Abstract
Simple Summary
Xylaria species are known for their medicinal value and production of a variety of bioactive compounds. They usually grow on rotten wood, fallen leaves, seeds, and fruits. Some species can be found growing on soil or associated with termite nests, which belong to subgenus Pseudoxylaria. They share with other Xylaria species a suite of morphological characteristics, including conspicuous or massive upright stromata with a light-coloured interior, a longer than wide ascal apical ring, bluing in an iodine reagent, and brown unicellular ascospores possessing a germ slit. In Thailand, there are only limited reports on Xylaria diversity and taxonomy, especially on species associated with termite nests. In the present study, we describe 12 new Xylaria taxa and report two species closely resembling known species from termite nests or soil. Their morphological and cultural characteristics are described and illustrated, and their nucleotide sequences of ITS rDNA, alpha-actin, and beta-tubulin genes were obtained. Phylogenetic inference based on these sequences confirmed that all taxa analyzed belong to subgenus Pseudoxylaria and differ from all other species with sequences available in public databases. Our study is the first to report on the novel Xylaria species associated with termite nests or growing on soil in Thailand. Subgenus Pseudoxylaria is likely highly diverse in the country.
Abstract
The diversity of Xylaria species associated with termite nests in northeast Thailand was investigated. Among the 14 taxa included in this study, 11 species and one variety were described as new, and another two species resemble the existing taxa, X. escharoidea and X. nigripes. The newly described taxa are X. chaiyaphumensis, X. conica, X. fulvescens, X. ischnostroma, X. margaretae, X. minima, X. reinkingii var. microspora, X. siamensis, X. sihanonthii, X. subintraflava, X. thienhirunae, and X. vinacea. Their morphological and cultural characteristics are described and illustrated, and their ITS, α-actin and β-tubulin sequences were analysed. A dichotomous key to the 17 species of Xylaria occurring in Thailand is provided.
Keywords: Pseudoxylaria, Xylariaceae, Ascomycota, taxonomy, systematics
1. Introduction
Xylaria Hill ex Schrank is the representative genus of the family Xylariaceae, being characterised by upright, cylindrical stromata with a light-coloured interior, eight-spored asci with an amyloid apical apparatus, brown unicellular ascospores possessing a germ slit, and a geniculosporium-like anamorph [1]. They are commonly found on fallen wood, leaves, fruits, seeds, dung, soil, and termite nests. These species associated with termite nests are mainly distributed in Africa and Asia, coinciding with the geographic range of the termite species belonging to the subfamily Macrotermitinae. Species associated with termite nests and growing on soil are classified in the subgenus Pseudoxylaria Boedijn [2]. Stromata of the species in subgenus Pseudoxylaria develop from abandoned fungus combs within termite nests and finally emerge on the soil surface [3]. The taxonomic characteristics of Xylaria species from termite nests and soil have long been discussed [3,4,5,6,7]. Recently, Hsieh et al. [8] described two new species, X. insolita and X. subescharoidea, from Taiwan, increasing the known species of subgenus Pseudoxylaria to 28.
In Thailand, there are only a few reports on Xylaria species of subgenus Pseudoxylaria. Thienhirun [9] studied the xylariaceous fungi throughout the country and reported only three species of Xylaria from termite nests: X. gracillima, X. cf. melanaxis, and X. cf. nigripes. Subsequently, Srihanant and Petcharat [10] surveyed the Xylaria species in oil palm and Pará rubber plantations in southern Thailand, and they found five species growing on soil that they identified as X. acuminatilongissima, X. atrodivaricata, X. escharoidea, X. nigripes, and X. tanganyikaensis. In the current study, we surveyed Xylaria species of subgenus Pseudoxylaria in northeast Thailand. Their morphological and culture characteristics were described, and their nucleotide sequences analysed to confirm their placement within subgenus Pseudoxylaria.
2. Materials and Methods
2.1. Sample Collection and Identification
Stromata of Xylaria species were collected from termite nests and soil in north-eastern Thailand during the rainy season (May–July) in the years 2015–2018. The morphological characteristics of stromatal surface and apex, perithecia, and ostioles were observed under a stereomicroscope (Carl Zeiss Stemi 508) and scanning electron microscope (SEM) (JEOL JSM-6610LVand JEOL JSM-IT500HR). The stromatal colour was recorded using Rayner’s colour chart [11]. Asci, ascospores, and conidia were mounted in water and Melzer’s iodine reagent for examination by bright-field microscopy (Olympus BX50) and differential interference contrast microscopy (Carl Zeiss Axio Imager A2). At least 40 ascospores and conidia per sample were examined. Cultures were obtained from the fresh inner tissue of stromata [6] or ascospores on potato dextrose agar (PDA) and oatmeal agar (OA). Cultures and anamorphs were observed after incubation at 30 °C for 7–21 days under 12 h fluorescent light. The studied specimens were deposited at Srinakharinwirot University Fungal Herbarium (SWUF), Bangkok, Thailand.
2.2. DNA Extraction, Amplification and Sequencing
Genomic DNAs were extracted from mycelia using a FavorPrep Plant Genomic DNA Extraction Mini Kit (Favorgen, Taiwan). The internal transcribed spacer region of nuclear rDNA (ITS) was amplified with the primer pair ITS5/ITS4 [12], beta-tubulin gene (TUB) was amplified with the primer pairs T1/T22 or T1/Bt2b, and Bt2a/T22 [13,14], while the alpha-actin gene (ACT) was amplified with the primer pair ACT-512F/ACT-738R [15]. Each PCR reaction consisted of 10–100 ng µL−1 of DNA template, 2 µM of each primer, 200 µM dNTP, 1.5 mM MgCl2, 1 × buffer, and 1 U µL−1 of Taq DNA polymerase (Qiagen, Germany). The PCR cycles contained an initial denaturation step at 94 °C for 5 min, 35 cycles of 94 °C for 1 min, 45–55 °C for 1 min, 72 °C for 1–1.30 min, and a final extension at 72 °C for 10 min [2,16]. The amplicons were directly sequenced or cloned using TOPclonerä TA kit (Enzynomics, Daejeon, Korea) following the manufacturer’s protocol. DNA sequencing was performed at the Apical Scientific Sdn. Bhd. company (Selangor, Malaysia) and the resulting chromatograms were manually checked using Chromas 2.4 (Technelysium Pty Ltd., South Brisbane, Australia).
2.3. ITS, Alpha-Actin and Beta-Tubulin Sequence Analysis
Two datasets were built, containing ACT-TUB and ITS sequences of Xylaria species associated with termite nest and related genera in the family Xylariaceae from GenBank, UNITE, and BOLD databases. They were aligned using MUSCLE software [17]. Several alignments of ACT-TUB sequences were performed merging or separating exon and intron regions. The final ACT-TUB datasets consisted of 1157 (exons + intons), 991 (exons only), and 165 (introns only) positions, while the ITS dataset had 747 positions. Gaps were treated as missing data. The phylogenetic trees were constructed using the maximum likelihood (ML) and Bayesian interference (BI) methods. ML trees were generated using the GTR+I+G model. The initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with the superior log-likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The ML phylogenetic analysis was conducted in MEGA X [18,19]. The Bayesian inference analyses were performed with MrBayes v3.2 [20] by Markov chain Monte Carlo (MCMC) sampling. Six simultaneous Markov chains were run sampled every 100th generation. The analysis was stopped when the standard deviation of split frequencies between the trees generated in the two independent runs went below 0.01 after 1,000,000 generations. Twenty-five percent of the 10,000 sampled trees were discarded; the remaining were used to compute a 50% majority rule consensus tree to obtain estimates for posterior probabilities. The phylograms were visualised in FigTree 1.4.2 [21]. ITS sequences were analysed and used to confirm the Xylaria species identification carried out in this study. The trees were run at the same parameters as the ACT-TUB analysis. All sequences produced were deposited in the GenBank database.
3. Results
3.1. Phylogenetic Analyses
The GenBank accession numbers of ITS, ACT, and TUB sequences obtained in this study are listed in Table 1. The trees obtained from ACT-TUB datasets, including either exon only, intron only, or both regions, showed similar topologies in ML and BI. Therefore, the phylogenetic tree, including both exons and introns generated by BI analysis, was presented herein (Figure 1), while the tree generated by ML analysis is provided in supplementary data (Figure S1). The phylogenetic trees obtained from the exon-only and intron-only analyses are provided in Figures S2 and S3, respectively (supplementary data). All Xylaria taxa studied in the present work were grouped together with known species of Xylaria subgenus Pseudoxylaria in clade TE [2], with a high Bayesian posterior probability (1.00) and likelihood bootstrap value (71%). These taxa are clearly different from each other and from known species. Species of Xylaria subgenus Pseudoxylaria lacking sequences available in the public databases produce morphologically different teleomorphic and anamorphic states. Sequences of Xylaria species found on other substrates were grouped in clades HY and PO, while clade NR contained species of Nemania and Rosellinia, in accordance with the results obtained previously by Hsieh et al. [2]. In TE clade, X. conica (SWUF18-4.4) and X. ischnostroma (SWUF18-22.1), two species with larger ascospores (>10 µm in length), were grouped together with high bootstrap support with X. fimbriata, the only non-Asian species in this clade. Xylaria subintraflava (SWUF16-4.3, SWUF17-22.2, SWUF17-24.2) was grouped together with X. intraflava; their close relatedness agrees with their similar morphological characteristics. Similarly, X. chaiyaphumensis (SWUF16-11.4) and X. thienhirunae (SWUF16-6.2), which have a similar ascospore shape, colour, and size, were also significantly related on the basis of results from phylogenetic inference, based on 84 ITS sequences from Xylaria species associated with termite nests or soil from known species (Figure 2 and Figure S4 for ML analysis). Only X. reinkingii var. microspora (SWUF17-19.1) showed a 100% similarity to Xylaria sp. ASMC3 (GenBank accession number EU164404), obtained from a fungus comb of Macrotermes carbonarius in Vietnam [22]. Notably, ITS sequences of X. cf. escharoidea collected from Thailand showed a 99% similarity to X. escharoidea (herbarium voucher HAST 658, epitype; EU179864) [5], while ACT and TUB sequences obtained from the same voucher showed 99% and 97% similarities to the GenBank accession numbers GQ853044 and GQ502709, respectively. In the same way, X. cf. nigripes (SWUF17-31.1) sequences obtained in the present study showed 90.63%, 80.12%, and 81.78% similarities to the GenBank accession numbers GU324755 (ITS), GQ853045 (ACT), and GQ502710 (TUB) sequences of X. nigripes (herbarium voucher HAST 653), respectively, while its morphological characteristics were all similar to X. nigripes [5], with minor differences in perithecial size. Additional samples of X. cf. escharoidea and X. cf. nigripes from different localities are required to evaluate the variability of these species and understand their genetic boundaries.
Table 1.
Taxa used in this study.
