Abstract
The versatility and applicability of thermoresponsive polymeric systems have led to great interest and a multitude of publications. Of particular significance, multifunctional poly(N-isopropylacrylamide) (PNIPAAm) systems based on PNIPAAm copolymerized with various functional comonomers or based on PNIPAAm combined with nanomaterials exhibiting unique properties. These multifunctional PNIPAAm systems have revolutionized several biomedical fields such as controlled drug delivery, tissue engineering, self-healing materials, and beyond (e.g., environmental treatment applications). Here, we review these multifunctional PNIPAAm-based systems with various cofunctionalities, as well as highlight their unique applications. For instance, addition of hydrophilic or hydrophobic comonomers can allow for polymer lower critical solution temperature modification, which is especially helpful for physiological applications. Natural comonomers with desirable functionalities have also drawn significant attention as pressure surmounts to develop greener, more sustainable materials. Typically, these systems also tend to be more biocompatible and biodegradable and can be advantageous for use in biopharmaceutical and environmental applications. PNIPAAm-based polymeric nanocomposites are reviewed as well, where incorporation of inorganic or carbon nanomaterials creates synergistic systems that tend to be more robust and widely applicable than the individual components.
Keywords: bioengineering, biomaterials, composites, drug delivery systems, stimuli-sensitive polymers
INTRODUCTION
Intelligent, smart, or responsive can all be used to describe polymers that possess the ability to respond to environmental changes in a regulated and reproducible manner. These changes are a response to physical or chemical alterations in the environment, such as shift in temperature, pressure, electric or magnetic field, ionic strength, or pH. Research involving the synthesis and applicability of stimuli-responsive polymers has escalated significantly over the last couple decades, catapulting these advanced materials to the forefront of several research fields, including biomedical and environmental.1-4 Systems that are temperature, or thermo, responsive have been one of the most prevalent for smart polymer design and are characterized by their thermoreversible properties in aqueous solutions.5-7 Thermoresponsive polymers exhibit a phase change at their critical solution temperature and such behavior can be attributed to disruption of intra and intermolecular interactions that cause the polymer to either expand or collapse within the aqueous solvent.8 Polymers with a lower critical solution temperature (LCST) will display phase separation (e.g., precipitation) above a specific temperature, while those with an upper critical solution temperature will display phase separation (e.g., precipitation) below a specific temperature.1,8 In hydrogel systems, where there is an increased or decreased swelling around this transition temperature, investigators sometimes refer to this swelling transition as the volume phase transition temperature (VPTT).
The most extensively studied thermoresponsive polymers are those containing poly(N-isopropylacrylamide) (PNIPAAm). For the purpose of this review, PNIPAAm will be used as the abbreviation for PNIPAAm. However, it should be noted that there is a multitude of abbreviations used in the literature for this compound: P(NIPAAm), pNIPAM, PNIPAm, PNIPAM, P(NIPA), and so forth. Characteristic behavior for these polymers consists of oscillation between hydrophilic and hydrophobic states by varying the temperature to below and above the polymer LCST, which usually ranges from 32 to 35 °C for pure PNIPAAm.9-11 Hydrogen bonding between water molecules and the functional units present in the PNIPAAm polymer is the determining factor of its LCST.12 Accordingly, above the LCST, PNIPAAm molecules will demonstrate aqueous insolubility.
One attractive quality that PNIPAAm possesses is the ease in which the functionality of the polymer can be designed and modified through the addition of comonomers or addition of nanoparticles, as shown in Figure 1. The resulting copolymers and nanocomposites are relatively easy to synthesize through various polymerization strategies.13 Incorporation of cofunctionality will often shift the polymer LCST and can produce enhanced materials with tunable attributes, depending on the desired application.14
Figure 1.
The potential for PNIPAAm functionalized polymers through addition of comonomers or incorporation into composites provides facile and highly purposeful polymeric systems, with vast potential in biomedical, environmental, and pharmaceutical applications.
Since its debut around 50 years ago, an astonishing amount of articles have reported on PNIPAAm polymers and their applicability in sectors such as environmental science, energy, physics, astronomy, and, most notably, biomedicine.13,15 This review intends to highlight some recent progress in the area of multifunctional thermoresponsive polymers as well as their unique applications. Since these multifunctional PNIPAAm systems have revolutionized several biomedical fields such as controlled drug delivery, tissue engineering, self-healing materials, and beyond (e.g., environmental treatment applications), it is important to review the most recent technologies to assess their pertinent contributions. In addition to systems containing functional comonomers, polymeric nanocomposite systems will also be discussed, in which PNIPAAm systems are combined with nanoparticulates to create an array of unique systems having vast potential. In this review, focus will primarily be on contributions made in last 10 years concerning PNIPAAm-based systems containing cofunctionality to enhance their applications as biomaterials and beyond.
SYNTHETIC SMART POLYMERS
Introducing PNIPAAm with a comonomer can help alter the thermoresponsive property of the synthesized copolymer in order to suit the desired application. In general, hydrophilic comonomers shift the LCST to a higher temperature, whereas hydrophobic comonomers shift the LCST downward and can be useful for many applications.16-21 Hydrophilic/hydrophobic comonomers have been reported to produce copolymers that are widely applicable as biomaterials and in environmental remediation technologies.22,23
Hydrophilic Comonomers
Hydrophilic monomers have been used to shift polymer LCST to higher temperatures, create multiresponse systems, or introduce functionality to increase affinity for target compounds.23-25 The introduction of hydrophilic comonomers into PNIPAAm-based polymers to increase the LCST has been utilized to expand the application of these responsive materials to systems where initial temperatures are greater than the LCST of neat PNIPAAm (e.g., greater than 37 °C for in vivo triggering applications).26 PNIPAAm copolymers with a hydrophilic, ionic comonomers have been widely studied as multiresponsive systems (i.e., pH as well as temperature responsive), and these stimuli-responsive materials can be used for drug delivery applications where drugs can be encapsulated by the polymer network and released upon response to pH or temperature stimulus. In other applications, the hydrophilic comonomer can provide increased affinity for target compounds, which can be useful for contaminant removal applications and for enhanced loading of drugs.
Mahida and Patel, who focused on removing hazardous dyes from water using a poly(NIPAAm/acrylic acid/N-allyisatin) nanohydrogel, incorporated a hydrophilic comonomer to facilitate the removal of harmful substances from contaminated water sources.27 The research described using a microemulsion polymerization with ethylene glycol dimethylacrylate as a crosslinker to form nanohydrogels. The nanohydrogels were added into a solution contaminated with dyes such as methylene blue, auramine O, and chrysoidine. The adsorption of these dyes was studied by varying parameters including time, temperature, pH, initial dye concentration, and adsorption dose, and it was reported that all three dyes were effectively removed (Table I).
