Doppler image revealing flow acceleration consistent with significant coarctation effect.
Central Message.
Intraoperative TEE confirmed the clinical diagnosis of coarctation by showing the presence of an unexpected residual dissection flap within the distal aortic arch and providing Doppler measurements.
Clinical Summary
A 49-year-old woman with Marfan syndrome was referred for surgical repair of a thoracoabdominal aortic aneurysm with expanding diameters secondary to a chronic DeBakey III aortic dissection diagnosed 16 years earlier, reaching a maximum diameter of 7.5 cm at the distal thoracic aorta (DTA) (see Figure 1, Figure 2, Figure 3). Prior written informed consent was obtained from the patient for the use of anonymized material for teaching purposes.
Figure 1.
Preoperative computed tomography scan image. Sagittal view showing the chronic dissection flap originating near the origin of the left subclavian area and extending downstream to the thoracic and abdominal aorta that has been progressively dilating with current diameters of 4.5 and 7.5 cm in the proximal and distal thoracic aorta, respectively. Note the presence of sternal wires from a previous open-and-close median sternotomy at the time of presentation with the acute DeBakey III aortic dissection.
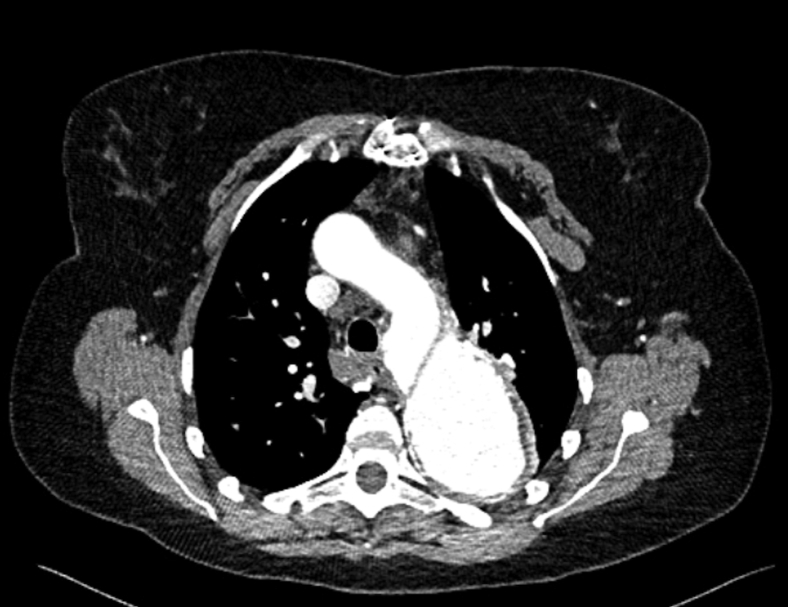
Figure 2.
Preoperative computed tomography scan image. Axial view showing the presence of a chronic dissection flap separating the true and the false aortic lumens, originating at the distal arch near the origin of the left subclavian artery.
Figure 3.
Preoperative computed tomography scan image. Coronal view showing the presence of a chronic dissection flap separating the true and the false aortic lumens, extending from the distal arch at the level of the left subclavian artery.
The dissection flap originated at the proximal DTA, distal to the origin of the left subclavian artery (LSA) and extended to the distal abdominal aorta, stopping above the iliac bifurcation. The patient's relevant medical history included a previous median sternotomy coinciding with acute aortic syndrome 16 years earlier due to misdiagnosis with DeBakey I or II aortic dissection. She underwent an elective Crawford extent II thoracoabdominal aortic aneurysm repair from the proximal DTA to the iliac bifurcation, with reimplantation of 2 pairs of lower intercostal arteries (T9-T11) and individual reimplantation of the visceral vessels (ie, celiac, superior mesenteric, right renal, and left renal arteries).