| Species | Collection Number | Country | GenBank Accession Number ITS | Reference | GenBank Accession Number Beta-Tubulin | Reference | GenBank Accession Number Alpha-Actin | Reference |
|---|---|---|---|---|---|---|---|---|
| Astrocystis bambusae | HAST 89021904 | Taiwan | - | - | GQ495942 | [2] | GQ449239 | [2] |
| A. mirabilis | HAST 94070803 | Taiwan | - | - | GQ495941 | [2] | GQ449238 | [2] |
| A. sublimbata | HAST 89032207 | Taiwan | - | - | GQ495940 | [2] | GQ449236 | [2] |
| Kretzschmaria clavus | JDR 114 | French Guiana | - | - | EF025611 | [2] | EF025596 | [2] |
| K. guyanensis | HAST 89062903 | Taiwan | GU300079 | [2] | GQ478214 | [2] | GQ408901 | [2] |
| K. sandvicensis | JDR 113 | USA, Hawaiian Islands | - | - | GQ478211 | [2] | GQ398234 | [2] |
| Nemania bipapillata | HAST 90080610 | Taiwan | - | - | GQ470221 | [2] | GQ389693 | [2] |
| N. diffusa | HAST 91020401 | Taiwan | - | - | GQ470220 | [2] | GQ389692 | [2] |
| N. illita | JDR 236 | USA | - | - | EF025608 | [2] | EF025593 | [2] |
| Poronia pileiformis | WSP 88113001 | Taiwan | GU324760 | [2] | GQ502720 | [2] | GQ455449 | [2] |
| Rosellinia buxi | JDR 99 | France | - | - | GQ470228 | [2] | GQ398228 | [2] |
| R. necatrix | HAST 89062904 | Taiwan | - | - | EF025603 | [2] | EF025588 | [2] |
| Xylaria acuminatilongissima | HAST 623 | Taiwan | EU178738 | [6] | GQ502711 | [2] | GQ853046 | [2] |
| X. adscendens | JDR 865 | Thailand | GU322432 | [2] | GQ487709 | [2] | GQ438746 | [2] |
| X. apoda | HAST 90080804 | Taiwan | GU322437 | [2] | GQ495930 | [2] | GQ438751 | [2] |
| X. arbuscula var. plenofissura | HAST 93082814 | Taiwan | GU339495 | [2] | GQ478225 | [2] | GQ421285 | [2] |
| X. atrodivaricata | HAST 95052001 | Taiwan | EU178739 | [6] | GQ502713 | [2] | GQ853048 | [2] |
| X. atrosphaerica | HAST 91111214 | Taiwan | GU322459 | [2] | GQ495953 | [2] | GQ452363 | [2] |
| X. bambusicola | JDR 162 | Thailand | GU300088 | [2] | GQ478223 | [2] | GQ408910 | [2] |
| X. brunneovinosa | HAST 720 | Taiwan | EU179862 | [6] | GQ502706 | [2] | GQ853041 | [2] |
| X. chaiyaphumensis | SWUF16-04.1 | Thailand | MT622777 | This study | - | - | - | - |
| X. chaiyaphumensis | SWUF16-11.4 | Thailand | MT622776 | This study | MW459236 | This study | MW459213 | This study |
| X. chaiyaphumensis | SWUF17-15.1 | Thailand | MT622774 | This study | - | - | MW459214 | This study |
| X. chaiyaphumensis | SWUF17-49.2 | Thailand | MT622775 | This study | - | - | MW459215 | This study |
| X. cirrata | HAST 664 | Taiwan | EU179863 | [6] | GQ502707 | [2] | GQ853042 | [2] |
| X. coccophora | HAST 786 | French Guiana | GU300093 | [2] | GQ487701 | [2] | GQ421289 | [2] |
| X. conica | SWUF18-4.3 | Thailand | MT622786 | This study | - | - | MW459223 | This study |
| X. conica | SWUF18-4.4 | Thailand | MT622787 | This study | MW459243 | This study | MW459224 | This study |
| X. coprinicola | 1145 | China | HM585020 | [23] | HM585018 | [23] | HM585017 | [23] |
| X. crozonensis | HAST 398 | France | GU324748 | [2] | GQ502697 | [2] | GQ455441 | [2] |
| X. cubensis | GENT 159 | Papua New Guinea | - | - | GQ502702 | [2] | GQ455446 | [2] |
| X. cubensis | HAST 515 | French West Indies | GU373810 | [2] | GQ502701 | [2] | GQ455445 | [2] |
| X. culleniae | JDR 189 | Thailand | GU322442 | [2] | GQ495935 | [2] | GQ438756 | [2] |
| X. curta | HAST 92092022 | Taiwan | GU322443 | [2] | GQ495936 | [2] | GQ438757 | [2] |
| X. escharoidea | HAST 658 | Taiwan | EU179864 | [6] | GQ502709 | [2] | GQ853044 | [2] |
| X. escharoidea | CLZhao TJ-1 | China | MK343687 | Unpublished | - | - | - | - |
| X. escharoidea | CLZhao TJ-2 | China | MK343688 | Unpublished | - | - | - | - |
| X. escharoidea | CLZhao TJ-3 | China | MK343689 | Unpublished | - | - | - | - |
| X. escharoidea | SWFU000021 | China | MK862248 | Unpublished | - | - | - | - |
| X. escharoidea | 274-CW107 | China | KU194333 | Unpublished | - | - | - | - |
| X. escharoidea | TF18 | China | MN509048 | Unpublished | - | - | - | - |
| X. escharoidea | Y-4 | China | KC462194 | Unpublished | - | - | - | - |
| X. cf. escharoidea | SWUF17-35.1 | Thailand | MT622792 | This study | MW459227 | This study | MW459200 | This study |
| X. cf. escharoidea | SWUF17-38.1 | Thailand | MT622793 | This study | - | - | - | - |
| X. cf. escharoidea | SWUF17-39.1 | Thailand | MT622794 | This study | MW459228 | This study | MW459201 | This study |
| X. cf. escharoidea | SWUF17-39.2 | Thailand | MT622795 | This study | - | - | - | - |
| X. fimbriata | HAST 491 | French West Indies | GU324753 | [2] | GQ502705 | [2] | GQ853040 | [2] |
| X. fulvescens | SWUF17-27.2 | Thailand | MT622780 | This study | MW459238 | This study | MW459218 | This study |
| X. griseosepiacea | HAST 641 | Taiwan | EU179865 | [6] | GQ502714 | [2] | GQ853049 | [2] |
| X. hypoxylon | HAST 95082001 | Taiwan | GU300095 | [2] | GQ487703 | [2] | GQ427195 | [2] |
| X. ianthinovelutina | HAST 553 | French West Indies | GU322441 | [2] | GQ495934 | [2] | GQ438755 | [2] |
| X. insolita | HAST 99090301-1251 | Taiwan | MN655979 | [8] | MN656983 | [8] | MN656985 | [8] |
| X. intracolorata | HAST 90080402 | Taiwan | GU324741 | [2] | GQ502690 | [2] | GQ452375 | [2] |
| X. intraflava | HAST 725 | Taiwan | EU179866 | [6] | GQ502718 | [2] | GQ853053 | [2] |
| X. ischnostroma | SWUF18-22.1 | Thailand | MT622788 | This study | MW459244 | This study | MW459225 | This study |
| X. juruensis | HAST 92042501 | Taiwan | GU322439 | [2] | GQ495932 | [2] | GQ438753 | [2] |
| X. laevis | HAST 95,072,910 | Taiwan | GU324747 | [2] | GQ502696 | [2] | GQ455440 | [2] |
| X. luteostromata var. macrospora | HAST 508 | French West Indies | GU324739 | [2] | GQ502688 | [2] | GQ452373 | [2] |
| X. margaretae | SWUF17-34.1 | Thailand | MT622778 | This study | - | - | MW459216 | This study |
| X. margaretae | SWUF17-34.2 | Thailand | MT622779 | This study | MW459237 | This study | MW459217 | This study |
| X. minima | SWUF18-3.2 | Thailand | MT622789 | This study | MW459245 | This study | MW459226 | This study |
| X. multiplex | HAST 580 | French West Indies | GU300098 | - | GQ487705 | [2] | GQ427198 | [2] |
| X. nigripes | HAST 653 | Taiwan | GU324755 | [2] | GQ502710 | [2] | GQ853045 | [2] |
| X. nigripes | Xn1 | China | MK748600 | Unpublished | - | - | - | - |
| X. nigripes | Xn-6 | China | JQ967448 | Unpublished | - | - | - | - |
| X. nigripes | Xn-7 | China | JQ979095 | Unpublished | - | - | - | - |
| X. nigripes | CWF | Taiwan | KJ627787 | Unpublished | - | - | - | - |
| X. nigripes | 420526MF0342 | China | MG712340 | Unpublished | - | - | - | - |
| X. nigripes | Hf-1A | Taiwan | JQ927570 | Unpublished | - | - | - | - |
| X. nigripes | 4-29 | China | HM050414 | Unpublished | - | - | - | - |
| X. nigripes | ZD17052101 | China | MN523323 | Unpublished | - | - | - | - |
| X. cf. nigripes | SWUF17-31.2 | Thailand | MT622790 | This study | MW459229 | This study | MW459202 | This study |
| X. cf. nigripes | SWUF17-36.1 | Thailand | MT622791 | This study | - | - | MW459203 | This study |
| X. ochraceostroma | HAST 401 | Taiwan | EU179869 | [6] | GQ502717 | [2] | GQ853052 | [2] |
| X. oligotoma | HAST 784 | French Guiana | GU300092 | [2] | GQ487700 | [2] | GQ421288 | [2] |
| X. ophiopoda | HAST 93082805 | Taiwan | GU322461 | [2] | GQ452365 | [2] | GQ495955 | [2] |
| X. plebeja | HAST 91122401 | Taiwan | GU324740 | [2] | GQ502689 | [2] | GQ452374 | [2] |
| X. polymorpha | JDR1012 | USA | GU322460 | [2] | GQ495954 | [2] | GQ452364 | [2] |
| X. reinkingii var. microspora | SWUF17-19.1 | Thailand | MT622769 | This study | MW459234 | This study | MW459209 | This study |
| X. ripicola | KA11-0060-1 | South Korea | NR153251 | [24] | - | - | - | - |
| X. ripicola | KA11-0060-2 | South Korea | KM817200 | [24] | - | - | - | - |
| X. siamensis | SWUF17-20.2 | Thailand | MT622765 | This study | MW459233 | This study | MW459208 | This study |
| X. siamensis | SWUF17-20.3 | Thailand | MT622766 | This study | - | - | - | - |
| X. siamensis | SWUF17-20.4 | Thailand | MT622767 | This study | - | - | - | - |
| X. siamensis | SWUF17-20.6 | Thailand | MT622768 | This study | - | - | - | - |
| X. sihanonthii | SWUF18-5.1 | Thailand | MT622784 | This study | MW459241 | This study | MW459221 | This study |
| X. sihanonthii | SWUF18-1.3 | Thailand | MT622785 | This study | MW459242 | This study | MW459222 | This study |
| X. striata | HAST 304 | Taiwan | GU300089 | [2] | GQ478224 | [2] | GQ421284 | [2] |
| X. subescharoidea | HAST 660 | Taiwan | GU324754 | [2] | GQ502708 | [8] | GQ853043 | [8] |
| X. subescharoidea | HAST 99060401-1188 | Taiwan | MN655980 | [8] | MN656984 | [8] | MN656986 | [8] |
| X. subintraflava | SWUF16-4.3 | Thailand | MT622762 | This study | MW459230 | This study | MW459204 | This study |
| X. subintraflava | SWUF16-11.1 | Thailand | MT622763 | This study | - | - | MW459205 | This study |
| X. subintraflava | SWUF17-9.2 | Thailand | MT622758 | This study | - | - | - | - |
| X. subintraflava | SWUF17-13.1 | Thailand | MT622759 | This study | - | - | - | - |
| X. subintraflava | SWUF17-22.2 | Thailand | MT622764 | This study | MW459231 | This study | MW459206 | This study |
| X. subintraflava | SWUF17-24.2 | Thailand | MT622757 | This study | MW459232 | This study | MW459207 | This study |
| X. subintraflava | SWUF18-9.1 | Thailand | MT622760 | This study | - | - | - | - |
| X. subintraflava | SWUF18-9.2 | Thailand | MT622761 | This study | - | - | - | - |
| X. terricola | YMJ 1375 | Taiwan | MF577042 | [25] | MF577044 | [25] | MF577045 | [25] |
| X. thienhirunae | SWUF16-6.2 | Thailand | MT622770 | This study | MW459235 | This study | MW459210 | This study |
| X. thienhirunae | SWUF16-7.2 | Thailand | MT622772 | This study | - | - | - | - |
| X. thienhirunae | SWUF16-10.1 | Thailand | MT622773 | This study | - | - | MW459211 | This study |
| X. thienhirunae | SWUF17-44.1 | Thailand | MT622771 | This study | - | - | MW459212 | This study |
| X. venustula | HAST 88113002 | Taiwan | GU300091 | [2] | GQ487699 | [2] | GQ421287 | [2] |
| X. vinacea | SWUF18-2.1 | Thailand | MT622781 | This study | MW459239 | This study | MW459219 | This study |
| X. vinacea | SWUF18-2.3 | Thailand | MT622782 | This study | - | - | - | - |
| X. vinacea | SWUF18-2.10 | Thailand | MT622783 | This study | MW459240 | This study | MW459220 | This study |
| X. sp. 1 | HAST 642 | Taiwan | GU324759 | [2] | GQ502719 | [2] | GQ853054 | [2] |
| X. sp. 3 | HAST 722 | Taiwan | GU324756 | [2] | GQ502712 | [2] | GQ853047 | [2] |
| X. sp. 4 | HAST 646 | Taiwan | GU324757 | [2] | GQ502715 | [2] | GQ853050 | [2] |
| X. sp. 5 | HAST 650 | Taiwan | GU324758 | [2] | GQ502716 | [2] | GQ853051 | [2] |
| Xylaria sp. | ASMC3 | Vietnam | EU164404 | [22] | - | - | - | - |
| Xylaria sp. | AAG5 | Africa | EU164400 | [22] | - | - | - | - |
| Xylaria sp. | HNHB | China | FN812862 | Unpublished | - | - | - | - |
| Xylaria sp. | D12 | China | KC414236 | Unpublished | - | - | - | - |
| Xylaria sp. | TF37 | China | MN526593 | Unpublished | - | - | - | - |
| Xylaria sp. | 4-E7-2 | China | FN812842 | Unpublished | - | - | - | - |
| Xylariaceae sp. | 342.D | South Africa | FJ425676 | [26] | - | - | - | - |
| Xylariaceae sp. | 341 | South Africa | FJ425675 | [26] | - | - | - | - |
Figure 1.