Table I.
Cofunctionalities and Main Application Areas of Multifunctional PNIPAAm Polymers and Composites Reviewed in This Article
| Cofunctional addition | Application | Author, year | Refs. | ||
|---|---|---|---|---|---|
| Syn-hydrophilic | AA, N-allyisatin | Removal of hazardous dyes | Mahida and Patel, 2016 | 27 | |
| 2-Aminoethyl methacrylamide | Flocculation of MFTs | Zhang et al., 2017 | 28 | ||
| AA | Copper ion adsorption | Chen et al., 2013 | 34 | ||
| Hydroxyethyl methacrylate | High elasticity and actuation | Liu et al., 2017 | 37 | ||
| AA | Drug delivery | Zhang et al., 2017 | 44 | ||
| Maleic acid | Actuator | Fundueanu et al., 2017 | 45 | ||
| N,N-dimethylacrylamide-b-lactide, N,N-dimethylacrylamide-b-ε-caprolactone | Drug delivery | Li et al., 2011 | 46 | ||
| Syn-hydrophobic | Maleic anhydride-β-cyclodextrin | Drug delivery | Wang et al., 2018 | 48 | |
| Azobenzene containing methacrylate | Release of Nile Red | Feng et al., 2010 | 49 | ||
| AA, MAA, EAA | Tunable pH and temperature-responsive system | Lu et al., 2013 | 50 | ||
| Chrysin multiacrylate, 4,4′-dihydroxybiphenyl diacrylate, curcumin multiacrylate, quercetin multiacrylate | Pollutant binding | Tang et al., 2017 | 46,51,52 | ||
| Natural | Sodium alginate | Oil/gas riggs, drug delivery | Ciocoiu et al., 2018 | 63 | |
| Hyaluronic acid | Transdermal drug delivery | Kim et al., 2018 | 64 | ||
| Chitosan | Drug delivery | Chen et al., 2013 | 67 | ||
| Ulvan | In situ hydrogel forming for biomedical devices | Morelli et al., 2016 | 68 | ||
| DNAzyme | Biocatalysts, biosensing | Li et al., 2018 | 70 | ||
| Nanomaterials | Gold | AuNRs | Biosensors and nanoreactors | Zhang et al., 2017 | 85 |
| Gold core nanoparticles | Optical photonic devices and soft robotics | Wei et al., 2019 | 86 | ||
| Carbon | GO | Molecular separation membranes | Liu et al., 2017 | 89 | |
| Multiwalled carbon nanotubes | Remote-controlled drug delivery | Satarkar et al., 2010 | 90 | ||
| GO | Electrochemical biosensor | Zhang et al., 2019 | 91,92 | ||
| GO sheets | Composite smart fibers | Ma et al., 2019 | 93 | ||
| Iron Oxide | IONPs with curcumin | Targeted drug delivery and hyperthermia treatment | Patra et al., 2015 | 98 | |
| IONPs | Remote-controlled microfluidic valve | Satarkar et al., 2009 | 99 | ||
| IONPs | Drug delivery | Li et al., 2016 | 102 | ||
| lONPs | Micro- and nanoscale medical devices | Frimpong et al., 2008 | 103,104 | ||
Zhang et al. reported the use of PNIPAAm for flocculation and dewatering of mature fine tailings (MFT).28 As seen in Figure 2, the flocculation of MFT in pure PNIPAAm has a higher initial settling rate (ISR) as one raises the temperature, most likely due to the hydrophobic interaction between PNIPAAm and the MFT particles. However, due to the neutral nature of PNIPAAm, it could not effectively bind to negative fine particles in the supernatant, leaving the solution quite turbid. This issue was addressed through addition of poly (acrylamide-st-diallyldimethylammonium choride) or poly(AAm-st-DADMAC). Zhang and group found that the P(AAm-st-DADMAC) successfully binds the fine negative particles and the solution becomes clear with high ISR. Additionally, PNIPAAm was copolymerized with a cationic comonomer, 2-aminoethylmethacrylamide hydrochloride (AEMA), forming poly(AEMA-st-NIPAm), which reportedly has the same effect with high ISR and resulting supernatant clarity.
Figure 2.
Effect of hydrophilic comonomer addition to PNIPAAm for the purpose of MFT flocculation.
Environmental pollution stemming from heavy metal waste discharge by industries such as battery manufacturing, electroplating, and metal finishing has been a recurring issue and efforts are being made to remove these specific contaminants from water.25,29-33 Chen et al. attempted to mediate this problem using PNIPAAm with acrylic acid (AA) to remove copper ions from aqueous systems.34 Using emulsion polymerization techniques, P(NIPAAm-co-AA) was synthesized, and the addition of AA was chosen in hopes of using it to chelate Cu2+ ions. The VPTT was reported to significantly decrease after Cu2+ adsorption. The Cu2+ adsorption data was fitted into Langmuir model and the maximum adsorption capacity, qm, obtained was 67.25 mg g−1. The adsorption kinetics did follow a pseudo-second-order kinetic model and thermodynamic studies showed that ΔG° was negative and ΔH° was positive indicating adsorption was favorable and endothermic.
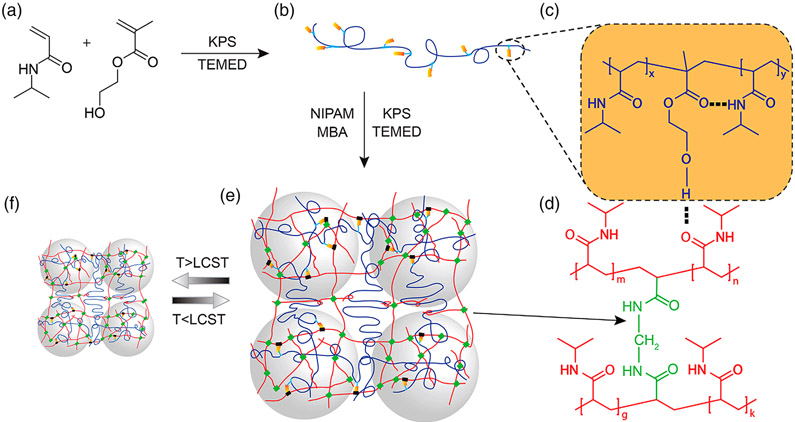
Fiber shape materials have a unique property of being flexible and long and can have great purpose in biomedical applications.35,36 Liu et al. synthesized a core–shell microfiber system with a sodium alginate core and semi interpenetrating polymer network shell consisting of PNIPAAm copolymerized with poly(n-isopropylacrylamide-co-hydroxyethylmethacrylate), as shown in Figure 3.37 The elasticity of these microfibers was examined by attaching a load of 3.6 g at one end and raising the environmental temperature to above LCST, where the microfiber collapsed in length, and subsequently lowering the temperature, finding the microfiber returning to its original length. It was therefore successfully demonstrated that these microfibers can function as an actuator, responding to thermal stimuli even with an attached load.
