Abstract
By constantly stimulating intestinal immunity, gut microbes play important regulatory roles, and their possible involvement in human physical and mental disorders beyond intestinal diseases suggests the importance of maintaining homeostasis in the gut microbiota. Both transplantation of fecal microbiota and dietary interventions have been shown to restore microbial homeostasis in recipients. In the current study with wild-type mice, we combined these two approaches to determine if transplanting fecal material from mice fed black raspberries (BRB, 5%) altered recipients’ immune system. The donors received a control or 5% BRB diet, and fecal transplantation was performed every other day 15 times into recipients fed control diet. Afterward, we used flow cytometry to analyze populations of CD3+ T, CD4+ T, CD8+ T cells, and NK cells among bone marrow cells, splenocytes, and peripheral blood mononuclear cells (PBMCs) collected from the recipients. We found that BRB-fecal material that contained both fecal microbiota and their metabolites increased NK cell populations among bone marrow cells, splenocytes, and PBMCs, and raised levels of CD8+ T cells in splenocytes. Our findings suggest that fecal transplantation can modulate the immune system and might therefore be valuable for managing a range of physical and mental disorders.
Keywords: Black raspberries, Fruits and vegetables, Fecal material transplantation, Immune system, Natural killer cells, CD8+ T cells
1 |. INTRODUCTION
The gut microbiota is a community of billions of commensal and transient microorganisms—and potential pathogens—that colonize the intestinal epithelial surface and constantly stimulate intestinal immunity (Quaranta, Sanguinetti, & Masucci, 2019). In mammals, such as humans and mice, 5–20% of all lymphocytes reside in the gut, where they are crucial regulators of the immune system (Ganusov & De Boer, 2007). Accordingly, it is not surprising that the gut microbiota has been implicated in intestinal diseases (Nishida et al., 2018; Rodiño-Janeiro, Vicario, Alonso-Cotoner, Pascua-García, & Santos, 2018; D’Odorico et al., 2018; Shen et al., 2018). In the last decades, however, there has been growing recognition that it might also be involved in many other human physical and mental clinical conditions, including cognition (Jiang, Li, Huang, Liu, & Zhao, 2017), neuropsychiatric disorders (Evrensel & Ceylan, 2016), organ transplantation (Xiao et al., 2018), obesity (Kang & Cai, 2017), cancer management (Chen, Wu, Jin, Wang, & Cao, 2019), autoimmune diseases (De Luca & Shoenfeld, 2019), female reproductive tract diseases (Quaranta et al., 2019), age-related pathological conditions (including atherosclerosis, type 2 diabetes, and Parkinson’s disease) (Vaiserman, Koliada, & Marotta, 2017), autism and mood disorders (Mangiola et al., 2016; Li, Han, Dy, & Hagerman, 2017; Choi & Cho, 2016; Yang, Tian, & Yang, 2018), and chronic fatigue syndrome (Xu et al., 2015; Aroniadis & Brandt, 2013).
As stated above, lymphocytes reside in the mammalian gut (5–20%) and spleen (~10%), bone marrow (~15%), and lymph nodes (~40%); these locales are large immune compartments (Ganusov & De Boer, 2007). Only ~2% of all lymphocytes are found in blood (Ganusov & De Boer, 2007). Natural killer (NK) cells are the effector lymphocytes of the innate immune system. They combat tissue damage by limiting the spread of infectious microbes and many types of tumor cells through the body. NK cells also play roles in hematopoietic stem cell transplantation and in reproduction (Vivier, Tomasello, Baratin, Walzer, & Ugolini, 2008). As regulatory cells, they interact with other immune cells, such as dendritic cells, macrophages, T cells, and endothelial cells, to limit or exacerbate immune responses (Vivier et al., 2008). In addition, T cells coordinate multiple aspects of adaptive immunity, including responses to pathogens, allergens, and tumors. T cells also respond to insults throughout the body and maintain immune homeostasis (Kumar, Connors, & Farber, 2018). In other words, T cells maintain immune responses, homeostasis, self-tolerance, and immunological memory. They function by expressing receptors that can recognize the wide range of antigens presented by pathogens, tumors, and the environment (Kumar et al., 2018).