Perioperative monitoring and surgical adjuncts included double-lumen endotracheal tube, right radial and right femoral arterial catheters, right internal jugular central venous line, echocardiogram leads, nasopharyngeal temperature probe, transesophageal echocardiography (TEE) probe, urinary catheter, vascath via right femoral vein connected to a rapid infusion system, forehead near-infrared spectometry electrodes, lumbar spinal drain for cerebrospinal fluid drainage and motor-evoked-potential electrodes in the skull and 4 limbs. TEE examination following induction of anesthesia revealed good biventricular function, moderate aortic insufficiency, and dilated DTA with a dissection flap originating just after the LSA.
Exposure was gained via a left thoraco-phreno-laparotomy along sixth intercostal space and midabdominal line down to level of the umbilicus. After mild heparinization (target activated clotting time, 250-300 seconds), left heart bypass (LHB) was established with the inflow cannula in the left inferior pulmonary vein and the outflow cannula in the infrarenal aorta. The operation was conducted under mild hypothermia (34°C) and sequential aortic crossclamping from proximal to distal aortic segments and visceral protection with selective perfusion of cold blood via the celiac and superior mesenteric arteries and intermittent administration of cold Custodiol HTK Solution (Essential Pharmaceutical Corp, Pomona, Calif) via the renal arteries. The ischemic times were as follow: celiac 40 minutes, superior mesenteric arteries 54 minutes, renal arteries 94 minutes, and iliac arteries 17 minutes, with a total LHB time of 286 minutes.
Once the proximal clamps were applied immediately after the origin of the LSA and the mid-DTA, a chronic thick dissection flap was identified between the true and the false aortic lumens immediately after the proximal clamp. The flap was resected until the proximal clamp area and the proximal aortic anastomosis was constructed. The aortic repair was conducted per our standard technique described elsewhere.1, 2, 3
Once the LHB was discontinued, a large pressure gradient (72 mm Hg) was noticed between the right radial and femoral arterial lines (Figure 4). The distal aortic graft pressure was measured directly inserting an 18-gauge needle connected to a manometer line into the Dacron (DuPont, Wilmington, Del) graft at the mid-descending thoracic level, confirming the pressure gradient. TEE revealed the presence of a residual thick dissection flap in the native aorta proximal to the anastomosis. Color Doppler in the area revealed marked flow acceleration and continuous wave Doppler confirmed a high systolic instantaneous peak pressure gradient of 62.37 mm Hg (Figure 5). The continuous wave Doppler waveform (Figure 5) also demonstrated persistence of the pressure gradient into diastole. These findings are compatible with a significant coarctation effect.4
Figure 4.
Once the left heart bypass was discontinued, a large pressure gradient (72 mm Hg) was noticed between the right radial and femoral arterial lines.
Figure 5.
Transesophageal echocardiography was used to investigate a large blood pressure difference after left heart bypass discontinuation. Use of color and continuous wave Doppler across the distal aortic arch revealed flow acceleration of 3.95 m/sec and a pressure gradient of 62.37 mm Hg occurring in both systole and diastole, which was consistent with a significant coarctation effect.
LHB was re-established. The aortic arch was clamped proximal to the origin of the LSA that was temporarily occluded. The aortic graft was opened 2 cm distal to the previous anastomosis and the surgical fenestration was extended, retrograde, toward the aortic arch. Once LHB was weaned again, the pressure gradient had resolved. No further discrepancy between arterial lines was noticed. The patient made an uneventful recovery and the postoperative computed tomography scan showed complete resection of the dissection flap (Figure 6).
Figure 6.
Postoperative computed tomography scan image. Axial view showing the complete repair with complete resection of the dissection flap. The proximal anastomosis was conducted beyond the origin of the left subclavian artery. Note the large apical hematoma in the pleural space that had to be evacuated on a second stage video-assisted thoracoscopic procedure.