Figure 3.
Synthesis of semi-IPN hydrogel networks: (a) NIPAAm and HEMA monomer addition. (b) Linear PNIPAAm-HEMA) copolymer. (c–e) Representation of hydrogel below polymer LCST in a swollen state. In the PNH gel, the second network was form by covalent crosslinking (green square) and networks are held together by hydrogen bonding (black rectangle) and chain entanglement. Gray spheres represent dense crosslinked regions within the hydrogel. (f) PNH hydrogel below polymer LCST in a collapsed state.
PNIPAAm comonomers have applications in various biomedical fields like drug delivery, gene delivery, tissue engineering.24,38-43 Zhang et al. synthesized a hydrogel with glycol chitosan and a dibenzaldehyde terminated copolymer, poly(n-isopropylacrylamide-co-acrylic acid).44 This hydrogel is pH responsive as well as thermoresponsive due to the presence of AA and NIPAAm, respectively. The presence of imine crosslinkages allows these hydrogels to display self-healable attributes and injectable capabilities. The hydrogel is noncytotoxic and can also support cell growth in its network. It was studied for controlled release of rhodamine B dye and cisplatin in a specific surrounding pH and temperature. Fundueanu et al. used a different strategy to synthesize a pH/temperature-responsive system by developing a PNIPAAm-co-maleic acid copolymer and investigating the effect of pH and triggering agents on the polymer network.45 It was found that the polymer lost thermoresponsive properties at pH 7.4, but when a trigger molecule is introduced, like diphenhydramine (DPH), the negatively charged carboxylic groups in the polymer would interact with the positively charged moieties of the DPH, thereby activating the microgels and causing them to collapse, as depicted in Figure 4. This demonstrated successful development of microgels that have controlled behavior upon introduction of stimuli, that is, pH change or introduction of a triggering agent.
Figure 4.
Conceptual representation of operating principle of maleic acid-functionalized PNIPAAm system in simulated physiological fluids: isotonic solution at pH = 5.7 [pathway (a)] and phosphate-buffered saline at pH = 7.4 [pathway (b)].
In their report, Li et al. synthesized temperature- and pH-responsive diblock copolymer micelles of poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide-b-lactide) (PID118-b-PLA59) and poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide-b-ε-caprolactone) (PID118-b-PCL60) by reversible addition fragmentation chain transfer (RAFT).46 The micelles were then characterized by a number of different techniques to determine their structural and physical properties. The VPTT of these micelles were found to be around 40 °C. To determine drug delivery applicability, micelles were loaded with the drug Adriamycin (ADR) and intracellular uptake of ADR was shown to significantly increase above the polymer VPTT. Flow cytometry and fluorescent microscopy demonstrated that the transport of ADR increased fourfold as compared to commercial ADR formation Taxotere.
Hydrophobic Comonomers
Hydrophobic monomers have been primarily used to shift polymer LCST to lower temperatures (e.g., closer to ambient temperatures), introduce functionality to increase affinity for target compounds, and so forth.16,21 PNIPAAm-based systems are utilized in numerous separation processes, making it advantageous to lower polymer LCST to near ambient temperatures in order to lower energy input for transition. In addition, the incorporation of hydrophobic comonomers can be used for hydrophobic contaminant separations in environmental remediation applications.47 Drug loading for hydrophobic compounds can be enhanced by having a hydrophobic moiety in the system, and these applications are also discussed below.48
Introducing a hydrophobic comonomer into a PNIPAAm network can aid in drug delivery applications where the drug is hydrophobic in nature. A similar concept was used by Wang et al. in development of poly(maleic anhydride-β-cyclodextrin-co NIPAAm) (poly(MAH-β-CD-co-NIPAAm) hydrogels.48 The gels have thermo and pH stimuli groups present within them. The model drug, naproxen sodium, was identified and captured by MAH-β-CD and can be released by a temperature or pH trigger as illustrated in Figure 5.
Figure 5.
(a) Maleic anhydride-ß-cyclodextrin functionalized PNIPAAm hydrogels exhibit swelling behavior below polymer LCST and are able to drug load; (b) Drug release occurs above polymer LCST; (c) Drug release in low pH similar to that of gastric juice; (d) Drug release in pH similar to that of intestinal fluid.
Feng et al. developed thermo and photoresponsive micelles with a hydrophilic component of poly(ethylene oxide) and a hydrophobic copolymer component consisting of PNIPAAm and azobenzene containing methacrylate.49 The hydrodynamic radius of the micelle was dependent on surrounding temperature and the encapsulated substance, Nile Red, was released by manipulating the hydrodynamic radius. It was also found that UV irradiation did not disrupt the micelle but rather changed the hydrophobicity, indicating that the micelle is also light responsive as well.
Lu et al. synthesized a series of homo- and copolymer brushes with PNIPAAm using surface-initiated RAFT, which contained 2-alkylacrylic acids with increasing hydrophobicity, that is, AA, methacrylic acid (MAA), and 2-ethylacrylic acid (EAA).50 The copolymers were denoted as P(AA-co-NIPAM), P(MAA-co-NIPAM), and P(EAA-co-NIPAM), respectively. When tested for their hydrophobicity and temperature responsiveness with varying the pH, it was found that P(AA-co-NIPAM) and P(MAA-co-NIPAM) showed large changes in swelling and contact angles in the pH range of 3–6 but were not very temperature sensitive. Conversely, P(EAA-co-NIPAM) was pH-responsive as well as thermo in the physiological pH range, making it a better fit than the P(AA-co-NIPAM) and P(MAA-co-NIPAM) polymers for use in biomedical applications.
The use of PNIPAAm-based copolymers for environmental remediation is also well known. Tang et al. have developed a PNIPAAm copolymer with acrylate forms of hydrophobic polyphenols like curcumin, quercetin, 4,4′-dihydropxybiphenyl, and chrysin for binding pollutants like polychlorinated biphenyls.47,51,52 A series of temperature-responsive crosslinked PNIPAAm copolymers were developed with varying contents of curcumin multiacrylate quercetin multiacrylate, 4,4′-dihydroxybiphenyldiacrylate, and chrysin multiacrylate. The LCST behavior of these hydrogels were examined, and it was determined that as concentration of the hydrophobic comonomer is increased, the LCST decreases. Temperature dependent swelling studies were also conducted, and it was found that the swelling ratio decreased with the increase in temperature as well.