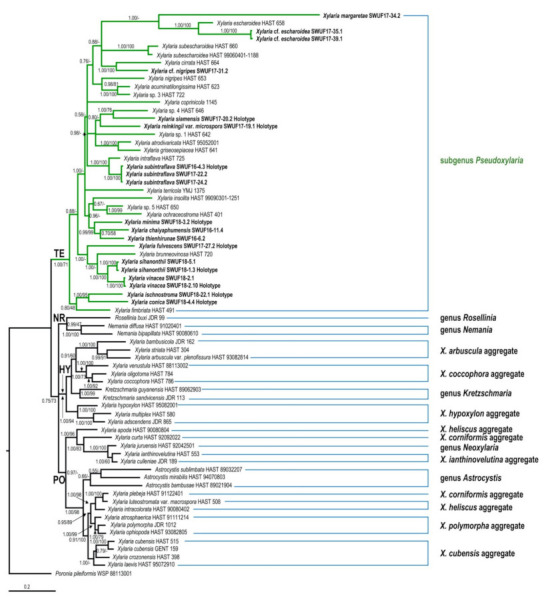
Phylogenetic tree inferred from Bayesian analysis based on ACT-TUB sequences of Xylaria and related genera. Branches are labelled with Bayesian posterior probabilities and bootstrap values from BI and ML analyses, respectively. Poronia pileiformis (WSP 88113001) is the outgroup.
Figure 2.
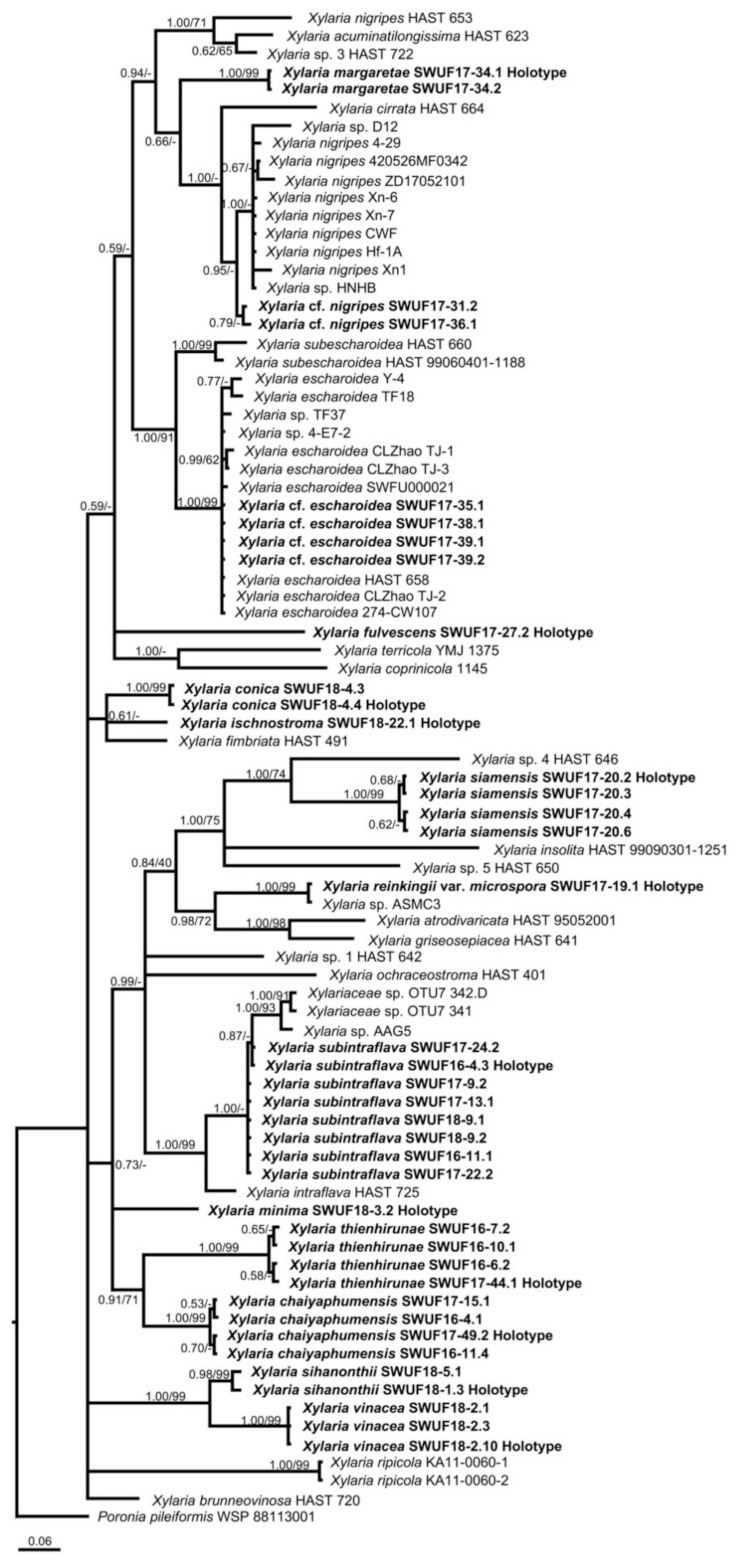
Phylogenetic tree inferred from Bayesian analysis based on a data set of ITS sequences of Xylaria and related species from termite nest or soil. Branches are labelled with Bayesian posterior probabilities and bootstrap values from BI and ML analyses, respectively. Poronia pileiformis (WSP 88113001) is the outgroup.
3.2. Taxonomy
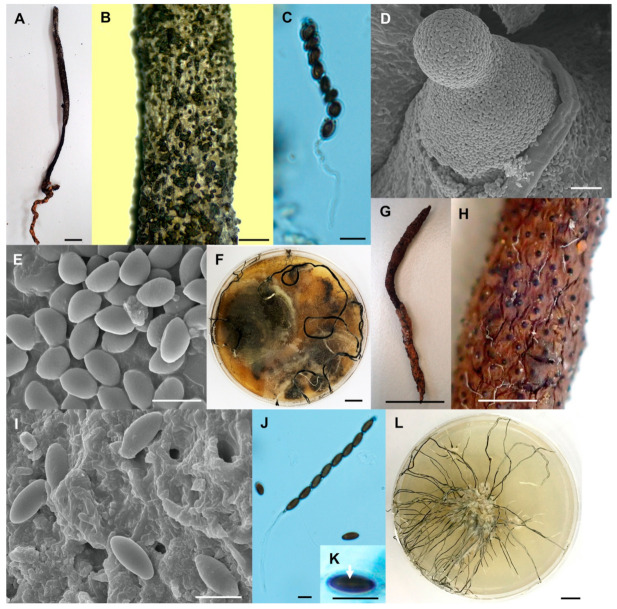
Xylaria chaiyaphumensis Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 3A–H.
Figure 3.

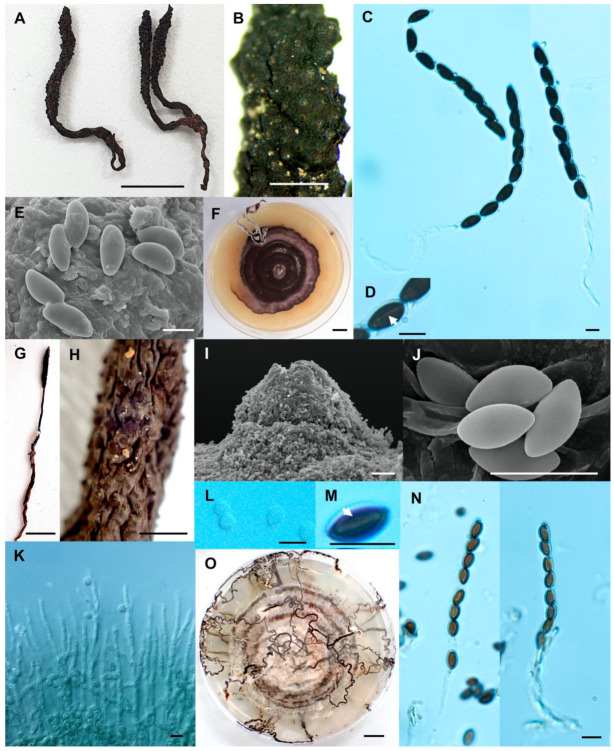
Xylaria chaiyaphumensis (SWUF17-49.2). (A) Stroma. (B) Wrinkled stromatal surface with ostioles. (C) Ascospores. (D) Ascus with ascospores. (E) Apical ring. (F) Germ slit (arrowed). (G) Wrinkled stromata surface with ostioles. (H) Colony on PDA in a 9 cm Petri dish at 4 weeks. Xylaria conica (SWUF18-4.4). (I) Natural habit of stroma. (J) Stromata. (K,L) Stromata surface with ostioles. (M) Ascospores. (N) Ascus with ascospores. (O) Apical ring. (P) Ascospore with germ slit (arrowed). (Q) Colony on PDA in a 9 cm Petri dish at 4 weeks. (C,G,L,M) by SEM; (D–F,N–P) by DIC. Scale bars (A,H–J,Q) = 1 cm; (B,K) = 1 mm; (C–F,M–P) = 5 µm; (G) = 20 µm; (L) = 100 µm.
MycoBank number: MB 839097.
Etymology—Chaiyaphumensis (Lat.): referring to Chaiyaphum Province of Thailand.
Type—Thailand, Dong Bang sub-district, Khon San District, Chaiyaphum province, on soil near termite nest, June 2017, Wangsawat N. SWUF17-49.2 (cultured) (holotype), GenBank accession: ITS = MT622775, α-act = MW459215; Dong Bang sub-district, Khon San District, Chaiyaphum province, on soil near termite nest, June 2017, Wangsawat N. SWUF17-4.1; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Wangsawat N. SWUF16-04.1 (cultured), GenBank accession: ITS = MT622777; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Wangsawat N. SWUF16-11.4 (cultured), GenBank accession: ITS = MT622776, α-act = MW459213, β-tub = MW459236; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Khaeng-raeng R. SWUF17-15.1 (cultured), GenBank accession: ITS = MT622774, α-act = MW459214.
Stromata—Cylindrical, unbranched, with an acuminate apex, 5.9–7.6 cm in total length above ground by 2.5–4 mm broad, 1.7–2.6 cm long at fertile parts, 3.2–4.5 cm long at stipes, with a tortuose rooting base. The surface has conspicuous to inconspicuous perithecial mounds, longitudinally wrinkled, continuous, brown to blackish-brown, becoming light brown on the apex, black and smooth on the stipes, texture woody, interior white to buff (45). Perithecia globose to obovoid, 0.3–0.7 mm diameter by 0.6–0.7 mm high. Ostioles conic-papillate, black, ≤0.1 mm broad at base. Asci with eight ascospores, cylindrical, 52.5–128.5 µm total length by 4–5.5 µm broad, the spore-bearing parts 30–42(–45) µm long, stipes 20–91 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 0.9–1.2 µm high by 0.9–1.5 µm broad.
Ascospores—Brown to dark brown, unicellular, lemon-shaped, nearly equilateral with narrowly rounded ends, sometimes pinched, smooth, 4–5(–5.7) × (2.5–)3–3.8 µm, with a straight germ slit 1/2 spore length.
Culture characteristics—Colonies reaching the edge of a 9 cm of Petri dish in 4 weeks, at first white and loose-cottony, becoming black surrounding the centre of colonies, azonate, mostly submerged, with diffuse margin. Reverse remaining uncoloured. Stromata cylindrical, tapering upward, unbranched to branched, flexuous, up to 9 cm long by 1–2 mm diameter, dispersed small stromata, white, becoming pale brown to black, white on the upper part. Anamorph not observed.
Notes—Xylaria chaiyaphumensis differs from the other species of Xylaria associated with termite nests in having lemon-shaped ascospores with a half spore-length germ slit. Stromata often exceed 3 mm diameter, and ascospores are mostly shorter than 5 µm.
-
2.
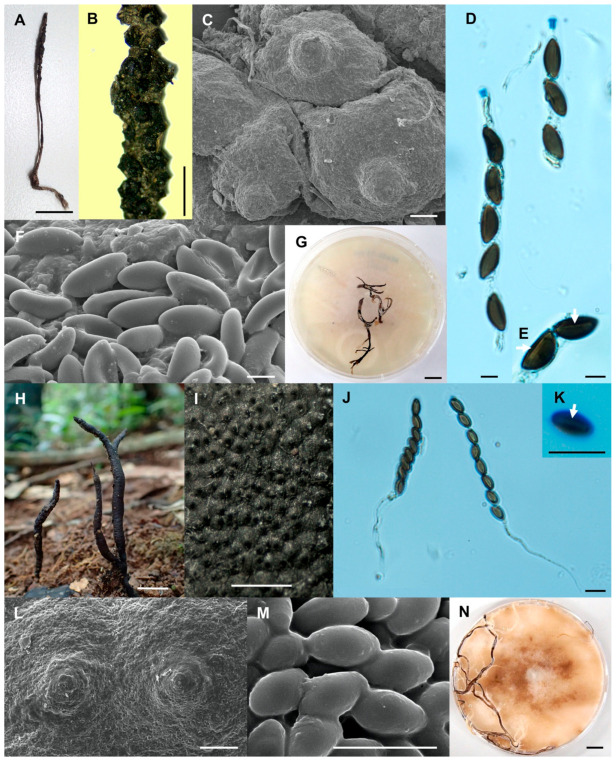
Xylaria conica Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 3I–Q.
