-mediated Cytotoxicity and FcgammaRIIIa Binding. Sci Rep. 2020;10(1):383. doi: 10.1038/s41598-019-57184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM. Antibody-drug conjugates: current status and future directions. Drug Discov Today. 2014;19(7):869–81. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Harper J, Mao S, Strout P, Kamal A. Selecting an Optimal Antibody for Antibody–Drug Conjugate Therapy: internalization and Intracellular Localization. In: Ducry L, editor. Antibody-Drug Conjugates 1045. Totowa (NJ): Humana Press; 2013. p. 41–49. [DOI] [PubMed] [Google Scholar]
- 126.Christiansen J, Rajasekaran AK. Biological impediments to monoclonal antibody-based cancer immunotherapy. Mol Cancer Ther. 2004;3:1493–501. [PubMed] [Google Scholar]
- 127.Awuah P, Bera TK, Folivi M, Chertov O, Pastan I. Reduced Shedding of Surface Mesothelin Improves Efficacy of Mesothelin-Targeting Recombinant Immunotoxins. Mol Cancer Ther. 2016;15(7):1648–55. doi: 10.1158/1535-7163.MCT-15-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gebleux R, Wulhfard S, Casi G, Neri ND. Antibody Format and Drug Release Rate Determine the Therapeutic Activity of Noninternalizing Antibody–Drug Conjugates. Mol Cancer Ther. 2015;14(11):2606–12. doi: 10.1158/1535-7163.MCT-15-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gebleux R, Stringhini M, Casanova R, Soltermann A, Neri D. Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int J Cancer. 2017;140(7):1670–79. doi: 10.1002/ijc.30569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dal Corso A, Gebleux R, Murer P, Soltermann A, Neri D. A non-internalizing antibody-drug conjugate based on an anthracycline payload displays potent therapeutic activity in vivo. J Control Release. 2017;264:211–18. doi: 10.1016/j.jconrel.2017.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giansanti F, Capone E, Ponziani S, Piccolo E, Gentile R, Lamolinara A, Di Campli A, Sallese M, Iacobelli V, Cimini A, et al. Secreted Gal-3BP is a novel promising target for non-internalizing Antibody-Drug Conjugates. J Control Release. 2019;294:176–84. doi: 10.1016/j.jconrel.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 132.Demetri GD, Luke JJ, Hollebecque A, Powderly JD, Spira AI, Subbiah V, Lai DW, Yue H, Kasichayanula S, Gulbranson S, et al. First-in-human phase 1 study of ABBV-085, an antibody-drug conjugate (ADC) targeting LRRC15, in sarcomas and other advanced solid tumors. J Clin Oncol. 2019;37(15_suppl):3004. doi: 10.1200/JCO.2019.37.15_suppl.3004. [DOI] [Google Scholar]
- 133.Purcell JW, Tanlimco SG, Hickson J, Fox M, Sho M, Durkin L, Uziel T, Powers R, Foster K, McGonigal T, et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody-Drug Conjugates. Cancer Res. 2018;78(14):4059–72. doi: 10.1158/0008-5472.CAN-18-0327. [DOI] [PubMed] [Google Scholar]
- 134.Zhao P, Zhang Y, Li W, Jeanty C, Xiang G, Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B. 2020;10(9):1589–600. doi: 10.1016/j.apsb.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K, Tsurutani J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141(8):1682–89. doi: 10.1002/ijc.30870. [DOI] [PubMed] [Google Scholar]
- 136.Mantaj J, Jackson PJ, Rahman KM, Thurston DE. From Anthramycin to Pyrrolobenzodiazepine (PBD)-Containing Antibody-Drug Conjugates (ADCs). Angew Chem Int Ed Engl. 2017;56(2):462–88. doi: 10.1002/anie.201510610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ross PL, Wolfe JL. Physical and Chemical Stability of Antibody Drug Conjugates: current Status. J Pharm Sci. 2016;105(2):391–97. doi: 10.1016/j.xphs.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 138.Wakankar AA, Feeney MB, Rivera J, Chen Y, Kim M, Sharma VK, Wang YJ. Physicochemical stability of the antibody-drug conjugate Trastuzumab-DM1: changes due to modification and conjugation processes. Bioconjug Chem. 2010;21(9):1588–95. doi: 10.1021/bc900434c. [DOI] [PubMed] [Google Scholar]
- 139.Beckley NS, Lazzareschi KP, Chih HW, Sharma VK, Flores HL. Investigation into temperature-induced aggregation of an antibody drug conjugate. Bioconjug Chem. 2013;24(10):1674–83. doi: 10.1021/bc400182x. [DOI] [PubMed] [Google Scholar]