NATURAL SMART POLYMERS
Interest toward development or incorporation of naturally occurring materials stems from a push to replace petroleum-based or synthetically engineered polymeric materials with greener, more sustainable ones.53 Not only are natural materials typically more biocompatible and biodegradable but they also tend to be extremely cost-effective due to their abundance and renewability. Accordingly, the importance of PNIPAAm biocompatibility and potential biodegradability is emphasized by their significant utilization in drug delivery and tissue engineering research.54-57 The viscoelastic behavior generally exhibited by PNIPAAm polymers is advantageous for interactions with living tissues or drug delivery systems.
Alginates, chitosan, cellulose, dextran, and starches are all sustainable and naturally occurring materials that have the potential to increase PNIPAAm mechanical properties and functionality all while remaining biocompatible and nontoxic, lending these materials to a multitude of applications such as sorbent development for water treatment and remediation, biomedical and pharmaceutical.58-62
Ciocoiu et al. grafted PNIPAAm side chains onto a sodium alginate backbone, resulting in copolymers that can cover a broad range NIPAAm composition and molecular weight.63 Thermothickening behavior was observed for temperatures over the LCST of PNIPAAm only if conditions of solution concentration and copolymer weight composition are optimized such that intramolecular hydrophobic interactions are more predominant than intermolecular interactions. Such materials could be particularly useful for drug delivery systems. Kim et al. prepared crosslinked PNIPAAm hydrogels with hyaluronic acid via radical polymerization and Michael addition in order to create a transdermal delivery system of luteolin for psoriasis skin disorder.64 A graphic representation of the synthesis process is shown in Figure 6. Hyaluronic acid is a linear polysaccharide found to have significant involvement in maintaining extracellular skin moisture and viscoelasticity.65,66 A 42.8% luteolin incorporation efficiency was achieved, and the highest drug release was observed at 25 °C and pH 5.5 while conditions similar to that of psoriasis skin conditions, 37 °C and pH 7.4, a gradual drug release was observed.
Figure 6.
Synthesis of a PNIPAAm-based hydrogel functionalized with hyaluronic acid for a transdermal drug delivery system.
Chen et al. reported successful preparation of a comb-shaped copolymer comprised of chitosan main chains with PNIPAAm side chains.67 In order to do so, phthaloyl-chitosan is functionalized with -bromosisobutyryl bromide to initiate PNIPAAm growth in the presence of catalyst CuCl/bpy. The LCST of the resulting chitosan-graft-PNIPAAm copolymer is reported to be 33 °C at pH 6.3 and 35 °C at pH 5.0, which is indicative of pH-responsive behavior as well as temperature responsive, most likely due to the chitosan NH2 groups.
Morelli et al. investigated ulvan-based hydrogels by grafting PNIPAAm chains onto acryloyl modified ulvan backbones via radical polymerization induced by UV irradiation through a “grafting-from” method.68 Ulvan is a sulphated polysaccharide that can be extracted from green seaweeds or algal biomass waste.69 These systems show promise for use as in situ hydrogel forming systems for biomedical applications.
Another highly functional system created by Li, Wang, and Guo, intended for biosensing applications, consists of DNAzyme functionalized PNIPAAm microgels prepared via one-step precipitation polymerization.70 DNAzymes are functional nucleic acids that have the ability to mimic catalytic activities of enzymes and have been reported as catalyzing agents in the presence of certain cofactors, such as bond formation or cleavage reactions.71,72 The spherical microgels are reported to have a layered structure with DNAzymes mainly located on the surface of a PNIPAAm core and exhibit highly catalytic activities in aqueous solution. These materials have high recyclability potential through temperature-mediated separation from solution. Additionally, the group demonstrates the ease in which different types of DNAzymes can be facilely prepared by using two typical catalytic DNA structures for validation, the Mg2+-dependent DNAzyme and the hemin-G-quadruplex horseradish peroxidase-mimicking DNAzyme, and establish retention of initial catalytic ability, 91 and 80%, respectively, after eight consecutive catalysis cycles. Such advancements have set the paradigm for effective and affordable DNA-based biocatalysts for bioassays and biosensors.
NANOCOMPOSITE SMART POLYMERS
Incorporation of inorganic or carbon nanomaterials with thermoresponsive polymers creates synergistic interactions in which the composite materials provide a more robust and complex design as compared to the individual pieces. Although PNIPAAm polymers and nanomaterial systems individually have potential for use in biomedical and environmental applications, they each have respective disadvantages limiting their broad applicability.73 For instance, simple polymer networks often do not contain the required mechanical and biological properties for several tissue engineering applications since it can be challenging to maintain polymer structural integrity throughout repeat hydrophilic/hydrophobic transitions, while also realizing efficient response rates.73 Additionally, there is a growing demand for smart polymer technologies in the fields of actuators, biosensors and biologic devices, where the polymer response to magnetic, optical, and/or electrical stimuli is the basis of measurement.73-75 However, most polymeric materials are comprised of inert and magnetically or electrically unresponsive components.75,76 Fortunately, the incorporation of NPs into smart polymer networks has produced innovative materials with a diverse set of functionalities that are capable of overcoming these challenges. Recently, there have been a few comprehensive reviews on magnetic nanoparticles and polymer nanocomposites that delve more extensively into composite systems and provide detailed accounts on remote-controlled nanocomposites and their applications.77,78 In 2015, a detailed review on magnetic nanoparticles and their composites was reported by Hauser et al., where they discuss interesting macro- to nanoscale technologies that have potential to utilize remote-controlled drug release through application of a static or alternating magnetic field (AMF).77 Satarkar, et al. also reviewed hydrogel nanocomposites that possess the unique ability to be heated through absorption of heat via remote-controlled application of AMF or IR.78
Gold Nanomaterials
Thermoresponsive polymeric systems modified with gold nanomaterials have drawn interest because of their enhanced optoelectrical, external stimuli-responsive properties.79,80 Additionally, and of particular importance for use in biomedical applications, gold nanomaterials have proven to be biocompatible and have shown success with in vivo studies.81-83 Gold-PNIPAAm nanocomposites not only exhibit the thermosensitive behavior seen with most PNIPAAm-based systems but also have the ability to exhibit a light-sensitive behavior due to the photothermal effect of the gold nanomaterials and its surface plasmon resonance.84
Zhang et al. utilize an electrospinning technique to prepare gold nanorod (AuNR)/PNIPAAm composite hydrogel films with the aim of developing composites that display fast thermal/optical responses, high heating rates, and high structural integrity.85 Additionally, AuNR/PNIPAAm films can be easily scaled up due to the high feed speed and coordinating productivity of the electrospinning process. For comparison, a casting film was also prepared using the same electrospun solution and simply using a glass template to form the polymer. The maximum swelling ratio for the electrospun composite film was measured as 8.7, whereas the casting film was only 3.9, highlighting the increased flexibility afforded through the electrospinning process. Incorporation of AuNRs is reported to enhance the thermal response rate of PNIPAAm, owing to the plasma effect of heat releasing gold. Additionally, AuNR incorporation increases hydrogel thermo/photoresponsivity, resulting in a high heating rate with fierce size change. These polymer nanocomposites could be most successfully utilized in applications such as “smart” sensors, nanoreactors, or as an artificial muscle.