In healthy adults, levels of CD3+ T cells, CD4+ T cells, and CD8+ T cells decrease with age (>50 years old), but levels of NK cells do not (Kokuina, Breff-Fonseca, Villegas-Valverde, & Mora-Díaz, 2019). Most studies of the functions of NK and T cells have focused on cancer, and they have reported associations between higher levels of NK and CD8+ T cells and favorable outcomes—in colorectal cancer (Xu et al., 2018; Sun, Fan, Wang, & Xu, 2017; Sconocchia et al., 2014), lung cancer (Soo et al., 2018), and ovarian cancer (Nelson, 2008), for example.
Fecal microbiota transplantation is transfer of microbiota in the stool from a healthy individual into the gastrointestinal tract of a person with a disease, such as infection with Clostridium difficile, in an attempt to increase microbial diversity and richness as well as to restore microbial homeostasis (Holleran et al., 2018). Dietary interventions (such as altering fiber intake) that induce rapid changes in certain nutrients can also modify the gut microbiota’s composition, richness, and diversity (Aron-Wisnewsky & Clément, 2016). It is conceivable, therefore, that combining fecal microbiota transplantation with dietary intervention could be an important strategy for rebalancing the gut microbiota and its production of metabolites to maintain health and prevent or manage disease (Shen, 2017). We have shown that black raspberries (BRBs) and their anthocyanin and fiber fractions altered the composition and diversity of gut microbiota in F-344 rats (Pan et al., 2017) and changed their metabolites in wild-type C57BL/6 (Pan et al., 2017) and ApcMin/+ mice (Pan et al., 2015) as well as colorectal cancer patients (Pan et al., 2015). We further showed that those metabolites were responsible for BRBs’ anticancer effects by modulating host immunity (Pan et al., 2020; Pan et al., 2017; Pan et al., 2017; Pan et al., 2018; Pan et al., 2018; Pan et al., 2019; Pan et al., 2018; Pan et al., 2017).
Our current study investigated the functional changes initiated by fecal material transplantation from wild-type mice fed BRBs. We showed that recipient wild-type mice on a control diet that received BRB fecal material had increased NK cell populations in bone marrow, spleen, and PBMCs, and boosted those of CD8+ T cells in spleen. Accordingly, our results suggest that material containing the microbiota/metabolites of a donor fed BRBs can modulate a recipient’s immune system. The possibility that this approach might be valuable for managing various physical and mental disorders warrants future investigation.
2 |. MATERIALS AND METHODS
2.1 |. Animal experiments
All protocols were carried out in accordance with the institutional guidelines for animal care dictated by the Medical College of Wisconsin Animal Care and Use Committee. C57BL/6 mice, aged 5–6 weeks, were purchased from the Jackson Laboratory. The donor mice to be used for fecal transplantation were fed either the control AIN-76a diet or the control diet supplemented with 5% BRBs for the entire duration of the study. Every other day, starting from Day 0, feces from donor mice were freshly collected every 2 hr for 8 hr. They were frozen immediately, and were dissolved in PBS, vortexed, and centrifuged the next day. The fecal supernatant was given to recipient mice via oral gavage. After 1 month of this fecal material transplantation (15 doses), all the mice were euthanized. Splenocytes, bone marrow cells, and PBMCs were collected and processed for flow cytometric analysis.
2.2 |. Isolation of murine splenocytes, bone marrow cells, and PBMCs
Mice were euthanized by CO2 asphyxiation. Spleen was collected and placed into a wet 40 μm cell strainer on top of a 50-mL centrifuge tube. Then the spleen was smashed in the cell strainer, which was rinsed with 5 mL of medium. The cell suspension collected in the tube was centrifuged at 1000 rpm for 5 min. The pellet was resuspended in 5 mL red blood cell lysis buffer (containing 0.15 M NH4Cl, 0.01 M KHCO3, and 0.1 Mm EDTA), and incubated at 4°C for 5 min. Then the cell suspension was centrifuged and rinsed with PBS (containing 5% FBS). Splenocytes in the final pellet were suspended in RPMI1640 medium containing 20% FBS for further application.