- 140.Mendelsohn BA, Barnscher SD, Snyder JT, An Z, Dodd JM, Dugal-Tessier J. Investigation of Hydrophilic Auristatin Derivatives for Use in Antibody Drug Conjugates. Bioconjug Chem. 2017;28(2):371–81. doi: 10.1021/acs.bioconjchem.6b00530. [DOI] [PubMed] [Google Scholar]
- 141.Shao S, Tsai MH, Lu J, Yu T, Jin J, Xiao D, Jiang H, Han M, Wang M, Wang J. Site-specific and hydrophilic ADCs through disulfide-bridged linker and branched PEG. Bioorg Med Chem Lett. 2018;28(8):1363–70. doi: 10.1016/j.bmcl.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 142.Satomaa T, Pynnonen H, Vilkman A, Kotiranta T, Pitkanen V, Heiskanen A, Herpers B, Price LS, Helin J, Saarinen J. Hydrophilic Auristatin Glycoside Payload Enables Improved Antibody-Drug Conjugate Efficacy and Biocompatibility. Antibodies (Basel). 2018;7:2. doi: 10.3390/antib7020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu J, Jiang F, Lu A, Zhang G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int J Mol Sci. 2016;17(4):561. doi: 10.3390/ijms17040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jain N, Smith SW, Ghone S, Current TB. ADC Linker Chemistry. Pharm Res. 2015;32(11):3526–40. doi: 10.1007/s11095-015-1657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bargh JD, Isidro-Llobet A, Parker JS, Spring DR. Cleavable linkers in antibody-drug conjugates. Chem Soc Rev. 2019;48(16):4361–74. doi: 10.1039/c8cs00676h. [DOI] [PubMed] [Google Scholar]
- 146.Perrino E, Steiner M, Krall N, Bernardes GJ, Pretto F, Casi G, Neri D. Curative properties of noninternalizing antibody-drug conjugates based on maytansinoids. Cancer Res. 2014;74(9):2569–78. doi: 10.1158/0008-5472.CAN-13-2990. [DOI] [PubMed] [Google Scholar]
- 147.Shefet-Carasso L, Benhar I. Antibody-targeted drugs and drug resistance--challenges and solutions. Drug Resist Updat. 2015;18:36–46. doi: 10.1016/j.drup.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 148.Verkade JMM, Wijdeven MA, Van Geel R, Janssen BMG, Van Berkel SS, Van Delft FL, Polar Sulfamide A. Spacer Significantly Enhances the Manufacturability, Stability, and Therapeutic Index of Antibody-Drug Conjugates. Antibodies (Basel). 2018;7:1. doi: 10.3390/antib7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dorywalska M, Dushin R, Moine L, Farias SE, Zhou D, Navaratnam T, Lui V, Hasa-Moreno A, Casas MG, Tran TT, et al. Molecular Basis of Valine-Citrulline-PABC Linker Instability in Site-Specific ADCs and Its Mitigation by Linker Design. Mol Cancer Ther. 2016;15(5):958–70. doi: 10.1158/1535-7163.MCT-15-1004. [DOI] [PubMed] [Google Scholar]
- 150.Simmons JK, Burke PJ, Cochran JH, Pittman PG, Lyon RP. Reducing the antigen-independent toxicity of antibody-drug conjugates by minimizing their non-specific clearance through PEGylation. Toxicol Appl Pharmacol. 2020;392:114932. doi: 10.1016/j.taap.2020.114932. [DOI] [PubMed] [Google Scholar]
- 151.Sochaj AM, Swiderska KW, Otlewski J. Current methods for the synthesis of homogeneous antibody-drug conjugates. Biotechnol Adv. 2015;33(6 Pt 1):775–84. doi: 10.1016/j.biotechadv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 152.Acchione M, Kwon H, Jochheim CM, Atkins WM. Impact of linker and conjugation chemistry on antigen binding, Fc receptor binding and thermal stability of model antibody-drug conjugates. MAbs. 2012;4(3):362–72. doi: 10.4161/mabs.19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiggins B, Liu-Shin L, Yamaguchi H, Ratnaswamy G. Characterization of cysteine-linked conjugation profiles of immunoglobulin G1 and immunoglobulin G2 antibody-drug conjugates. J Pharm Sci. 2015;104(4):1362–72. doi: 10.1002/jps.24338. [DOI] [PubMed] [Google Scholar]
- 154.Sun X, Ponte JF, Yoder NC, Laleau R, Coccia J, Lanieri L, Qiu Q, Wu R, Hong E, Bogalhas M, et al. Effects of Drug-Antibody Ratio on Pharmacokinetics, Biodistribution, Efficacy, and Tolerability of Antibody-Maytansinoid Conjugates. Bioconjug Chem. 2017;28(5):1371–81. doi: 10.1021/acs.bioconjchem.7b00062. [DOI] [PubMed] [Google Scholar]