Wei and Serpe also take advantage of Au nanoparticle (AuNP) optical properties by synthesized AuNP@PNIPAAm core/shell systems through seed mediation free-radical polymerization.86 Thermoresponsiveness of the nanoparticles was characterized with photon correlation spectroscopy by measuring the hydrodynamic radius as a function of temperature, with an observed volume phase transition at 32 °C, confirming little to no hindrance from the AuNP core on the PNIPAAm shell. The duo goes on to fabricate a light-responsive photonic device by layering the particles between thin sheets of Au. As such, the temperature and specific light wavelength responsiveness can be attributed to the PNIPAAm shell and AuNP core, respectively. These optical devices have great potential for locally triggered drug delivery platforms, active optics or soft robotics.
Carbon Nanomaterials
Carbon 1D (e.g., nanotubes) and 2D [e.g., graphene oxide (GO)] nanomaterials have become materials of interest for a variety of fields. For instance, GO has lately been incorporated into hydrogel systems because of its desirable attributes such as flexible architecture and electrochemical properties.87,88 Liu et al. has reported synthesis of a nanograting membrane comprised of PNIPAAm covalently bound to GO via free-radical polymerization, as shown in Figure 7.89 The resulting temperature-responsive membrane has significantly reduced permeance at higher temperatures −12.41 m−2 h−1 bar−1 at 25 °C to 1.81 m−2 h−1 bar−1 at 50 °C, giving rise to a high gating ratio of around 7. Regulation of temperature thereby enables the smart GO membrane to separate small molecules of varying sizes and shows promise for many applications in fields such as fluid transport systems, microfluidic chip systems, and molecular separation devices.
Figure 7.
Synthesis of a PNIPAAm-based thermoresponsive GO membrane and schematic representation of its temperature-driven water gating capabilities. Reprinted from Ref. 89. This work is licensed under Creative Commons 4.0.
Satarkar et al. reported the synthesis of nanocomposite systems containing PNIPAAm-based hydrogels with multiwalled carbon nanotubes.90 The nanocomposite LCST was fine-tuned for use in physiological application through addition of varying amounts of acrylamide, which shifted LCST to higher temperatures. This system provided yet another example of how hydrophilic monomer incorporation can alter LCST temperatures. The addition of carbon nanotubes decreased hydrogel swelling behavior but was shown to enhance mechanical properties. Upon application of a 13.56 MHz radiofrequency field, the nanocomposite temperature was raised significantly and showed direct correlation to the amount of carbon nanotube loading. As such, these materials show great promise for use in applications from tissue engineering to cancer treatment and remote-controlled drug delivery technologies.
Zhang et al. have fabricated a variety of interesting PNIPAAm– GO nanocomposites.91,92 One system uses a covalent “grafting-from” methodology in order to combine PNIPAAm-GO with glassy carbon electrodes that will possess temperature-related switching capabilities used to achieve switchable electrocatalysis toward 1,4-dihydro-β-nicotinamide adenine dinucleotide and dopamine biomolecules.91 Incorporation of GO successfully promotes electron mobility in the electrode while PNIPAAm equips it with a temperature-based switching effect. Another method reported a very interesting dual-responsive and thermal-responsive reduced GO (rGO) system in which thermoresponsive PNIPAAm is embedded with imidazolium polymeric ionic liquid via copolymerization reaction. Afterward, the formed copolymer was noncovalently anchored to rGO through cation-π coupling between the polymeric ionic liquid units and graphene during an in situ reduction. The resulting functionalized rGO is responsive to both temperature and anion stimuli where the function of modified anions can be promoted and inhibited in response to the temperature stimuli. It is proposed that this type of preparation strategy can be used to create smart rGO materials as multifunctional systems in the field of electrochemistry and catalysis. Ma et al. covalently grafted PNIPAAm onto edges of GO sheets using a facial method crosslinked by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide in aqueous solution.93 Subsequently, a PNIPAAm-grafted-graphene composite fiber was prepared through wet spinning of the polymer dispersion followed by chemical reduction via hydroiodic acid. The synthesized smart fiber shows significant potential for incorporations into intelligent strain sensors, textiles, actuators, wires, and switches.
Iron Oxide Nanomaterials
Iron oxide nanoparticles (IONPs) have a wide variety of applications spanning through multiple domains such as biomedical, catalysis, and environmental fields.94-96 Their magnetic property can be exploited in a variety of ways: (1) special manipulation through use of a static magnetic field, (2) heat generation in response to an AMF for local destabilization, (3) tunable size formation for desired application.97 From an environmental standpoint, for example, incorporation of IONPs into polymeric systems could address some of the concerns that arise when using conventional water sorbents by providing a quick method of sorbent removal and efficient form of recycling. IONP composites are also an attractive choice for nanocarriers in several biomedical applications. As such, Patra et al. have developed PNIPAAm coated IONPs for use as a targeted delivery system of curcumin and treatment of hyperthermia.98 Microwave radiation was used to modify the surface of IONPs with carboxymethyl cellulose, folic acid, and PNIPAAm-co-poly(glutamic acid), shifting the LCST of the copolymer to an appropriate drug delivery temperature of 40 °C. Successful and high-capacity (89%) drug loading was achieved followed by efficient drug release at the desired conditions. Additionally, targeting behavior and overall efficiency of the system was examined through construction of a simple magnetic capturing system in which it was found that around 99% of the NPs accumulated around a static magnet in 2 min.
Our group has extensively studied magnetic hydrogel nanocomposites that can be manipulated through remote-controlled heating via application of an AMF.99-101 These nanocomposite systems have been shown to be especially useful in microfluidic devices or for drug delivery technologies. For example, Satarkar et al. presented a very interesting application of a PNIPAAm and IONP nanocomposite as a remote-controlled microfluidic valve.99 As illustrated in Figure 8, expansion and collapse of the synthesized nanocomposite was controlled through AMF exposure and corresponds to closing and opening of a valve on the Y-junction channel of a microfluidic chip. In order to assess reversible valve transitions, multiple ON–OFF cycles were induced along with analysis of pressure at the inlet and successful reproducible thermoresponsive behavior was observed.
Figure 8.
Conceptual illustration of a remote-controlled hydrogel nanocomposite valve responding to an AMF with ON–OFF behavior through swollen and collapsed states.