MycoBank number: MB 839099.
Etymology—Conica (Lat.): referring to conical elevations.
Type—Thailand, Pang Ta Wai sub-district, Pang Sila Thong District, Kamphaeng Phet province, on termite nests, June 2018, Wangsawat N. SWUF18-4.4 (cultured) (holotype), GenBank accession: ITS = MT622787, α-act = MW459224, β-tub = MW459243; Pho Thong sub-district, Pang Sila Thong District, Kamphaeng Phet province, on termite nests, June 2018, Thamvithayakorn P. SWUF18-4.3 (cultured), GenBank accession: ITS = MT622786, α-act = MW459223; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nest, May 2016, Wangsawat N. SWUF16-1.2.
Stromata—Cylindrical, unbranched with blunt apex, 3–5 cm in total length above ground by 1.5–2.5 mm broad, 0.9–2.4 cm long at fertile parts, 2.1–2.6 cm long at stipes, with a tortuose rooting base. The surface with mostly conspicuous to inconspicuous perithecial mounds, black, black and smooth stipes, texture woody, interior white. Perithecia obovoid to globose, 0.3–0.5 mm diameter by 0.3–0.9 mm high. Ostioles conic-papillate, black, 0.2–0.3 mm broad at base. Asci with eight spores, cylindrical, 150–168.5(–217.7) µm total length by 6.4–8.8 µm broad, the spore-bearing parts 73–110.5 µm long, stipes 59–79(–114) µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 4–4.5 µm high by 2–3 µm broad.
Ascospores—Brown to dark brown, unicellular, ellipsoid, inequilateral with narrowly rounded ends, smooth, (10–)10.8–12.3(–13.2) × 4.5–6(–6.4) µm, with a straight, spore-length germ slit on flattened side.
Culture characteristics—Colonies not reaching the edge of a 9 cm Petri dish in 8 weeks, attaining 4–6 cm diameter, at first white, cottony, immediately becoming dark brown, zonate, mostly submerged, with diffuse margin. Reverse pale brown. Stromata and anamorph not observed.
Note—This fungus differs from other Xylaria species associated with termite nests in having ascospores longer than 10 µm and larger perithecia. The perithecial mounds exposed from the stromatal surface have a conical shape. Although the ascospore size of X. conica is close to that of X. radicans (11.5–14 × 4–5 µm) [6], it has larger perithecia and a straight, spore-length germ slit. Xylaria radicans has perithecia 0.2–0.3 mm diameter and a straight germ slit shorter than spore length.
-
3.
Xylaria cf. escharoidea (Berk.) Fr. Figure 4A–F.
Figure 4.
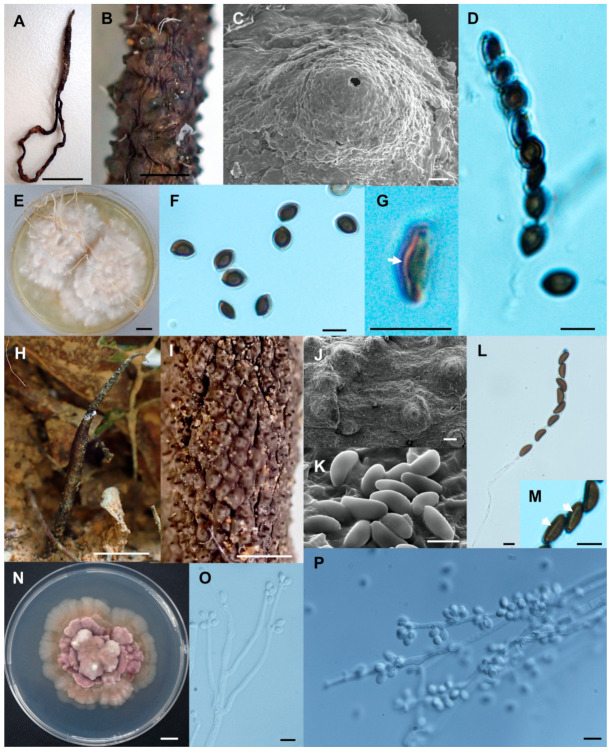
Xylaria cf. escharoidea (SWUF17–38.1). (A) Stroma. (B) Stromatal surface with ostioles. (C) Ascus with ascospores. (D) Ostiole. (E) Ascospores. (F) Colony on PDA in a 9 cm Petri dish at 4 weeks. Xylaria fulvescens (SWUF17-27.2). (G) Stroma. (H) Wrinkled stromatal surface with ostioles. (I) Ascospores. (J) Ascus and ascospores. (K) Germ slit (arrowed). (L) Colony on PDA in a 9 cm Petri dish at 4 weeks. (D,E,I) by SEM; (C,J,K) by DIC. Scale bars (A,F–G,L) = 1 cm; (B,H) = 1 mm; (C,E,I–K) = 5 µm; (D) = 20 µm.
Type—Thailand, Dong Bang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-35.1 (cultured), GenBank accession: ITS = MT622792, α-act = MW459200, β-tub = MW459227; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Phosri C. SWUF17-38.1 (cultured), GenBank accession: ITS = MT622793; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Phosri C. SWUF17-39.1 (cultured), GenBank accession: ITS = MT622794, α-act = MW459201, β-tub = MW459228; Phosri C. SWUF17-39.2 (cultured), GenBank accession: ITS = MT622795.
The teleomorph is as described in Rogers et al. [5]. Apical rings are inverted hat-shaped, 1.4–1.7 µm high by 0.8–1.0 µm broad.
Culture characteristics–Colonies covering 9 cm Petri dish in 1 week, at first white, cottony-softly, becoming dark green to black, azonate, with diffuse margin. Reverse turning green-brown to dark green to black, due to release of exudate from mycelium. Stromata arise in 2–3 weeks, cylindrical, tapering upward, unbranched or branched, flexuous, up to 17 cm long by 1 mm diameter, white, becoming dark green to black. Anamorph not observed.
Note—This fungus differs from X. escharoidea [5] in having slightly larger perithecia, 0.3–0.5 mm diameter, and significantly different sequences of β-tub gene (97% similarity). Additional samples from different locations are required to clarify the identity of this species in future investigation.
-
4.
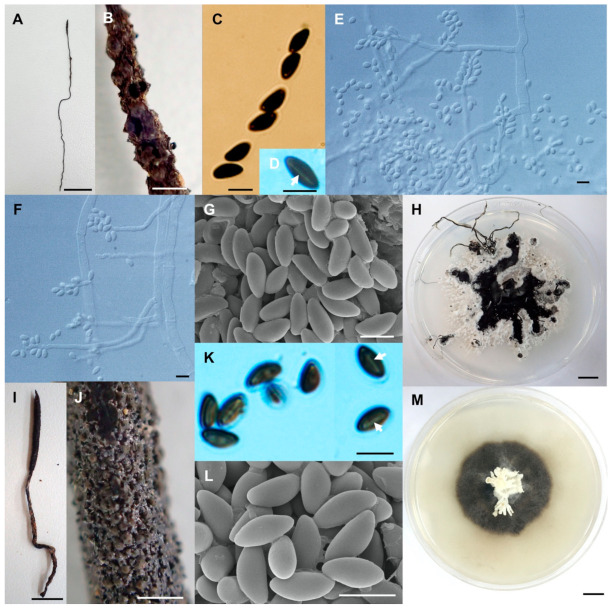
Xylaria fulvescens Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 4G–L.
MycoBank number: MB 839101.
Etymology—Fulvescens (Lat.): referring to the fulvous stromatal surface colour.
Type—Thailand, Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-27.2 (cultured) (holotype), GenBank accession: ITS = MT622780, α-act = MW459218, β-tub = MW459238.
Stromata—Cylindrical, unbranched, with acuminate apex, 3.9 cm in total length above ground by 1.5–2.5 mm broad, 1–2.3 cm long at fertile parts, 1.6–2.8 cm long at stipes. Surface smooth with mostly embedded perithecia, wrinkled, brown to fulvous (43), black and smooth stipes, texture woody, interior white to cream. Perithecia subglobose to globose, 0.3–0.4 mm diameter by 0.4–0.5 mm high. Ostioles papillate, black, ≤0.1 mm broad at base. Asci with eight ascospores, cylindrical, 46.5–77(–88) µm total length by 3.4–5.0 µm broad, the spore-bearing parts 40–54 µm long, stipes 6.5–37 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 1.1–1.7 µm high by 0.9–1.6 µm broad.
Ascospores—Light brown to dark brown, unicellular, ellipsoid, nearly equilateral with narrowly rounded ends, smooth, (4.5–)5–6.2 × 2.2–3 µm, with straight 3/4 spore length germ slit.
Culture characteristics—Colonies not reaching the edge of a 9 cm Petri dish in 4 weeks, attaining 3–4 cm diameter in 8 weeks, at first white, becoming pale brown, azonate, mostly submerged, with diffuse margin. Reverse remaining uncoloured. Stromata forming after 10 days, cylindrical, arising abundantly from the centre and elongating reach the edge of the plate, tapering upward, unbranched, flexuous, up to 10 cm long by <1 mm diameter, white, becoming smoke grey to black, white on the tips. Anamorph not observed.
Note—Xylaria fulvescens is similar to X. ochraceostroma [6] in stromatal surface and size but differs in having larger perithecia (0.3–0.4 mm diam), a smaller ascal apical apparatus (≤2 µm) and lighter stromatal surface colour. In addition, ascospores of X. fulvescens are ellipsoid and nearly equilateral, while those of X. ochraceostroma are ellipsoid and inequilateral.
-
5.
Xylaria ischnostroma Wangsawat N, Ju, Y.-M., Phosri C., Whalley A.J.S. & Suwannasai N., sp. nov. Figure 5A–G.
Figure 5.
Xylaria ischnostroma (SWUF18-22.1). (A) Stroma. (B) Stromatal surface with ostioles. (C) Ostioles. (D) Ascospores and apical rings. (E) Ascospores with germ slits (arrowed). (F) Ascospores. (G) Colony on PDA in a 9 cm Petri dish at 4 weeks. Xylaria margaretae (SWUF17-34.1). (H) Natural habit of stromata. (I) Wrinkled stromata surface with ostioles. (J) Asci with ascospores. (K) Germ slit (arrowed). (L) Ostioles. (M) Ascospores. (N) Colony on PDA in a 9 cm Petri dish at 4 weeks. (C,F,L,M) by SEM; (D,E,J,K) by DIC. Scale bars (A,G,H,N) = 1 cm; (B,I) = 1 mm; (C,L) = 100 µm; (D–F,J,K,M) = 5 µm.
MycoBank number: MB 839102.
Etymology—Ischnostroma (Lat.): referring to its slim stromata.
Type—Thailand, Pho Thong sub-district, Pang Sila Thong District, Kamphaeng Phet Province, on termite nests, June 2018, Wangsawat N. SWUF18-22.1 (cultured) (holotype), GenBank accession: ITS = MT622788, α-act = MW459225, β-tub = MW459244.
Stromata—Cylindrical, unbranched, acuminate apices, 5.7 cm in total length above ground by 1–1.7 mm broad, 1.7 cm long at fertile parts, 4.0 cm long at stipes, with a tortuose rooting base. The surface with conspicuous perithecial mounds, of mostly naked perithecia, single to combined, blackish-brown, black and smooth stipes, texture woody, white to cream inner tissue. Perithecia globose, 0.5–0.6 mm diameter by 0.6–0.8 mm high. Ostioles conic-papillate, black, 0.1 mm broad at base. Asci with eight spores, cylindrical, 73–111 µm total length by 5.8–7.0 µm broad, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 1.5–2 µm high by 2–3 µm broad.
Ascospores—Light brown to brown to dark brown, unicellular, ellipsoid, nearly inequilateral with narrowly rounded ends, some pinched at the ends, smooth, 10.3–11.6(–12.1) × 4.8–5.6 µm, with straight full spore length germ slit on flattened side.
Culture characteristics—Colonies covering a 9 cm Petri dish in 4 weeks, whitish, mostly submerged, azonate, with diffuse margin. Reverse pale brown. Stromata arising after 6 weeks or not produced, cylindrical, tapering upward, with several branches, up to 7.5 cm long by 1 mm diameter, at first white, becoming black. Anamorph not observed.
Note—Xylaria ischnostroma can be easily recognised because of its naked perithecial mounds and large ascospores. Although the stromatal features of this fungus are similar to those of X. rhizomorpha and X. furcata [5], these species have ascospores measuring 4.5–5 × 2–2.5 µm and 3.5–4.5(–5.5) × (2–)2.5–3 µm, respectively. The ascospore size of X. ischnostroma is closer to that of X. radicans [5] (11.5–14 × 4–5 µm), but their stromatal features are obviously different.
-
6.