- 155.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 156.Lu G, Nishio N, Van Den Berg NS, Martin BA, Fakurnejad S, van Keulen S, Colevas AD, Thurber GM, Rosenthal EL. Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat Commun. 2020;11(1):5667. doi: 10.1038/s41467-020-19498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ponte JF, Lanieri L, Khera E, Laleau R, Ab O, Espelin C, Kohli N, Matin B, Setiady Y, Miller ML, et al. Antibody Co-Administration Can Improve Systemic and Local Distribution of Antibody–Drug Conjugates to Increase In Vivo Efficacy. Mol Cancer Ther. 2021;20(1):203–12. doi: 10.1158/1535-7163.Mct-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 159.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109(40):16101–06. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zimmerman ES, Heibeck TH, Gill A, Li X, Murray CJ, Madlansacay MR, Tran C, Uter NT, Yin G, Rivers PJ, et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug Chem. 2014;25(2):351–61. doi: 10.1021/bc400490z. [DOI] [PubMed] [Google Scholar]
- 161.Rabuka D, Rush JS, deHart GW, Wu P, Bertozzi CR. Site-specific chemical protein conjugation using genetically encoded aldehyde tags. Nat Protoc. 2012;7(6):1052–67. doi: 10.1038/nprot.2012.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu Z, Ramakrishnan B, Li J, Wang Y, Feng Y, Prabakaran P, Colantonio S, Dyba MA, Qasba PK, Dimitrov DS. Site-specific antibody-drug conjugation through an engineered glycotransferase and a chemically reactive sugar. MAbs. 2014;6(5):1190–200. doi: 10.4161/mabs.29889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thompson P, Ezeadi E, Hutchinson I, Fleming R, Bezabeh B, Lin J, Mao S, Chen C, Masterson L, Zhong H, et al. Straightforward Glycoengineering Approach to Site-Specific Antibody-Pyrrolobenzodiazepine Conjugates. ACS Med Chem Lett. 2016;7(11):1005–08. doi: 10.1021/acsmedchemlett.6b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schumacher FF, Nunes JP, Maruani A, Chudasama V, Smith ME, Chester KA, Baker JR, Caddick S. Next generation maleimides enable the controlled assembly of antibody-drug conjugates via native disulfide bond bridging. Org Biomol Chem. 2014;12(37):7261–69. doi: 10.1039/c4ob01550a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Badescu G, Bryant P, Bird M, Henseleit K, Swierkosz J, Parekh V, Tommasi R, Pawlisz E, Jurlewicz K, Farys M, et al. Bridging disulfides for stable and defined antibody drug conjugates. Bioconjug Chem. 2014;25(6):1124–36. doi: 10.1021/bc500148x. [DOI] [PubMed] [Google Scholar]
- 166.Bryant P, Pabst M, Badescu G, Bird M, McDowell W, Jamieson E, Swierkosz J, Jurlewicz K, Tommasi R, Henseleit K, et al. In Vitro and In Vivo Evaluation of Cysteine Rebridged Trastuzumab-MMAE Antibody Drug Conjugates with Defined Drug-to-Antibody Ratios. Mol Pharm. 2015;12(6):1872–79. doi: 10.1021/acs.molpharmaceut.5b00116. [DOI] [PubMed] [Google Scholar]
- 167.Wagh A, Song H, Zeng M, Tao L, Das TK. Challenges and new frontiers in analytical characterization of antibody-drug conjugates. MAbs. 2018;10(2):222–43. doi: 10.1080/19420862.2017.1412025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beck A, D’Atri V, Ehkirch A, Fekete S, Hernandez-Alba O, Gahoual R, Leize-Wagner E, Francois Y, Guillarme D, Cianferani S. Cutting-edge multi-level analytical and structural characterization of antibody-drug conjugates: present and future. Expert Rev Proteomics. 2019;16(4):337–62. doi: 10.1080/14789450.2019.1578215. [DOI] [PubMed] [Google Scholar]
- 169.Kozak KR, Tsai SP, Fourie-O’Donohue A, dela Cruz Chuh J, Roth L, Cook R, Chan E, Chan P, Darwish M, Ohri R, et al. Total antibody quantification for MMAE-conjugated antibody-drug conjugates: impact of assay format and reagents. Bioconjug Chem. 2013;24(5):772–79. doi: 10.1021/bc300491k. [DOI] [PubMed] [Google Scholar]
- 170.Cusumano A, Guillarme D, Beck A, Fekete S. Practical method development for the separation of monoclonal antibodies and antibody-drug-conjugate species in hydrophobic interaction chromatoraphy, part 2: optimization of the phase system. J Pharm Biomed Anal. 2016;121:161–73. doi: 10.1016/j.jpba.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 171.Debaene F, Boeuf A, Wagner-Rousset E, Colas O, Ayoub D, Corvaia N, Van Dorsselaer A, Beck A, Cianferani S. Innovative native MS methodologies for antibody drug conjugate characterization: high resolution native MS and IM-MS for average DAR and DAR distribution assessment. Anal Chem. 2014;86(21):10674–83. doi: 10.1021/ac502593n. [DOI] [PubMed] [Google Scholar]