Discussion
Intraoperative TEE use is recommended for procedures involving the thoracic aorta4 to confirm preoperative diagnosis, assess surgical results, and detect unexpected findings or complications. The literature describes the role of TEE in diagnosis of dissection complications such as aortic regurgitation and arterial vessel involvement. As such, TEE has been incorporated into the clinical practice recommendations of the European Association of Echocardiography.5
In this case, the TEE was rapidly utilized to identify the morphology and location of an unusual complication; namely, a pseudo-coarctation due to incomplete surgical fenestration of the dissection flap beyond the proximal clamp area by illuminating the residual flap within the distal arch. This case is an example of how improvements in patient outcome may be achieved by appropriately timed echocardiography-guided surgery.
In cases of chronic aortic dissection, it is important to perform a surgical fenestration consisting of surgical resection of the thick septum to prevent malperfusion or selective pressurization of 1 of the lumens. Among the risks of leaving a dissection flap at the site of the anastomosis, as we experienced, is insufficient expansion of the true lumen to allow adequate flow with resultant high proximal pressure. Although feasible based on the preoperative computed tomography scan, it is not always possible to guarantee the surgical result with complete expansion of the true lumen because application of the proximal clamp might distort the anatomy and make the anastomosis challenging.
Clamping distal to the LSA poses some benefits that will increase risk of respiratory complications, such as preserving its contribution to spinal cord blood flow and avoiding the trajectory of the left recurrent laryngeal nerve. The presence of a large distal arch aneurysm, mandates clamping the arch, which also increases the risk of iatrogenic retrograde type A aortic dissection related to a sudden increase in the proximal blood pressure.1, 2, 3 Another potential complication of the proximal clamp and transection of the aorta at the proximal and mid-DTA level is a lesion in the esophageal wall, which would be devastating.1, 2, 3
Other intraoperative methods to detect this complication are epiaortic ultrasound and direct pressure measurement with a gauge needle and pressure line inserted in the proximal and distal segments. Postoperatively, this complication would have been detected by the computed tomography scan or on an invasive aortogram (Video 1).
Video 1.

Professor Aung Oo discusses the importance of both antegrade and retrograde surgical fenestrations for chronic aortic dissections and the importance of transesophageal echocardiography in detecting intraoperative complications. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00083-3/fulltext.
Lessons Learned
Suspicion of this complication should arise in patients with large aneurysms with reduced space for the application of the crossclamp. In these scenarios, the anatomy is easily distorted and careful identification of the 2 lumens before constructing the anastomosis is essential to allowing resection of any residual flap and/or avoiding exclusion of 1 of the lumens while constructing the anastomosis. Improvements in patient outcome may be achieved by appropriately timed echocardiography-guided surgery.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Professor Aung Oo discusses the importance of both antegrade and retrograde surgical fenestrations for chronic aortic dissections and the importance of transesophageal echocardiography in detecting intraoperative complications. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00083-3/fulltext.
References
- 1.Lopez-Marco A., Adams B., Oo A. Thoracoabdominal aneurysmectomy: operative steps for Crawford extent II repair. J Thorac Cardiovasc Surg Tech. 2020;3:25–36. doi: 10.1016/j.xjtc.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouzounian M., LeMaire S.A., Weldon S., Coselli J.S. Open repair of thoracoabdominal aneurysm: step-by-step. Oper Tech Thorac Cardiovasc Surg. 2018;23:2–20. [Google Scholar]
- 3.Chiesa R., Rinaldi E. Thoracoabdominal aortic aneurysms open repair: a multimodal approach. J Vis Surg. 2018;4:128. [Google Scholar]
- 4.Goldstein S.A., Evangelista A., Abbara S., Arai A., Asch F.M., Badano L.P., et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:119–182. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista A., Flachskampf F.A., Erbel R., Antonini-Canterin F., Vlachopoulos C., Rocchi G., et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010;11:645–658. doi: 10.1093/ejechocard/jeq056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Professor Aung Oo discusses the importance of both antegrade and retrograde surgical fenestrations for chronic aortic dissections and the importance of transesophageal echocardiography in detecting intraoperative complications. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00083-3/fulltext.