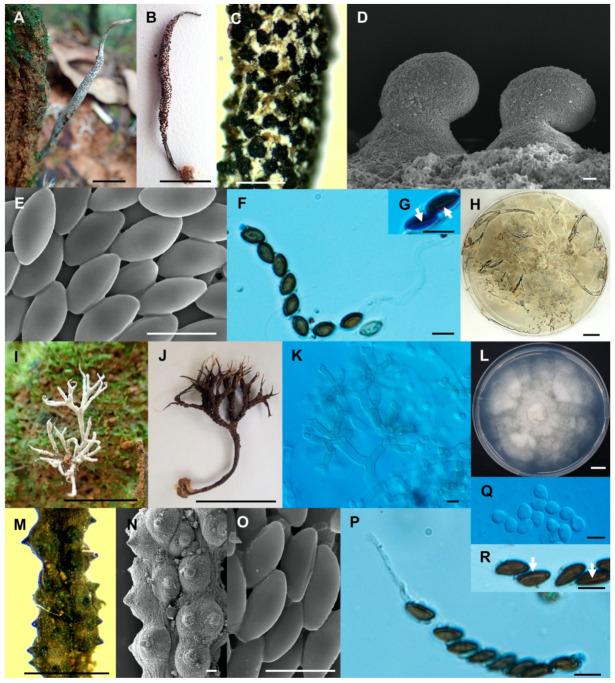
Xylaria margaretae Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 5H–N.
MycoBank number: MB 839103.
Etymology—Margaretae (Lat.): referring to honour British mycologist Dr. Margaret Whalley, who had worked on Xylariaceae for more than 30 years.
Type—Thailand, Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-34.1 (cultured) (holotype), GenBank accession: ITS = MT622778, α-act = MW459216; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-34.2 (cultured), GenBank accession: ITS = MT622779, α-act = MW459217, β-tub = MW459237.
Stromata—Cylindrical, unbranched or dichotomously branched once at tip, acuminate apices, 5.8–10 cm in total length above ground by 2–4 mm broad, 4–4.4 cm long at fertile parts, 1.4–6 cm long at stipes, with a tortuose rooting base sometimes. Surface with conspicuous to inconspicuous perithecial mounds, wrinkled, dark brick (60) to sepia (63) to fuscous black (104), black stipes, texture soft, interior buff (45). Perithecia obovoid to globose, 0.2–0.6 mm diameter by 0.3–0.5 mm high. Ostioles conic-papillate, black, 0.1–0.2 mm broad at base. Asci with eight ascospores, cylindrical, 66–99 µm total length by 3.4–5.2 µm broad, the spore-bearing parts 27–34 µm long, stipes 33–70 µm long, with apical rings straining blue in Melzer’s iodine reagent, inverted hat-shaped, 0.7–1.2 µm high by 0.7–1.2 µm broad.
Ascospores—Light brown to dark brown, unicellular, ellipsoid, inequilateral with narrowly rounded ends, smooth, (3.2–)3.8–5 × (1.8–)2–2.5 µm, with a full or nearly full spore length germ slit on convex side.
Culture characteristics—Colonies covering a 9 cm Petri dish in 1 week, at first white, cottony-softly, becoming red-brown, azonate, with diffuse margin. Reverse turning reddish-brown, due to release of exudate from mycelium. Stromata arise in 1–2 weeks or not produced, cylindrical, tapering upward, unbranched, sometimes dichotomously branched, flexuous, up to 13 cm long by 1 mm diameter, white, becoming reddish-brown. Anamorph not observed.
Note—Xylaria margaretae has unbranched or dichotomously branched stromata with acuminate apices similar to those of X. atrodivaricata [6], but stromata of X. margaretae are larger (>2 mm broad) with longer fertile parts. Perithecia of X. atrodivaricata are 0.2–0.3 mm in diameter by 0.3 mm high, while those of X. margaretae are 0.2–0.6 mm in diameter by 0.3–0.5 mm high. In addition, the ascal apical apparatus of X. margaretae is slightly smaller than that of X. atrodivaricata. In the present study, we found only one sample with dichotomously branched stromata. The ITS sequences of X. atrodivaricata and X. margaretae are only 89.53% similar, also supporting the idea that they are different species.
-
7.
Xylaria minima Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 6A–H.
Figure 6.
Xylaria minima (SWUF18-3.2). (A) Stroma. (B) Stromatal surface with ostioles. (C) Ascospores and apical ring. (D) Germ slit (arrowed). (E,F) Anamorph. (G) Ascospores. (H) Colony on PDA in a 9 cm Petri dish at 4 weeks. Xylaria cf. nigripes (SWUF17–31.2). (I) Stroma. (J) Stromata surface with ostioles. (K) Ascospores with germ slits (arrowed). (L) Ascospores. (M) Colony on PDA in a 9 cm Petri dish at 4 weeks. (G,L) by SEM; (D,E,F,K) by DIC. Scale bars (A,H,I,M) = 1 cm; (B,J) = 1 mm; (C,D,E–G,K,L) = 5 µm.
MycoBank number: MB 839104.
Etymology—Minima (Lat.): referring to its tiny stromata.
Type—Thailand, Pho Thong sub-district, Pang Sila Thong District, Kamphaeng Phet province, on termite nests, June 2018, Wangsawat N. SWUF18-3.2 (cultured) (holotype), GenBank accession: ITS = MT622789, α-act = MW459226, β-tub = MW459245; Pho Thong sub-district, Pang Sila Thong District, Kamphaeng Phet province, on termite nests, June 2018, Khaeng-raeng R. SWUF18-3.1; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nest, June 2017, Suwannasai N. SWUF17-1.1; Sakaerat Environmental Research Station Sakaerat Biosphere Reserves, Nakhon Ratchasima province, on termite nest, October 2018, Wangsawat N. SWUF18-2.4.
Stromata—Cylindrical, unbranched, acuminate apex, 4.7–7.5 cm in total length above ground by 0.5–1 mm broad, 0.5–1 cm long at fertile parts, 3.1–6.6 cm long at stipes. Surface with conspicuous perithecial mounds, hairy, longitudinally wrinkled, blackish-brown, white-cream on the apex, black and hairy stipes, texture soft, white to cream inner tissue. Perithecia globose, 0.2–0.4 mm diameter by 0.2–0.4 mm high. Ostioles slightly conic-papillate, black, 0.1 mm broad at base.
Asci with eight spores, cylindrical, 49.5–60 µm total length by 4.2–5 µm broad, the spore-bearing parts 36–43 µm long, stipes 13.5–17 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 1.1–1.4 µm high by 1.4–2 µm broad.
Ascospores—Brown to dark brown, unicellular, ellipsoid-inequilateral, with narrowly rounded ends, smooth, 5.8–6.8 × 2.7–3.2 µm, with straight 3/4 spore length germ slit on flattened side.
Culture characteristics—Colonies on OA not reaching the edge of a 9 cm Petri dish in 4 weeks, at first white, cottony, becoming black, azonate, mostly submerged with diffuse margin. Reverse uncoloured. Immature stromata forming in 1 week, cylindrical, unbranched, up to 1.5 cm long by 1–2 mm diameter, white at first, becoming black-brown to black. White at tip due to conidia production. Anamorph—Conidiophores in upright, densely arranged palisades, some dichotomously branched from base, smooth, hyaline. Conidiogenous cells terminal, cylindrical, (20–)25–36(–48) × 1.8–3.5(–5.8) µm, smooth or slightly roughened, bearing several terminal and lateral denticulate conidial secession scars. Conidia produced holoblastically in sympodial sequence, hyaline, smooth, subglobose to obovoid, (3.2–)3.5–4.1(–4.7) × 2.1–2.9 µm, with a flattened base indicating former point of attachment to conidiogenous cell.
Note—Xylaria minima has solitary to confluent perithecial mounds similar to those of X. ischnostroma, but their ascospore sizes are different. Xylaria minima has smaller ascospores, resembling those of X. piperiformis, 5.5–7 × 3 × 3.5–4(–4.5) µm [5]. Xylaria piperiformis differs in having a cuboid ascal apical ring (1.5 µm) and a full-length germ slit on the flattened narrow side.
-
8.
Xylaria cf. nigripes (Klotzsch) M. C. Cooke Figure 6I–M.
Specimens examined—Thailand, Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-31.2 (cultured), GenBank accession: ITS = MT622790, α-act = MW459202, β-tub = MW459229; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Phomphet N. SWUF17-36.1 (cultured), GenBank accession: ITS = MT622791, α-act = MW459203.
Stromata—Cylindrical, unbranched, acuminate and fertile apex, 6.4 cm in total length above ground by 2–5 mm broad, 2.4–3.9 cm long at fertile parts, 3.5–4 cm long at stipes, with a tortuose rooting base; surface with inconspicuous perithecial mounds, luteous (12), black stipes, woody and luteous interior layer. Perithecia obovoid, 0.2–0.4 mm diameter by 0.4–0.6(–0.8) mm high. Ostioles papillate, black, 0.1 mm broad at base. Asci absent. Ascospores light brown to dark brown, unicellular, ellipsoid, nearly equilateral with narrowly rounded ends, smooth, (4.17–)4.4–5.4 × 2.5–3.0 µm, with straight full spore length germ slit on flattened side.
Culture characteristics—Colonies covering a 9 cm Petri dish in 1 week, at first white, cottony-softly, becoming dark green to black, azonate, with diffuse margin. Reverse pale brown. Stromata arise in 2–3 weeks or not produced, cylindrical, tapering upward, unbranched or dichotomously branched, less than 2 cm long by 1–3 mm diameter, white to cream. Anamorph not observed.
Note—Xylaria cf. nigripes is quite similar to X. nigripes [5], due to its cylindrical fertile headed stromata, with a similar surface colour and hard texture. Ascospore shape and size are in the same range, (3.5–)4.5–5(–6) × 2–3 µm. However, this fungus differs from X. nigripes in having conspicuous perithecial mounds and slightly larger perithecia (0.2–0.4 mm diameter by 0.4–0.6(–0.8) mm high), while the perithecia of X. nigripes are more or less embedded and measure 0.1–0.2 mm diameter. In previous works, Thienhirun [9] identified some specimens from the Phetchaburi, Kanchanaburi, and Chanthaburi provinces in Thailand as X. cf. nigripes, but these had slightly smaller perithecia (0.2 mm diam) and smaller ascospores (3.8–4.3 × 1.9–2.5 µm) than our specimens.
-
9.
Xylaria reinkingii var. microspora Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, var. nov. Figure 7A–H.
Figure 7.
Xylaria reinkingii var. microspora (SWUF17-19.1). (A) Natural habit of stroma. (B) Stroma. (C) Stromatal surface with ostioles. (D) Ostioles with ascospores. (E) Ascospores. (F) Ascus with ascospores. (G) Germ slits (arrowed). (H) Colony on PDA in a 9 cm Petri dish at 4 weeks. Xylaria siamensis (SWUF17-20.3). (I) Natural habit of immature stromata. (J) Stroma. (K) Anamorph. (L) Colony on OA in a 9 cm Petri dish at 4 weeks. (M,N) Wrinkled stromata surface with ostioles. (O) Ascospores. (P) Ascus with ascospores. (Q) Conidia. (R) Ascospores and germ slits (arrowed). (D,E,N,O) by SEM; (F–G,K,P–R) by DIC. Scale bars (A,B,H–J,L) = 1 cm; (C,M) = 1 mm; (D) = 20 µm; (E–G,K,O–R) = 5 µm; (N) = 100 µm.
MycoBank number: MB 839105.
Etymology–Microspora (Lat.): a small-ascospored variety of Xylaria reinkingii C.G. Lloyd.
Type—Thailand, Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-19.1 (cultured) (holotype), GenBank accession: ITS = MT622769, α-act = MW459209, β-tub = MW459234; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-19.2; Sakaerat Environmental Research Station Sakaerat Biosphere Reserves, Nakhon Ratchasima province, on termite nests, May 2013, Suwannasai N. SWUF13-1.
Stromata—Cylindrical, unbranched, with an acuminate apex, 2.2–12.3 cm in total length above ground, 1–4 mm broad, 0.9–5 cm long at fertile parts, 0.8–10 cm long at stipes, with a tortuose rooting base. The surface rough with mostly conspicuous perithecia mound, black, white powder outer layer, cracked into scales, black and smooth on the stipes, texture woody, interior buff (45), Perithecia globose, 0.2–0.5 mm diameter by 0.3–0.4 mm high. Ostioles conic-papillate, black, 0.1 mm diameter at base, 80–90 µm high from stroma. Asci with eight ascospores, cylindrical, 55–65 µm total length by 3.5–5 µm broad, the spore-bearing parts 34.5–45 µm long, stipes 13.5–27 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 0.95–1.3(–1.67) µm high × (0.85–)1–1.5 µm broad.
Ascospores—Brown to dark brown, unicellular, ellipsoid, nearly equilateral to equilateral with narrowly rounded ends, some pinched at the ends, smooth, 5.4–6(–6.3) × 2.4–3.0 µm, with straight germ slit nearly full spore length on convex side.