Li et al. successfully designed a dual-responsive polymer capsule system with a magnetic iron oxide core.102 A crosslinked polymerization method was employed to develop a drug carrier system that undergoes volume phase transition by either modulating environmental temperature or introducing glutathione to degrade the polymer capsule for hydrophilic or hydrophobic drug release. Doxorubicin hydrochloride (DOX) is used as the model hydrophilic drug and curcumin is used as the model hydrophobic drug in order to examine the capsules thermoresponsive or polymer biodegradation release capabilities. It was reported that DOX is rapidly released when environmental temperature is increased.
Frimpong et al. reported on core-shell structures and composite network systems using PNIPAAm and magnetite nanoparticles.103,104 Synthesis of a core-shell system was described in which magnetite IONPs are functionalized with PNIPAAm. Iron IONPs were first obtained through a one-pot coprecipitation method which produces either hydrophobic, oleic acid-coated or hydrophilic, citric acid-coated nanoparticles. Afterward, ligand exchange was conducted with various bromine alkyl halides and bromosilane in order to provide initiating sites for functionalization with PNIPAAm via atom transfer radical polymerization. It was demonstrated that the degree of polymeric coating varied with different ligand exchanges and was shown to increase with reaction time. These magnetic and temperature-responsive core-shell polymeric composites have shown significant promise for a multitude of biomedical applications.
CONCLUSIONS
This review highlights examples from recent years that demonstrate advanced materials with enhanced properties using PNIPAAm frameworks with unique cofunctionality. A diverse toolbox of innovative and interesting thermoresponsive polymers and polymer nanocomposites capable of unique properties and responses to address needs in material design for biomedical devices, drug delivery, environmental remediation, and beyond. Although PNIPAAm-based polymers have been reported on for decades, recent progression of highly functionalized thermoresponsive copolymer and composite systems have the capability to revolutionize the use of these materials. The potential to create unique and versatile temperature-responsive polymers is endless with the introduction of additional cofunctionalities beyond that of the thermoresponsive polymer. With this review, we have only begun to scratch the surface of what can be realized through advancement of these materials. The importance of continued materials optimization and transition into use cannot be stressed enough, as the most pertinent health challenges we face cannot be successfully addressed without them.
ACKNOWLEDGMENTS
Research reported was supported by NIEHS/NIH grant P42ES007380. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Biography

E. Molly Frazar is currently pursuing a Ph.D. in Chemical Engineering at the University of Kentucky. She has completed a Bachelor of Science in Chemistry at the University of Texas in Austin, Texas, as well as a Bachelor of Science in Chemical Engineering at the University of Kentucky. Her current research focuses on development of interesting and efficient sorbents for water remediation and treatment.

Rishabh A. Shah is currently a Chemical Engineering Ph.D. student in the Department of Chemical and Materials Engineering at the University of Kentucky. He completed his undergraduate degree in Chemical Engineering at Institute of Chemical Technology, Mumbai, India. Currently, he is working on development and testing of novel stimuli-responsive polymer networks.

Thomas D. Dziubla is the Professor and Chair of the Department of Chemical and Materials Engineering at the University of Kentucky. He has extensive experience in the synthesis of characterization of functional polymeric materials and biomaterials for a wide variety of applications, including the development of red/ox active polymers for the regulation of cellular oxidative stress related disorders.

J. Zach Hilt is the Gill Eminent Professor of Chemical Engineering in the College of Engineering at the University of Kentucky. As an investigator of polymers, nanoparticles, and nanocomposites, his research program pursues the rational design and development of innovative materials with applications in controlled drug delivery, environmental treatment, and beyond.
REFERENCES
- 1.Phillips DJ; Gibson MI Polym. Chem 2015, 6, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei M; Gao Y; Li X; Serpe MJ Polym. Chem 2017, 8, 127. [Google Scholar]
- 3.Koetting MC; Peters JT; Steichen SD; Peppas NA Mater. Sci. Eng 2015, 93, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens DE; Jian Y; Fang JE; Slaughter BV; Chen YH; Peppas NA Macromolecules. 2007, 40, 7306. [Google Scholar]
- 5.Kim YJ; Matsunaga YT J. Mater. Chem. B 2017, 5, 4307. [DOI] [PubMed] [Google Scholar]
- 6.Liu R; Fraylich M; Saunders BR Colloid Polym. Sci 2009, 287, 627. [Google Scholar]
- 7.Jochum FD; Theato P Chem. Soc. Rev 2013, 42, 7468. [DOI] [PubMed] [Google Scholar]
- 8.Ganesh VA; Baji A; Ramakrishna S RSC Adv. 2014, 4, 53352. [Google Scholar]
- 9.Schild HG Prog. Polym. Sci 2003, 17, 163. [Google Scholar]