Bone marrow cells were flushed out from femurs and tibias with 5 mL DMEM. The cell suspension collected in the tube was centrifuged at 1000 rpm for 5 min. The pellet was resuspended in 5 mL red blood cell lysis buffer (containing 0.15 M NH4Cl, 0.01 M KHCO3, and 0.1 Mm EDTA), and incubated at 4°C for 5 min. Then the cell suspension was centrifuged and rinsed with PBS (containing 5% FBS). Bone marrow cells in the final pellet were suspended in RPMI1640 medium containing 20% FBS for further application.
Circulating blood was collected using BD Vacutainer® spray-coated K2EDTA Tubes, and centrifuged at 1500 rpm for 5 min to obtain plasma. The pellet was resuspended in 20 mL red blood cell lysis buffer (containing 0.15 M NH4Cl, 0.01 M KHCO3, and 0.1 Mm EDTA), and incubated at room temperature for 30 min with vortexing every 10 min. Then the cell suspension was centrifuged and rinsed with PBS (containing 5% FBS). PBMCs in the final pellet were suspended in RPMI1640 medium containing 20% FBS for further application.
2.3 |. Flow cytometry
Splenocytes, bone marrow cells, and PBMCs were stained with surface-marker antibodies (BD Biosciences, Franklin Lakes, NJ). The gating strategies for different immune cells were as follows: CD3+ T cells: CD45+CD3+; CD4+ T cells: CD45+CD3+CD4+; CD8+ T cells: CD45+CD3+CD8+; NK cells: CD45+CD3−NKp46+. The samples were analyzed on an LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ), and FlowJo (Tree Star, Ashland, OR) was used to analyze the results. The data were presented as percentage of positive cells.
2.4 |. Statistical analysis
GraphPad Prism was used to analyze populations of CD3+ T cells, CD4+ T cells, CD8+ T cells, and NK cells in bone marrow and among splenocytes and PBMCs (unpaired, two-tailed t-test). A P value <.05 was considered statistically significant.