Culture characteristics—Colonies reaching the edge of a 9 cm Petri dish in 4 weeks, at first white, softly and loose-cottony, becoming pale yellow, azonate, with diffuse margins. Reverse uncoloured. Stromata forming after 1 week, cylindrical, tapering upward, flexuous, unbranched or sometimes branched, up to 8 cm long by ≥1 mm diameter, black at base, becoming blackish or grey on surface, Smoke grey at tips. Anamorph not observed.
Note—This variety differs from X. reinkingii [6] in having smaller ascospores (≤6 µm) and a smaller apical apparatus of asci (≤2 µm).
-
10.
Xylaria siamensis Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 7I–R.
MycoBank number: MB 839096.
Etymology—Siamensis (Lat.): referring to Thailand.
Type—Thailand, Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-20.2 (cultured) (holotype), GenBank accession: ITS = MT622765, α-act = MW459208, β-tub = MW459233; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-20.1; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-20.3, GenBank accession: ITS = MT622766; Dong Bang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Phosri C. SWUF17-20.4, GenBank accession: ITS = MT622767; Dong Bang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Phosri C. SWUF17-20.6, GenBank accession: ITS = MT622768.
Stromata—Cylindrical, highly branched with one or more tines, some dichotomously branched, bearing two acuminate apices, white at tip, 2–6.7 cm in total length above ground, 0.2–1.5 mm broad, 0.3–1 cm long at fertile parts, 1.3–5.3 cm long at stipes. Surface with conspicuous to half-exposed perithecial mounds, wrinkles, immature white, dark brick (60) to black when mature, overlain with some reddish-brown granules at base of stroma, interior white, black and smooth on the stipes. Texture woody to soft. Perithecia globose, 0.2–0.4 mm diameter by 0.2–0.3 mm high, crowded, highly conspicuous. Ostioles coarsely conic-papillate, black, ≤ 0.1 mm broad at base. Asci with eight ascospores, cylindrical, 45.4–74(–114.7) µm total length by 3.2–4.8 µm broad, the spore-bearing parts 30.5–42.5 µm long, stipes (5.4–)21–40.5(–83.8) µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 1.5–1.8 µm high × 1–1.5 µm broad. Paraphyses abundant.
Ascospore—Brown to dark brown, ellipsoid-inequilateral, with narrowly rounded ends, smooth, 5.0–6.0 × 2.5–3.5 µm, with a straight germ slit spore-length on flattened side.
Culture characteristics—Colonies on OA and PDA not reaching the edge of a 9 cm Petri dish in 4 weeks, at first white, becoming pale yellow, cottony in the centre, zonate, mostly submerged with diffuse margins. Reverse uncoloured. Stromata and anamorph not observed on culture media. Anamorph—Anamorphic characters observed on stromatal surface in nature. Conidiophores upright, dichotomously branched several times from base, smooth, hyaline, 3.5–5.3 µm at the main stipe. Conidiogenous cells terminal, flask-shaped, geniculate, 7.5–11.5 × 2.6–4.6 µm, smooth, bearing several terminal and lateral denticulate conidial secession scars. Conidia produced holoblastically in sympodial sequence, hyaline, smooth, obovoid, 4–4.6(–5) × 3–4 µm, with a flattened base indicating former point of attachment to conidiogenous cell.
Note—Xylaria siamensis is similar to X. insolita in having highly branched stromata and in the ascospore size ranges, but it mainly differs from X. insolita by a repeatedly dichotomous branching pattern and a dark brick to black stromatal surface colour without a greyish-brown outer layer ruptured by perithecial mounds. In addition, ascospores of X. siamensis are ellipsoid-inequilateral with narrowly rounded ends, while those of X. insolita have one end narrowly rounded and slightly beaked and the other end broadly rounded. The stromata of X. siamensis are similar to those of X. furcata and X. furcata var. hirsuta [5] in being highly dichotomously branched, but the stromata and the perithecia of X. siamensis are slightly smaller. The ascospores of X. siamensis are >5 µm, larger than those of X. furcata. Collection SWUF17-20.2 is finely pubescent between perithecia, but its ITS sequence is not different from that of the other collections.
-
11.
Xylaria sihanonthii Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 8A–F.
Figure 8.
Xylaria sihanonthii (SWUF18-1.3). (A) Stromata. (B) Stromatal surface with ostioles. (C) Asci with ascospores. (D) germ slits (arrowed). (E) Ascospores. (F) Colony on PDA in a 9 cm Petri dish at 4 weeks. Xylaria subintraflava (PK17-24.2). (G) Stroma. (H) Wrinkled stromata surface with ostioles. (I) Ostiole. (J) Ascospores. (K) Anamorph. (L) Conidia. (M) Germ slit (arrowed). (N) Asci and ascospores. (O) Colony on PDA in a 9 cm Petri dish at 4 weeks. (E,I,J) by SEM; (C,D,K–N) by DIC. Scale bars (A,F,G,O) = 1 cm; (B,H) = 1 mm; (C–E,J–N) = 5 µm; (I) = 20 µm.
MycoBank number: MB 839107.
Etymology—Sihanonthii (Lat.): referring to honour Thai mycologist Prof. Dr. Prakitsin Sihanonth, who had worked on fungi in Thailand for more than 30 years.
Type—Thailand, Nong Lat sub-district, Waritchaphum District, Sakon Nakhon province, on termite nests, July 2018, Wangsawat N. SWUF18-1.3 (cultured) (holotype), GenBank accession: ITS = MT622785, α-act = MW459222, β-tub = MW459242; Phang Khon sub-district, Phanh Khon District, Sakon Nakhon province, on termite nests, July 2018, Phosri C. SWUF18-5.1 (cultured), GenBank accession: ITS = MT622784, α-act = MW459221, β-tub = MW459241; Dong Bang sub-district, Khon San District, Chaiyaphum province, on termite nest, May 2016, Wangsawat N. SWUF16-29.1.
Stromata—Cylindrical, dichotomously branched one time at stipe, some unbranched with acuminate apices, 2.7–6.6 cm in total length above ground by 2–5 mm broad, 1–2.8 cm long at fertile parts, 2.1–3.8 cm long at stipes, some with two rooting bases. Surface with conspicuous perithecial mounds, blackish-brown to black, black and smooth stipes, texture woody, interior white. Perithecia obovoid, 0.3–0.6 mm diameter by 0.4–0.7 mm high. Ostioles conic-papillate, black, ≤0.1mm broad at base. Asci with eight spores, cylindrical, 77–120 µm total length by 4.4–6.1 µm broad, the spore-bearing parts 48–62(–70.5) µm long, stipes 19–49(–63) µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 1.2–1.7 µm high by 1.5–2.3 µm broad.
Ascospores—Light brown to dark brown, unicellular, ellipsoid, inequilateral with narrowly rounded ends, smooth, 7.5–9.5 × 3.5–4.5 µm, with straight full spore length germ slit on flattened side.
Culture characteristics—Colonies not reaching the edge of a 9 cm Petri dish in 8 weeks, at first white and loose-cottony, immediately becoming purple to brown vinaceous (84) zonate, with white concentric zones, mostly submerged, with diffuse margin. Reverse pale brown. Stromatal production beginning after 10 days, cylindrical, tapering upward, unbranched or branched, flexuous, up to 7 cm long by 1–2 mm diameter, black-purple, white at upper part, grey at top. Anamorph not observed.
Note—Xylaria sihanonthii has an ascospore size in the same range as X. kedahae (7.5–9 × 3–3.5 × 4–4.5 µm), X. micrura (7–8 × 3.5–4 µm) [6] and X. brasiliensis (7–8 × 3.5–4 µm) [5], but the shape of ascospores and stromata are different. Xylaria kedahae has ascospores with pinched ends. Xylaria micrura has a hyaline sheath surrounding ascospores. Xylaria brasiliensis has a gelatinous sheath surrounding ascospores and a less than spore-length germ slit.
-
12.
Xylaria subintraflava Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 8G–O.
MycoBank number: MB 839095.
Etymology—Subintraflava (Lat.): referring to its resemblance to Xylaria intraflava.
Type—Thailand, Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Wangsawat N. SWUF16-4.3 (cultured) (holotype), GenBank accession: ITS = MT622762, α-act = MW459204, β-tub = MW459230; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Wangsawat N. SWUF16-11.1 (cultured), GenBank accession: ITS = MT622763, α-act = MW459205; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-9.2 (cultured), GenBank accession: ITS = MT622758; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-13.1 (cultured), GenBank accession: ITS = MT622759; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Suwannasai N. SWUF17-22.2 (cultured), GenBank accession: ITS = MT622764, α-act = MW459206, β-tub = MW459231; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Suwannasai N. SWUF17-24.2 (cultured), GenBank accession: ITS = MT622757, α-act = MW459207, β-tub = MW459232; Pang Ta Wai sub-district, Pang Sila Thong District, Kamphaeng Phet province, on termite nests, May 2018, Wangsawat N. SWUF18-9.1 (cultured), GenBank accession: ITS = MT622760; Pang Ta Wai sub-district, Pang Sila Thong District, Kamphaeng Phet province, on termite nests, May 2018, Wangsawat N. SWUF18-9.2 (cultured), GenBank accession: ITS = MT622761.
Stromata—Cylindrical, unbranched, with an acuminate apex, 2.1–7.9 cm long above ground by 1.5–3 mm broad, with fertile parts 0.85–1.7 cm long, 0.4–5.4 cm long at stipes, with a tortuose rooting base; surface smooth except roughened around ostioles, with slight perithecial mounds, longitudinally wrinkled, continuous, blackish-brown, fawn (87), dark brick (60), some with glossy on fertile part, dark brick (60) to black on smooth stipes, interior pale luteous (11). Perithecia obovoid to globose, 0.3–0.7 mm diameter by 0.3–0.8 mm high. Ostioles conic-papillate, black, ≤0.1 mm broad at base. Asci with eight ascospores, cylindrical, 40–70 µm total length by 4–5 µm broad, the spore-bearing parts 25–31 µm long, stipes 11–38 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 0.8–1µm high × 0.8–1 µm broad. Paraphyses abundant.
Ascospores—Brown to dark brown, unicellular, ellipsoid, equilateral with narrowly rounded ends, smooth, 3.5–5 × 1.8–2.5 µm, with a straight germ slit 3/4 spore length.
Culture characteristics—Colonies on OA not reaching the edge of a 9 cm of Petri dish in 4 weeks, at first white, becoming brown patched, mostly submerged, zonate with diffuse margin. Reverse uncoloured. Immature stromata forming after 1 week, cylindrical, unbranched, up to 1 cm long by ≤0.1 mm diameter, black to grey at base, grey on surface. Smoke grey at tip and some on aerial mycelia due to production of conidia. Anamorph—Conidiophores in upright, densely arranged palisades, dichotomously branched several times from base, smooth, hyaline to light brown. Conidiogenous cells terminal, cylindrical, geniculate, 9.5–17(–25) × 1.6–2.4 µm, smooth or roughened, bearing several terminal and lateral denticulate conidial secession scars. Conidia produced holoblastically in sympodial sequence, hyaline to light brown, smooth, globose to subglobose 2.9–3.9 × 2.8–3.2 µm, with a flattened base indicating former point of attachment to conidiogenous cell.
Note—Xylaria subintraflava is similar to X. intraflava [6] in having slight perithecial mounds, a wrinkled stromatal surface, and small ascospores ≤5 µm long. It differs from X. intraflava by having unbranched stromata with one acuminate apex, larger perithecia up to 0.7 mm diameter, ascospores ellipsoid, nearly equilateral with narrowly rounded ends that are not pinched, and a small ascal apical apparatus less than 1 µm high. Furthermore, the inner stromatal tissue is pale luteous (11), while that of X. intraflava is pure yellow (14). The pairwise comparison of ITS sequences between X. subintraflava and X. intraflava revealed a 94.75% similarity.
-
13.
Xylaria thienhirunae Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 9A–G.
Figure 9.
Xylaria thienhirunae (SWUF17-44.1). (A) Stroma. (B) Wrinkled stromatal surface with ostioles. (C) Ostiole. (D) Ascus and ascospores. (E) Colony on PDA in a 9 cm Petri dish at 4 weeks. (F) Ascospores. (G) Germ slit (arrowed). Xylaria vinacea (SWUF18-2.10). (H) Natural habit of stromata. (I,J) Wrinkled stromata surface with ostioles. (K) Ascospores. (L) Ascus with ascospores. (M) Ascospores with germ slits (arrowed). (N) Colony on PDA in a 9 cm Petri dish at 2 weeks. (O,P) Anamorphs. (C,J,K) by SEM; (D,F,G,L,M,O,P) by DIC. Scale bars (A,E,H,N) = 1 cm; (B,I) = 1 mm; (C) = 20 µm; (D,F,G,K,M,O,P) = 5 µm; (J) = 100 µm.
MycoBank number: MB 839098.
Etymology—Thienhirunae (Lat.): to honour Dr. Surang Thienhirun, a long-term Thai mycologist who contributed to studies of Thai Xylariaceae.