- 10.Zhang J; Peppas NA Macromolecules. 2000, 33, 102. [Google Scholar]
- 11.Brazel CS; Peppas NA Macromolecules. 1995, 28, 8016. [Google Scholar]
- 12.Boutris C; Chatzi EG; Kiparissides C Polymer. 1997, 38, 2567. [Google Scholar]
- 13.Lanzalaco S; Armelin E Gels. 2017, 3, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rzaev ZMO; Dinçer S; Pişkin E Prog. Polym. Sci 2007, 32, 534. [Google Scholar]
- 15.Heskins M; Guillet JE J. Macromol. Sci. Part A Pure Appl. Chem 1968, 2, 1441. [Google Scholar]
- 16.Zhang H; Marmin T; Cuierrier É; Soldera A; Dory Y; Zhao Y Polym. Chem 2015, 6, 6644. [Google Scholar]
- 17.Guilherme MR; Da Silva R; Rubira AF; Geuskens G; Muniz EC React. Funct. Polym 2004, 61, 233. [Google Scholar]
- 18.Schmalijohann D; Oswald J; Jørgensen B; Nitschke M; Beyerlein D; Werner C Biomacromolecules. 2003, 4, 1733. [DOI] [PubMed] [Google Scholar]
- 19.Harmon ME; Kuckling D; Frank CW Macromolecules. 2003, 36, 162. [Google Scholar]
- 20.Zhang XZ; Wu DQ; Chu CC Biomaterials. 2004, 25, 3793. [DOI] [PubMed] [Google Scholar]
- 21.Picos-Corrales LA; Licea-Claverie A; Cornejo-Bravo JM; Schwarz S; Arndt KF Macromol. Chem. Phys 2012, 213, 301. [Google Scholar]
- 22.Ma X; Tang XJ Colloid Interface Sci. 2006, 299, 217. [DOI] [PubMed] [Google Scholar]
- 23.Huang G; Gao J; Hu Z; St. John JV; Ponder BC; Moro D J. Control. Release 2004, 94, 303. [DOI] [PubMed] [Google Scholar]
- 24.Schmaljohann D Adv. Drug Deliv. Rev 2006, 58, 1655. [DOI] [PubMed] [Google Scholar]
- 25.Ju XJ; Zhang SB; Zhou MY; Xie R; Yang L; Chu LY J. Hazard. Mater 2009, 167, 114. [DOI] [PubMed] [Google Scholar]
- 26.Henderson E; Lee BH; Cui Z; McLemore R; Brandon TA; Vernon BLJ Biomed. Mater. Res.— Part A 2009, 90, 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahida VP; Patel MP Arab. J. Chem 2016, 9, 430. [Google Scholar]
- 28.Zhang D; Thundat T; Narain R Langmuir. 2017, 33, 5900. [DOI] [PubMed] [Google Scholar]
- 29.Tofighy MA; Mohammadi TJ Hazard. Mater 2011, 185, 140. [DOI] [PubMed] [Google Scholar]
- 30.Hua M; Zhang S; Pan B; Zhang W; Lv L; Zhang Q J. Hazard. Mater 2012, 211–212, 317. [DOI] [PubMed] [Google Scholar]
- 31.Ozbas Z; Sahin CP; Esen E; Gurdag G; Kasgoz H J. Environ. Chem. Eng 2016, 4, 1948. [Google Scholar]
- 32.Wang JJ; Liu F Adv. Mater. Res 2013, 643, 83. [Google Scholar]
- 33.Kasgoz H; Ozbas Z; Esen E; Sahin CP; Gurdag G J. Appl. Polym. Sci 2013, 130, 4440. [Google Scholar]
- 34.Chen JJ; Ahmad AL; Ooi BS J. Environ. Chem. Eng 2013, 1, 339. [Google Scholar]
- 35.Zhang H; Niu Q; Wang N; Nie J; Ma G Eur. Polym. J 2015, 71, 440. [Google Scholar]
- 36.Slemming-Adamsen P; Song J; Dong M; Besenbacher F; Chen M Macromol. Mater. Eng 2015, 300, 1226. [Google Scholar]
- 37.Liu Y; Zhang K; Ma J; Vancso GJ ACS Appl. Mater. Interfaces 2017, 9, 901. [DOI] [PubMed] [Google Scholar]
- 38.Li G; Song S; Zhang T; Qi M; Liu J Int. J. Biol. Macromol 2013, 62, 203. [DOI] [PubMed] [Google Scholar]
- 39.Molina M; Giulbudagian M; Calderón M Macromol. Chem. Phys 2014, 215, 2414. [Google Scholar]
- 40.Hoare TR; Kohane DS Polymer (Guildf). 2008, 49, 1993. [Google Scholar]
- 41.Twaites BR; De Las Heras Alarcón C; Cunliffe D; Lavigne M; Pennadam S; Smith JR; Górecki DC; Alexander C J. Control. Release 2004, 97, 551. [DOI] [PubMed] [Google Scholar]
- 42.Twaites BR; De Las Heras Alarcón C; Lavigne M; Saulnier A; Pennadam SS; Cunliffe D; Górecki DC; Alexander C J. Control. Release 2005, 108, 472. [DOI] [PubMed] [Google Scholar]
- 43.Leobandung W; Ichikawa H; Fukumori Y; Peppas NA J. Appl. Polym. Sci 2003, 87, 1678. [Google Scholar]
- 44.Zhang Y; Fu C; Li Y; Wang K; Wang X; Wei Y; Tao L Polym. Chem 2017, 8, 537. [Google Scholar]
- 45.Fundueanu G; Constantin M; Bucatariu S; Ascenzi P Polymer (Guildf). 2017, 110, 177. [Google Scholar]
- 46.Li W; Li J; Gao J; Li B; Xia Y; Meng Y; Yu Y; Chen H; Dai J; Wang H; Guo Y Biomaterials. 2011, 32, 3832. [DOI] [PubMed] [Google Scholar]
- 47.Tang S; Floy M; Bhandari R; Sunkara M; Morris AJ; Dziubla TD; Hilt JZ ACS Omega. 2017, 2, 8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y; Yang N; Wang D; He Y; Chen L; Zhao Y Polym. Degrad. Stab 2018, 147, 123. [Google Scholar]
- 49.Feng Z; Lin L; Yan Z; Yu Y Macromol. Rapid Commun 2010, 31, 640. [DOI] [PubMed] [Google Scholar]
- 50.Lu Y; Zhuk A; Xu L; Liang X; Kharlampieva E; Sukhishvili SA Soft Matter. 2013, 9, 5464. [Google Scholar]
- 51.Tang S; Floy M; Bhandari R; Dziubla T; Hilt J Gels. 2017. 3, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang S; Bhandari R; Delaney SP; Munson EJ; Dziubla TD; Hilt JZ Mater. Today Commun 2017, 10, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Valdez O; Champagne P; Cunningham MF Prog. Polym. Sci 2018, 76, 151. [Google Scholar]
- 54.Bawa P; Pillay V; Choonara YE; du Toit LC Biomed. Mater 2009, 4, 022001. [DOI] [PubMed] [Google Scholar]
- 55.Prabaharan M J. Biomater. Appl 2008, 23, 5. [DOI] [PubMed] [Google Scholar]
- 56.Xiao L; Isner AB; Hilt JZ; Bhattacharyya D J. Appl. Polym. Sci 2013, 128, 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baroli B J. Pharm. Sci 2007, 96, 2197. [DOI] [PubMed] [Google Scholar]
- 58.Cheaburu CN; Ciocoiu O; Staikos G; Vasile C J. Appl. Polym. Sci 2013, 127, 3340. [Google Scholar]
- 59.Petrusic S; Jovancic P; Lewandowski M; Giraud S; Grujic S; Ostojic S; Bugarski B; Koncar V J. Mater. Sci 2013, 48, 7935. [Google Scholar]