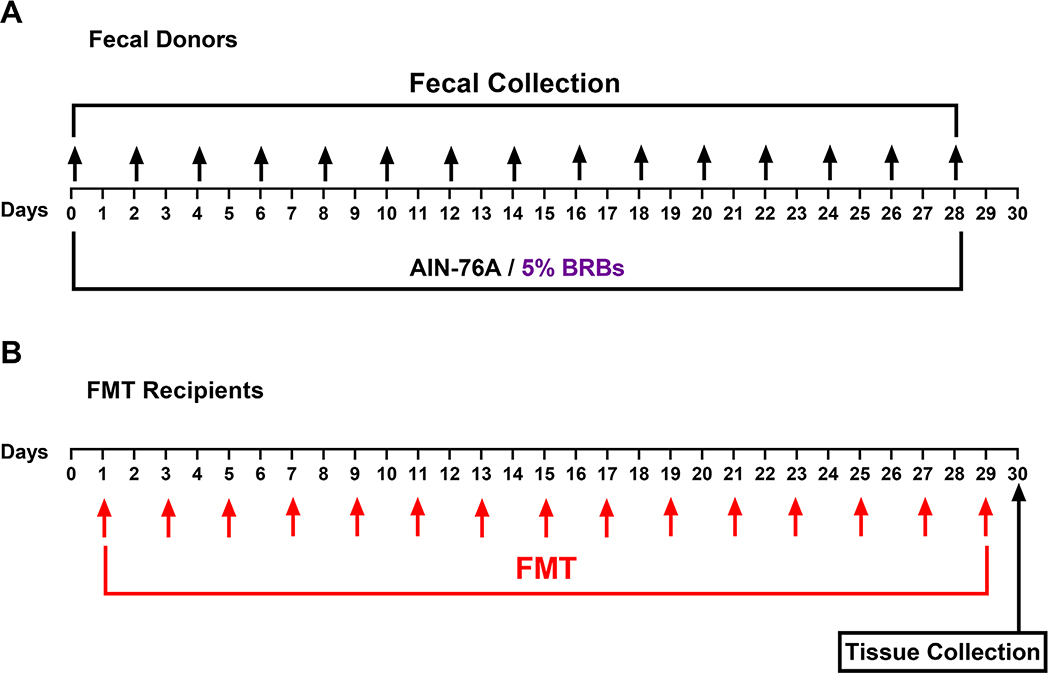
3 |. RESULTS AND DISCUSSION
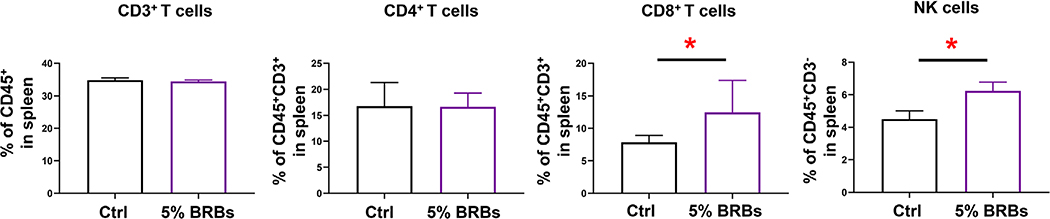
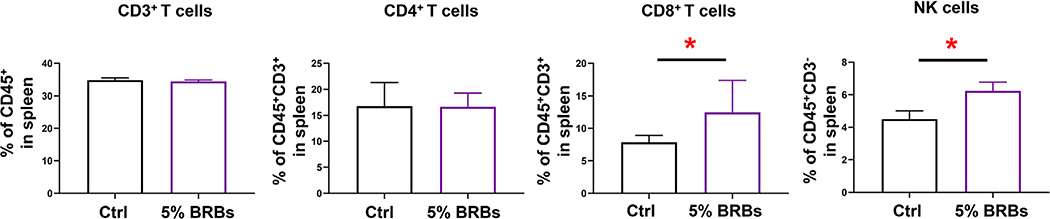
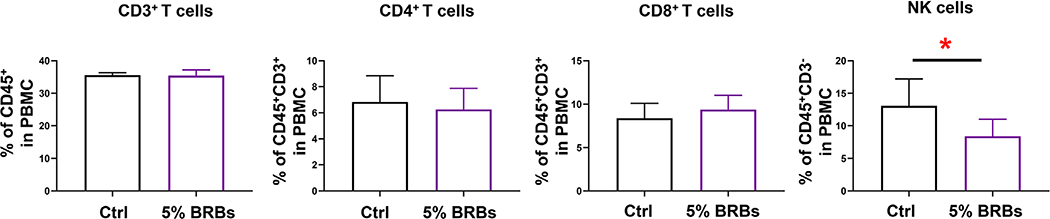
Because the gut microbiota has been implicated in many physical and mental clinical conditions and is known to play crucial regulatory roles in the immune system (Chen et al., 2019; Choi & Cho, 2016; Aroniadis & Brandt, 2013), restoring its hemeostasis to rebalance the immune system could be an attractive approach to managing a range of clinical conditions. Fecal microbiota transplantation (Chen et al., 2019; Choi & Cho, 2016; Aroniadis & Brandt, 2013) and dietary interventions (Aron-Wisnewsky & Clément, 2016) are among the most effective approaches that can restore homeostasis of the gut mictobiota. In the current study, we combined these two approaches to investigate if transplantation of fecal material from BRB-fed wild-type mice alters recipients’ immune systems. The experimental design is shown in Figure 1. Recipient mice on the control diet received fecal material from wild-type mice fed the control diet or the 5% BRB diet. Transplantations were performed every other day 15 times. We found no significant differences in populations of CD3+ T cells and CD4+ T cells in bone marrow (Figure 2), splenocytes (Figure 3), or PBMCs (Figure 4) regardless of whether the donor mice had eaten the control diet or the BRB diet. However, the recipients who received fecal transplantations from the BRB-fed donors had significantly larger populations of CD8+ T cells in their splenocytes (Figure 3)—though not in their bone marrow (Figure 2) or among their PBMCs (Figure 4)—compared with those who received transplantations from the donors on control diet. Interestingly, populations of NK cells expanded in all three organs (Figures 2–4).