Type–Thailand, Thung Na Lao sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-44.1 (cultured) (holotype), GenBank accession: ITS = MT622771, α-act = MW459212; Dong Klang sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-1.3; SWUF17-18.1; SWUF17-18.2; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Khaeng-raeng R. SWUF16-6.2 (cultured), GenBank accession: ITS = MT622770, α-act = MW459210, β-tub = MW459235; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Phosri C. SWUF16-7.2 (cultured), GenBank accession: ITS = MT622772; Thung Na Lao sub-district, Khon San District, Chaiyaphum province, on termite nests, May 2016, Phosri C. SWUF16-10.1 (cultured), GenBank accession: ITS = MT622773, α-act = MW459211.
Stromata—Cylindrical, unbranched, with an acuminate apex (0.5–0.9 cm long), 3–9 cm in total length above ground by 1–3 mm broad, 1.2–1.7 cm long at fertile parts, 1.4–6.5 cm long at stipes, with a tortuose rooting base. The surface with conspicuous to inconspicuous perithecial mounds, longitudinally wrinkled, continuous, dark brick (6), black, becoming slightly blackish-brown on the apex, black and smooth on the stipes, texture woody, interior pale luteous (11). Perithecia globose to obovoid, 0.5–0.8 mm diameter by 0.8–1 mm high. Ostioles conic-papillate, black, 0.1 mm broad at base. Asci with eight ascospores, cylindrical, 42–49 µm total length by 4.5–5.6 µm broad, the spore-bearing parts 30–36 µm long, stipes 8.8–16.6 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 0.9–1.4 µm high by 0.9–1.5 µm broad.
Ascospores—Light brown to dark brown, unicellular, lemon-shaped, nearly equilateral with narrowly rounded, frequently pinched at the ends, smooth, 4–5.2(–5.7) × 2.7–4 µm, with a straight germ slit spore-length.
Culture characteristics—Colonies reaching the edge of a 9 cm of Petri dish in 4 weeks, at first white and loose-cottony, becoming cream, mostly submerged, with diffuse margin. Reverse remaining uncoloured. Stromata cylindrical, tapering upward, unbranched to branched, flexuous, up to 8 cm long by 1–2 mm diameter, dispersed small stromata, white, becoming cream. Anamorph not observed.
Note—Xylaria thienhirunae is similar to X. chaiyaphumensis in having similarly-sized lemon-shaped ascospores. However, X. thienhirunae differs from X. chaiyaphumensis in having larger perithecia (up to 0.8–1 mm high) and half-exposed perithecial mounds (ca. 0.1 mm broad at base). There are 2–3 ostioles/mm in X. thienhirunae, while there are 3–4 ostioles/mm in X. chaiyaphumensis. In addition, the asci of X. thienhirunae have shorter stipes of asci (only 8.8–16.6 µm long), and its ascospores are frequently pinched at the ends. The germ slit of X. thienhirunae ascospores is straight and full length, while the germ slit of the X. chaiyaphumensis ascospores is half of the spore length.
-
14.
Xylaria vinacea Wangsawat N, Y.-M. Ju, Phosri C, Whalley AJS & Suwannasai N, sp. nov. Figure 9H–P.
MycoBank number: MB 839106.
Etymology—Vinacea (Lat.): referring to the vinaceous colour of mycelium in culture medium.
Type—Thailand, Nong Lat sub-district, Waritchaphum District, Sakon Nakhon province, on termite nests, July 2018, Wangsawat N. SWUF18-2.10 (cultured) (holotype), GenBank accession: ITS = MT622783, α-act = MW459220, β-tub = MW459240; Phang Khon sub-district, Phanh Khon District, Sakon Nakhon province, on termite nests, July 2018, Phosri C. SWUF18-2.1 (cultured), GenBank accession: ITS = MT622781, α-act = MW459219, β-tub = MW459239; Nong Lat sub-district, Waritchaphum District, Sakon Nakhon province, on termite nests, July 2018, Phosri C. SWUF18-2.3 (cultured), GenBank accession: ITS = MT622782; Thung Phra sub-district, Khon San District, Chaiyaphum province, on termite nests, June 2017, Wangsawat N. SWUF17-7.
Stromata—Fusoid to cylindrical, unbranched, with acuminate apex, 3–5 cm in total length above ground by 2.5–3 mm broad, 1.6–3.5 cm long at fertile parts, 0.9–3.3 cm long at stipes. Surface with conspicuous to mostly slight perithecial mounds, wrinkled, dark brick (60), black and smooth stipes, texture woody, interior white to cream. Perithecia obovoid, 0.3–0.5 mm diameter by 0.3–0.7 mm high. Ostioles papillate, black, 0.2–0.3 mm broad at base. Asci with eight spores, cylindrical, 69.5–120 µm total length by 3.8–5.2 µm broad, the spore-bearing parts 35–59 µm long, stipes 24–66.6 µm long, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped, 1.2–1.8 µm high by 1.7–2.2(–2.4) µm broad.
Ascospores—Light brown to dark brown, unicellular, ellipsoid, inequilateral with narrowly rounded ends, smooth, 6.7–8 × 2.8–3.7 µm, with straight, full spore-length to nearly spore-length germ slit on flattened side.
Culture characteristics—Colonies on OA and PDA mostly similar. Colonies not reaching the edge of a 9 cm Petri dish in 4 weeks, at first white and loose-cottony, becoming brown vinaceous (84), zonate, with brown and white concentric zones, mostly aerial mycelium, with lobed margin. Reverse uncoloured. Immature stromata cylindrical, unbranched, sometimes branched, up to 1.5 cm long by 1–2 mm diameter, black or black-purple, white to grey at tip and upper part of mycelium due to conidial production. Anamorph—Conidiophores in upright, dichotomously branched several times from base, smooth, hyaline. Conidiogenous cells terminal, cylindrical, 20–39 × 1.8–3.2(–3.9) µm, smooth, bearing several terminal and lateral denticulate conidial secession scars. Conidia produced holoblastically in sympodial sequence, hyaline, smooth, obovoid, 3.2–3.9(–4.4) × 2.2–3 µm, with a flattened base indicating former point of attachment to conidiogenous cell.
Note—Xylaria vinacea is similar to X. fimbriata [6,27] in stromatal shape and ascospore size. However, X. vinacea differs from X. fimbriata in having an acuminate apex, larger perithecia (0.3–0.5 mm diameter × 0.3–0.7 mm high) and papillate ostioles. Xylaria fimbriata has a fimbriate stromatal apex, smaller perithecia (2.5–3 mm diameter) and coarsely papillate to conic-papillate ostioles. The ascospore size of X. vinacea is closer to that of X. kedahae (7.5–9 × 3–3.5 × 4–4.5 µm) [5], but the stromatal shape and size are different. The stromata of X. kedahae have three cylindrical fertile branches, and their diameter reaches up to 4 mm. The ascospore ends of X. kedahae are usually minutely pinched, while the ascospores of X. vinacea have narrowly rounded ends (Table 2).
Table 2.
A key to species of Xylaria associated with termite nests and soil in Thailand.
| 1. Ascospores with a median germ pore, (3.6–)3.9–4.7(–5) × (2.35–)2.5–3.2(–3.5) µm | X. escharoidea |
| 1. Ascospores with a germ slit | 2 |
| 2. Stromata usually repeatedly branched, with prominent perithecial mounds, ascospores shorter than 6 µm | 3 |
| 2. Stromata unbranched or sparingly branched, perithecia naked or presenting either inconspicuous or conspicuous perithecial mounds | 4 |
| 3. Stromatal surface white when immature, becoming blackish at maturity; ascospores ellipsoid-inequilateral, germ slit straight spore-length or nearly on flattened side, 5.0–6.0 × 2.5–3.5 µm | X. siamensis |
| 3. Stromatal surface dull coloured, becoming blackish at maturity; ascospores short fusoid-inequilateral, 3.5–5.0 × 2.0–3.0 µm | X. atrodivaricata * |
| 4. Ascospores > 10 µm, germ slit straight full spore-length | 5 |
| 4. Ascospores < 10 µm, germ slit straight 3/4 or full spore-length | 7 |
| 5. Stromal surface whitish to greyish with black ostioles; ascospores blackish-brown,(11–)14.0–19.4 × (6.5–)7.0–10.0 µm | X. tanganyikaensis * |
| 5. Stromatal surface blackish-brown to black with black ostioles; ascospores light brown, brown to dark brown, frequently < 14 µm | 6 |
| 6. Perithecia immersed, usually with prominent perithecial mounds; ascospores ellipsoid-inequilateral with narrowly rounded ends, (10–)10.8–12.3(–13.2) × 4.5–6(–6.4) µm; apical apparatus 4–4.5 × 2–3 µm | X. conica |
| 6. Perithecia naked or nearly so; ascospores ellipsoid-inequilateral with narrowly rounded ends, some pinched at the ends,10.3–11.6(–12) × 4.8–5.6 µm; apical apparatus 1.5–2 × 2–3 µm | X. ischnostroma |
| 7. Stromatal surface white at maturity; ascospores 5.4–6(–6.3) × 2.4–3.0 µm, straight germ slit nearly spore-length on convex side | X. reinkingii var. microspora |
| 7. Stromatal surface other than white, usually dull coloured at maturity | 8 |
| 8. Stromata usually more or less cylindrical, often exceeding 3 mm in diameter | 9 |
| 8. Stromata usually slender, fusiform to cylindrical, rarely exceeding 3 mm in diameter | 12 |
| 9. Stromatal surface blackish-brown to black or dark brick; ascospores mostly longer than 5 µm | 10 |
| 9. Stromatal surface ochraceous to fawn, luteous, greyish or dull black; ascospores mostly shorter than 5 µm | 11 |
| 10. Perithecia presenting very conspicuous mounds, blackish-brown; ostioles conic-papillate; ascospores 7.5–9.5 × (3.2–)3.5–4.5 µm | X. sihanonthii |
| 10. Perithecia immersed, brown; ostioles papillate; ascospores 6.7–8 × 2.8–3.7 µm | X. vinacea |
| 11. Stromata acuminate at the apex, unbranched, ochraceous to yellowish-brown on surface; ascospores inequilateral, 4–5 × 1.8–2.5 µm | X. acuminatilongissima * |
| 11. Stromata usually blunt or, infrequently, mucronate at the apex, greyish-brown on surface; ascospores slightly inequilateral to nearly equilateral, 3.5–5 × 2–3 µm | X. nigripes * |
| 12. Ascospores mostly > 5 µm | 13 |
| 12. Ascospores mostly < 5 µm | 14 |
| 13. Stromata very thin, 0.5–1 mm broad; perithecia naked or so on, hairy; ascospores 5.8–6.8 × 2.7–3.2 µm | X. minima |
| 13. Stromata slender, perithecia immersed, forming conspicuous mounds, without hair; ascospores (4.5–)5–6.2 × 2.2–3 µm | X. fulvescens |
| 14. Ascospores short, fusoid, pinched at the ends | 15 |
| 14. Ascospores ellipsoid-inequilateral to nearly equilateral with narrowly rounded ends | 16 |
| 15. Perithecia 0.3–0.7 mm diameter, 3–4 ostioles/mm; ostioles conic-papillate; ascospores fusoid with pinched ends, 4–5(–5.7) × (2.5–)3–3.8 µm; germ slit of half-full ascospore length | X. chaiyaphumensis |
| 15. Perithecia 0.5–0.8 mm diameter, 2–3 ostioles/mm; ostioles conic-papillate; ascospores fusoid with pinched ends, 4–5.2(–5.7) × 2.7–4 µm; germ slit full ascospore length | X. thienhirunae |
| 16. Stromatal surface longitudinally wrinkled with long stipes, unbranched with fertile parts; ascospores 3.5–5 × 1.8–2.5 µm | X. subintraflava |
| 16. Stromatal surface wrinkled with acuminate at apex, without fertile parts, unbranched or two-branched at apex; ascospores (3.2–)3.8–5 × (1.8–)2–2.5 µm | X. margaretae |
* See Srihanant and Petcharat [10].
4. Discussion
In the present study, 12 new taxa of Xylaria subgenus Pseudoxylaria from northeast Thailand are described and compared with closely related species based on morphological and molecular data. The phylogenetic trees were based on a combined ACT-TUB dataset to support the classification at a supraspecific level [2,28], and complemented by the analysis of an ITS dataset, which is the most commonly used locus for barcoding fungi at the species level [16,29]. Therefore, with the 12 taxa described here for the first time, 17 species and one variety of subgenus Pseudoxylaria are now known in Thailand, i.e., X. acuminatilongissima, X. atrodivaricata, X. chaiyaphumensis, X. conica, X. escharoidea, X. cf. escharoidea, X. fulvescens, X. ischnostroma, X. margaretae, X. minima, X. nigripes, X. cf. nigripes, X. reinkingii var. microspora, X. siamensis, X. sihanonthii, X. subintraflava, X. tanganyikaensis, X. thienhirunae, and X. vinacea [9,10].