- 172.Cao M, De Mel N, Jiao Y, Howard J, Parthemore C, Korman S, Thompson C, Wendeler M, Liu D. Site-specific antibody-drug conjugate heterogeneity characterization and heterogeneity root cause analysis. MAbs. 2019;11(6):1064–76. doi: 10.1080/19420862.2019.1624127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Botzanowski T, Erb S, Hernandez-Alba O, Ehkirch A, Colas O, Wagner-Rousset E, Rabuka D, Beck A, Drake PM, Cianferani S. Insights from native mass spectrometry approaches for top- and middle- level characterization of site-specific antibody-drug conjugates. MAbs. 2017;9(5):801–11. doi: 10.1080/19420862.2017.1316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wakankar A, Chen Y, Gokarn Y, Jacobson FS. Analytical methods for physicochemical characterization of antibody drug conjugates. MAbs. 2011;3(2):161–72. doi: 10.4161/mabs.3.2.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Y. Drug-to-Antibody Ratio (DAR) by UV/Vis Spectroscopy. In: Durcy L, editor. Methods in Molecular Biology (Methods and Protocols) 1045. Totowa (NJ): Humana Press. 2013. [DOI] [PubMed] [Google Scholar]
- 176.Friese OV, Smith JN, Brown PW, Rouse JC. Practical approaches for overcoming challenges in heightened characterization of antibody-drug conjugates with new methodologies and ultrahigh-resolution mass spectrometry. MAbs. 2018;10(3):335–45. doi: 10.1080/19420862.2018.1433973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luo Q, Chung HH, Borths C, Janson M, Wen J, Joubert MK, Wypych J. Structural Characterization of a Monoclonal Antibody-Maytansinoid Immunoconjugate. Anal Chem. 2016;88(1):695–702. doi: 10.1021/acs.analchem.5b03709. [DOI] [PubMed] [Google Scholar]
- 178.Said N, Gahoual R, Kuhn L, Beck A, Francois YN, Leize-Wagner E. Structural characterization of antibody drug conjugate by a combination of intact, middle-up and bottom-up techniques using sheathless capillary electrophoresis - Tandem mass spectrometry as nanoESI infusion platform and separation method. Anal Chim Acta. 2016;918:50–59. doi: 10.1016/j.aca.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 179.Galush WJ, Wakankar AA. Formulation development of antibody-drug conjugates. Methods Mol Biol. 2013;1045:217–33. doi: 10.1007/978-1-62703-541-5_13. [DOI] [PubMed] [Google Scholar]
- 180.Goyon A, Sciascera L, Clarke A, Guillarme D, Pell R. Extending the limits of size exclusion chromatography: simultaneous separation of free payloads and related species from antibody drug conjugates and their aggregates. J Chromatogr A. 2018;1539:19–29. doi: 10.1016/j.chroma.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 181.Li Y, Gu C, Gruenhagen J, Zhang K, Yehl P, Chetwyn NP, Medley CD. A size exclusion-reversed phase two dimensional-liquid chromatography methodology for stability and small molecule related species in antibody drug conjugates. J Chromatogr A. 2015;1393:81–88. doi: 10.1016/j.chroma.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 182.Birdsall RE, McCarthy SM, Janin-Bussat MC, Perez M, Haeuw JF, Chen W, Beck A. A sensitive multidimensional method for the detection, characterization, and quantification of trace free drug species in antibody-drug conjugate samples using mass spectral detection. MAbs. 2016;8(2):306–17. doi: 10.1080/19420862.2015.1116659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Adem YT, Schwarz KA, Duenas E, Patapoff TW, Galush WJ, Esue O. Auristatin antibody drug conjugate physical instability and the role of drug payload. Bioconjug Chem. 2014;25(4):656–64. doi: 10.1021/bc400439x. [DOI] [PubMed] [Google Scholar]
- 184.Zhang X, Chemmalil L, Ding J, Mussa N, Li Z. Imaged capillary isoelectric focusing in native condition: a novel and successful example. Anal Biochem. 2017;537:13–19. doi: 10.1016/j.ab.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 185.International Conference on Harmonization (ICH) guidelines Q6B Specifications: Test procedures and acceptance criteria for biotechnological/biological products . https://www.ich.org/page/quality-guidelines [Google Scholar]