- 60.Bao HQ; Li L; Leong W; Gan LH J. Phys. Chem. B 2010, 114, 10666. [DOI] [PubMed] [Google Scholar]
- 61.Lai J-Y; Luo L-J Eur. J. Pharm. Biopharm 2017, 113, 140. [DOI] [PubMed] [Google Scholar]
- 62.Acciaro R; Aulin C; Wågberg L; Lindström T; Claesson PM; Varga I Soft Matter. 2011, 7, 1369. [Google Scholar]
- 63.Ciocoiu ON; Staikos G; Vasile C Carbohydr. Polym 2018, 184, 118. [DOI] [PubMed] [Google Scholar]
- 64.Kim AR; Lee SL; Park SN Int. J. Biol. Macromol 2018, 118, 731. [DOI] [PubMed] [Google Scholar]
- 65.Collins MN; Birkinshaw C Carbohydr. Polym 2013, 92, 1262. [DOI] [PubMed] [Google Scholar]
- 66.Hemshekhar M; Thushara RM; Chandranayaka S; Sherman LS; Kemparaju K; Girish KS Int. J. Biol. Macromol 2016, 86, 917. [DOI] [PubMed] [Google Scholar]
- 67.Chen C; Liu M; Gao C; Lü S; Chen J; Yu X; Ding E; Yu C; Guo J; Cui G Carbohydr. Polym 2013, 92, 621. [DOI] [PubMed] [Google Scholar]
- 68.Morelli A; Betti M; Puppi D; Chiellini F Carbohydr. Polym 2016, 136, 1108. [DOI] [PubMed] [Google Scholar]
- 69.Wang H-MD; Li X-C; Lee D-J; Chang J-S Bioresour. Technol 2017, 244, 1407. [DOI] [PubMed] [Google Scholar]
- 70.Li F; Wang C; Guo W Adv. Funct. Mater 2018, 28, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Achenbach JC; Chiuman W; Cruz RPG; Li Y Curr. Pharm. Biotechnol 2004, 5, 321. [DOI] [PubMed] [Google Scholar]
- 72.Lu Y Chem. A Eur. J 2002, 8, 4588. [DOI] [PubMed] [Google Scholar]
- 73.Zhao F; Yao D; Guo R; Deng L; Dong A; Zhang J Nanomater. (Basel, Switzerland) 2015, 5, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pastoriza-Santos I; Kinnear C; Pérez-Juste J; Mulvaney P; Liz-Marzán L Nat. Rev. Mater 2018, 3, 375. [Google Scholar]
- 75.Peppas NA; Hilt JZ; Khademhosseini A; Langer R Adv. Mater 2006, 18, 1345. [Google Scholar]
- 76.Annabi N; Tamayol A; Uquillas JA; Akbari M; Bertassoni LE; Cha C; Camci-Unal G; Dokmeci MR; Peppas NA; Khademhosseini A Adv. Mater 2014, 26, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hauser AK; Wydra RJ; Stocke NA; Anderson KW; Hilt JZ J. Control. Release 2015, 219, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satarkar N; Biswal D; Hilt J Soft Matter. 2010, 6, 2364. [Google Scholar]
- 79.Luo C; Dong Q; Qian M; Zhang H Chem. Phys. Lett 2016, 664, 89. [Google Scholar]
- 80.Udomluck N; Baipaywad P; Lee S; Park H J. Control. Release 2015, 213, E85. [DOI] [PubMed] [Google Scholar]
- 81.Elbialy NS; Fathy MM; Khalil WM Int. J. Pharm 2015, 490, 190. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X-D; Wu H-Y; Wu D; Wang Y-Y; Chang J-H; Zhai Z-B; Meng A-M; Liu P-X; Zhang L-A; Fan F-Y Int. J. Nanomedicine 2010, 5, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owens DE III; Eby JK; Jian Y; Peppas NA J. Biomed. Mater. Res. Part A 2007, 83A, 692. [DOI] [PubMed] [Google Scholar]
- 84.Alvarez-Puebla RA; Contreras-Cáceres R; Pastoriza-Santos I; Pérez-Juste J; Liz-Marzán LM Angew. Chem. Int. Ed. Engl 2009, 48, 138. [DOI] [PubMed] [Google Scholar]
- 85.Zhang CL; Cao FH; Wang JL; Yu ZL; Ge J; Lu Y; Wang ZH; Yu SH ACS Appl. Mater. Interfaces 2017, 9, 24857. [DOI] [PubMed] [Google Scholar]
- 86.Wei M; Serpe MJ Part. Part. Syst. Charact 2019, 36, 1. [Google Scholar]
- 87.Zhu C; Lu Y; Peng J; Chen J; Yu S Adv. Funct. Mater 2012, 22, 4017. [Google Scholar]
- 88.Wang L; Lian W; Yao H; Liu H ACS Appl. Mater. Interfaces 2015, 7, 5168. [DOI] [PubMed] [Google Scholar]
- 89.Liu J; Wang N; Yu LJ; Karton A; Li W; Zhang W; Guo F; Hou L; Cheng Q; Jiang L; Weitz DA; Zhao Y Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satarkar NS; Johnson D; Marrs B; Andrews R; Poh C; Gharaibeh B; Saito K; Anderson KW; Hilt JZ J. Appl. Polym. Sci 2010, 117, 1813. [Google Scholar]
- 91.Zhang H; Zhang Q; Zhang L; Pei T; Li E; Wang H; Zhang Q; Xia L Chem. A Eur. J 2019, 25, 1535. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Q; Zhang J; Liu B; Zhang L; Dong L; Li E; Zhang H; Xia L Chem. Commun 2017, 53, 6367. [DOI] [PubMed] [Google Scholar]
- 93.Ma H; Zhang H; Cao J; Tong M; Zhao J; Li Y; Xu H; Wu W Compos. Sci. Technol 2019, 169, 186. [Google Scholar]
- 94.Gupta AK; Gupta M Biomaterials. 2005, 26, 3995. [DOI] [PubMed] [Google Scholar]
- 95.Gutierrez AM; Dziubla TD; Hilt JZ Rev. Environ. Health 2017, 32, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohno K; Mori C; Akashi T; Yoshida S; Tago Y; Tsujii Y; Tabata Y Biomacromolecules. 2013, 14, 3453. [DOI] [PubMed] [Google Scholar]
- 97.Kango S; Kalia S; Celli A; Njuguna J; Habibi Y; Kumar R Prog. Polym. Sci 2013, 38, 1232. [Google Scholar]
- 98.Patra S; Roy E; Karfa P; Kumar S; Madhuri R; Sharma PK ACS Appl. Mater. Interfaces 2015, 7, 9235. [DOI] [PubMed] [Google Scholar]
- 99.Satarkar NS; Zhang W; Eitel RE; Hilt JZ Lab Chip. 2009, 9, 1773. [DOI] [PubMed] [Google Scholar]
- 100.Satarkar NS; Zach Hilt J Acta Biomater. 2008, 4, 11. [DOI] [PubMed] [Google Scholar]
- 101.Satarkar NS; Hilt JZ J. Control. Release 2008, 130, 246. [DOI] [PubMed] [Google Scholar]
- 102.Li A; Ma H; Feng S; Liu J RSC Adv. 2016, 6, 33138. [Google Scholar]
- 103.Frimpong RA; Hilt JZ Nanotechnology. 2008, 19, 175101. [DOI] [PubMed] [Google Scholar]
- 104.Frimpong RA; Fraser S; Zach Hilt J J. Biomed. Mater. Res. Part A 2007, 80, 1. [DOI] [PubMed] [Google Scholar]