Pseudoxylaria was originally proposed by Boedijn [30] as a genus to separate Xylaria nigripes from all other species of Xylaria. Since all Xylaria species from termite nests form a distinct clade within Xylaria [2,31,32,33], a case could be made to transfer them to the genus Pseudoxylaria. Nevertheless, we prefer to consider Pseudoxylaria a subgenus of Xylaria due to the fact that there are no definite morphological characteristics to warrant the separation between species of Pseudoxylaria and Xylaria. Previously, all species of Pseudoxylaria seemed to share a tiny ascospore size shorter than 8 μm, but with the discovery of X. conica and X. ischnostroma in the present study, which have ascospores longer than 10 μm, this trait seems no longer useful to separate Pseudoxylaria and Xylaria. In addition, some species of Pseudoxylaria, such as X. guepini, X. coprinicola, and X. ripicola, are not associated with termite nests but soil. Last but not least, we believe that we only know a small fraction of Xylaria species associated with termite nests, and therefore additional studies are necessary to confirm that all species are nested inside Pseudoxylaria.
Thailand is located within the tropical climatic zone with various types of forest ecosystems that are suitable for termite growth. Termite nests can be easily found in forests, farmlands, and rural shelters. The whole family Macrotermitinae includes up to 12 genera and 373 species worldwide [34], but in Thailand, five genera (Ancistrotermes, Hypotermes, Macrotermes, Microtermes and Odontotermes) and 46 species of termites in the subfamily Macrotermitinae can be found throughout the country [35]. Despite most of the diversity of the termite subfamily Macrotermitinae being located in Africa, only three species of Xylaria subgenus Pseudoxylaria have been documented from that continent: X. arenicola, X. furcata var. hirsuta, and X. escharoidea. In contrast, approximately 40 species of Xylaria subgenus Pseudoxylaria are known in Asia, where much less diversity of Macrotermitinae has been reported, suggesting that African species of Xylaria associated with termite nests are severely understudied. Results of the present study have shown that the stromata of Xylaria species are diverse in shape and size as well as in their perithecial surface and cultural characteristics. The ascospores size ranges from 4 to 19 µm long, and they vary in colour from light brown to dark brown. These results agree with those reported by Hsieh et al. [2]. The morphology of the teleomorph and anamorph states, as well as the cultural characteristics, of Xylaria species associated with termite nests, are apparently highly diverse, to such a degree that is nearly comparable with the morphological diversification found among Xylaria species associated with other substrates [2], in accordance with the phylogenetic structure inferred from DNA data.
In the present study, we found two species, X. cf. escharoidea and X. cf. nigripes, closely resembling X. escharoidea and X. nigripes, respectively, which are widely distributed throughout Asia and elsewhere [5]. The morphological characteristics of our species are similar to the descriptions of X. escharoidea and X. nigripes, but the nucleotide sequences showed differences, producing longer branches in the phylogenetic tree. Although both X. escharoidea and X. nigripes were previously recorded in Thailand [9,10], no cultures were obtained. To clarify these problems, more samples from different locations are required for further investigation.
5. Conclusions
The currently known diversity of Xylaria subgenus Pseudoxylaria in Thailand consists in 17 species and one variety, representing 43% of the 40 species known in the world. Nonetheless, this high diversity likely represents only a small fraction of the subgenus Pseudoxylaria in Thailand because the investigations were conducted mainly in the northeast region. By now, all species of Xylaria associated with termite nests belong to Pseudoxylaria, but the only known synapomorphic trait of this clade, the small ascospore size <8 µm, is not present in all species, suggesting that additional studies are necessary to confirm its most suitable taxonomic status.
Acknowledgments
We would like to thank Rungphet Khaeng-raeng (Faculty of Science and Technology, Pibulsongkram Rajabhat University), Natcha Promphet, Pisit Thamvithayakorn, Jakkrit Khamjerm and Mahakit Prasobkiatkit for the sample survey. We are honoured to dedicate this article in memory of Jack D. Rogers, the leading world authority on the Xylariaceae who sadly passed away on 14 June 2021.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10070575/s1, Figure S1: Phylogenetic tree inferred from ML analysis based on a dataset of ACT-TUB sequences of Xylaria species associated with termite nests. Branches are labelled with likelihood bootstrap support 1000 replications. Poronia pileiformis (WSP 88113001) is the outgroup. Figure S2: Phylogenetic tree inferred from BI analysis based on a dataset of exon ACT-TUB sequences of Xylaria species and related genera. Branches are labelled with Bayesian posterior probabilities and bootstrap values from BI and ML analyses, respectively. Poronia pileiformis (WSP 88113001) is the outgroup. Figure S3: Phylogenetic tree inferred from BI analysis based on a dataset of intron ACT-TUB sequences of Xylaria species and related genera. Branches are labelled with Bayesian posterior probabilities and bootstrap values from BI and ML analyses, respectively. Poronia pileiformis (WSP 88113001) is the outgroup. Figure S4: Phylogenetic tree inferred from ML analysis based on a dataset of ITS sequences of Xylaria species associated with termite nests. Branches are labelled with likelihood bootstrap support 1000 replications. Poronia pileiformis (WSP 88113001) is the outgroup.
Author Contributions
Conceptualization, Y.-M.J., A.J.S.W. and N.S.; Data curation, N.W.; Formal analysis, N.S.; Funding acquisition, N.W. and N.S.; Investigation, N.W.; Methodology, N.W.; Project administration, N.S.; Resources, N.W., Y.-M.J., C.P. and N.S.; Software, N.S.; Supervision, Y.-M.J., A.J.S.W. and N.S.; Validation, Y.-M.J., C.P., A.J.S.W. and N.S.; Visualization, C.P. and A.J.S.W.; Writing—original draft, N.W. and N.S.; Writing—review & editing, Y.-M.J., C.P. and A.J.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Royal Golden Jubilee Ph.D. programme, grant number PHD/0218/2559.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analysed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/, accessed on 16 June 2020; https://www.mycobank.org/page/Type%20specimens%20search, accessed on 23 March 2021; https://www.github.com/niwanawangsawat/Aligment-sequence-files.git, accessed on 17 June 2021.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Batra L.R., Batra S.W.T. Termite-fungus mutualism. In: Batra L.R., Montclair N.J., editors. Insect-Fungus Symbiosis-Nutrition, Mutualism and Commensalism. Allanheld, Osmun & Co.; New York, NY, USA: 1979. pp. 117–163. [Google Scholar]
- 2.Hsieh H.-M., Lin C.-R., Fang M.-J., Rogers J.D., Fournier J., Lechat C., Ju Y.-M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenet. Evol. 2010;54:957–969. doi: 10.1016/j.ympev.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Petch T. Termite fungi: A resume. Ann. Roy. Bot. Gard. 1913;5:303–341. [Google Scholar]
- 4.Dennis R.W.G. Xylarioideae and Thamnomycetoideae of Congo. Bull. Jard. Bot. l’État Brux. 1961;31:109. doi: 10.2307/3667330. [DOI] [Google Scholar]
- 5.Rogers J.D., Ju Y.-M., Lehmann J. Some Xylaria species on termite nests. Mycologia. 2005;97:914–923. doi: 10.1080/15572536.2006.11832783. [DOI] [PubMed] [Google Scholar]
- 6.Ju Y.-M., Hsieh H.-M. Xylaria species associated with nests of Odontotermes formosanusin Taiwan. Mycologia. 2007;99:936–957. doi: 10.1080/15572536.2007.11832525. [DOI] [PubMed] [Google Scholar]
- 7.Rogers J.D. The Xylariaceae: Systematic, Biological and Evolutionary Aspects. Mycologia. 1979;71:1–42. doi: 10.1080/00275514.1979.12020984. [DOI] [Google Scholar]
- 8.Hsieh H.-M., Chou J.-C., Ju Y.-M. Xylaria insolita and X. subescharoidea: Two newly described species collected from a termite nesting site in Hua-lien, Taiwan. Bot. Stud. 2020;61:11–19. doi: 10.1186/s40529-020-00287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thienhirun S. Ph.D. Thesis. Liverpool John Moores University; Liverpool, UK: 1997. A preliminary account of the Xylariaceae of Thailand. [Google Scholar]
- 10.Srihanant N., Petcharat V. Some Xylaria species in oil palm and Pará rubber plantation in southern Thailand. Khon. Kaen. Agri. J. 2015;43:163–169. [Google Scholar]
- 11.McKnight K.H., Rayner R.W. A Mycological Colour Chart. Mycologia. 1972;64:34. doi: 10.2307/3758035. [DOI] [Google Scholar]
- 12.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Shinsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 13.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 15.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 16.Suwannasai N., Martín M.P., Phosri C., Sihanonth P., Whalley A.J.S., Spouge J.L. Fungi in Thailand: A Case Study of the Efficacy of an ITS Barcode for Automatically Identifying Species within the Annulohypoxylon and Hypoxylon Genera. PLoS ONE. 2013;8:e54529. doi: 10.1371/journal.pone.0054529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stecher G., Tamura K., Kumar S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Hoehna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambaut A. FigTree v1.4.2, a Graphical Viewer of Phylogenetic Trees. [(accessed on 17 June 2021)];2014 Available online: http://tree.bio.ed.ac.uk/software/figtree/0.
- 22.Guedegbe H.J., Miambi E., Pando A., Houngnandan P., Rouland-Lefevre C. Molecular diversity and host specificity of termite-associated Xylaria. Mycologia. 2009;101:686–691. doi: 10.3852/08-182. [DOI] [PubMed] [Google Scholar]
- 23.Ju Y.-M., Hsieh H.-M., He X.-S. Xylaria coprinicola, a new species that antagonizes cultivation of Coprinus comatus in China. Mycologia. 2011;103:424–430. doi: 10.3852/10-215. [DOI] [PubMed] [Google Scholar]
- 24.Kim C.S., Jo J.W., Kwag Y.-N., Oh S.-O., Lee S.-G., Sung G.-H., Han J.-G., Oh J., Shrestha B., Kim S.-Y., et al. New Records of Xylaria Species in Korea: X. ripicola sp. nov. and X. tentaculata. Mycobiology. 2016;44:21–28. doi: 10.5941/MYCO.2016.44.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou W.-N., Hsieh H.-M., Ju Y.-M. Xylaria terricola sp. nov., a terrestrial anamorphic Xylaria species found in Taiwan. Fungal Sci. 2017;32:1–8. [Google Scholar]
- 26.Visser A.A., Ros V.I., De Beer Z.W., Debets A.J., Hartog E., Kuyper T.W., Læssoe T., Slippers B., Aanen D.K. Levels of specificity of Xylaria species associated with fungus-growing termites: A phylogenetic approach. Mol. Ecol. 2009;18:553–567. doi: 10.1111/j.1365-294X.2008.04036.x. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd C.G. Mycological notes 51. Mycol. Writ. 1917;5:717–732. [Google Scholar]
- 28.Hsieh H.-M., Ju Y.-M., Rogers J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia. 2005;97:844–865. doi: 10.1080/15572536.2006.11832776. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z.-B., Zhou M., Yuan Y., Dai Y.-C. Global Diversity and Taxonomy of Sidera (Hymenochaetales, Basidiomycota): Four New Species and Keys to Species of the Genus. J. Fungi. 2021;7:251. doi: 10.3390/jof7040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boedijn K.B. On a new family of the Sphaeriales. Persoonia. 1959;1:15–19. [Google Scholar]
- 31.U’Ren J.M., Miadlikowska J., Zimmerman N.B., Lutzoni F., Stajich J.E., Arnold A.E. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota) Mol. Phylogenet. Evol. 2016;98:210–232. doi: 10.1016/j.ympev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Wendt L., Sir E.B., Kuhnert E., Heitkämper S., Lambert C., Hladki A.I., Romero A.I., Luangsa-Ard J.J., Srikitikulchai P., Persoh D., et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018;17:115–154. doi: 10.1007/s11557-017-1311-3. [DOI] [Google Scholar]
- 33.Konta S., Hyde K.D., Phookamsak R., Xu J.C., Maharachchikumbura S.S.N., Daranagama D.A., McKenzie E.H.C., Boonmee S., Tibpromma S., Eungwanichayapant P.D., et al. Polyphyletic genera in Xylariaceae (Xylariales): Neoxylaria gen. nov. and Stilbohypoxylon. Mycosphere. 2020;11:2629–2651. doi: 10.5943/mycosphere/11/1/17. [DOI] [Google Scholar]
- 34.Krishna K., Grimaldi D.A., Krishna V., Engel M.S. Treatise on the Isoptera of the world 4. Termitidae (Part one) Bull. Am. Mus. Nat. Hist. 2013;377:977–1494. [Google Scholar]
- 35.Sornnuwat Y., Vongkaluang C., Takematsu Y. A systematic key to termites of Thailand. Kasetsart J. 2004;38:349–368. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analysed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/, accessed on 16 June 2020; https://www.mycobank.org/page/Type%20specimens%20search, accessed on 23 March 2021; https://www.github.com/niwanawangsawat/Aligment-sequence-files.git, accessed on 17 June 2021.