FIGURE 1.
Experimental protocol to determine if fecal material from BRB-fed wild-type mice alters immune cell levels in wild-type mice. (A) Collection of donor feces. (B) Fecal material transplantation (FMT) in the recipients
FIGURE 2.
Populations of CD3+ T, CD4+ T, CD8+ T, and NK cells among bone marrow cells from recipient mice that received control stool or BRB fecal material. *P < .05
FIGURE 3.
Populations of CD3+ T, CD4+ T, CD8+ T, and NK cells in splenocytes from recipient mice that received control stool or BRB fecal material. *P < .05
FIGURE 4.
Populations of CD3+ T, CD4+ T, CD8+ T, and NK cells among peripheral blood mononuclear cells from recipient mice that received control stool or BRB fecal material. *P < .05
It has been shown that levels of CD8+ T cells decrease in older people (>50 years old) (Kokuina et al., 2019) and that higher levels of NK and CD8+ T cells associate with favorable outcomes in cancers such as colorectal (Xu et al., 2018; Sun et al., 2017; Sconocchia et al., 2014), lung (Nishida et al., 2018), and ovarian (Nelson, 2008). Our results suggest the possibility of boosting immunity by transplanting fecal material from healthy individuals who have consumed BRBs.
In the current study, we decided not to treat the recipient mice with antibiotics to kill their own bacteria before fecal material was transplanted, because one study had shown that antibiotics induce mild inflammation in the gastrointestinal tract of wild-type mice (Rossen et al., 2015). In addition, antibiotic treatment alone can suppress tumor development (Goldin, Venditti, & Geran, 1985; Pushalkar et al., 2018). In contrast, antibiotic use increases the risk of human colorectal cancer (Shen et al., 2018). Also, patients with ulcerative colitis do not receive antibiotics to kill their own bacteria before fecal microbiota transplantation (Paramsothy et al., 2017; Moayyedi et al., 2015; Rossen et al., 2015). In human clinical trials, the donor feces can be transplanted by colonoscopy and enemas, and upper gastrointestinal delivery routes such as duodenal or gastric tubes or orally ingested frozen capsules (Imdad et al., 2018). Among them, the colonic route, colonoscopy and enemas, is the most efficacious (Imdad et al., 2018). Interestingly, using oral gavage approach, human or mouse fecal microbiota are able to stably colonized into mice (Vaiserman et al., 2017; Nagao-Kitamoto et al., 2016), suggesting that some of those microbiota survive after passing through the stomach with gastric acid.
We should point out that the fecal material we transplanted contained not only microbiota but also microbial metabolites from the donor’s gut as well as intact BRB components. Future studies are needed to investigate the gut microbiota that are changed by BRBs. Indeed, our previous publication reported that feces from wild-type mice fed 5% BRBs had significantly higher levels of amino acids, lipids, xenobiotics, cofactors, and vitamins than feces from wild-type mice on a control diet (Pan et al., 2017). Dietary BRBs also increased benzoate metabolites, such as 3-hydroxybenzoate and 3,4-dihydroxybenzoate, in feces of those mice, serving as evidence that BRBs modulate the gut’s microbiota (Pan et al., 2017). The benzoate metabolites could have been derived from the polyphenols and anthocyanins in BRBs, which are absorbed by the large intestine and metabolized by the gut microbiota (Talavéra et al., 2004). We suspect that transplanting the gut microbiota with its metabolites would be more effective than transferring the microbiota alone, because gut microbes grow better when fecal material contains substrates derived from BRBs. Indeed, using FMT approach, consumption of ginger was shown to beneficially change gut microbiota that prevented obesity in mice (Wang, Wang, Li, Hu, & Chen, 2020). Because dietary nutrients have been suggested to alter gut microbiota (Li, Wang, Wang, Hu, & Chen, 2019), such approach could well apply to many other commonly consumed foods (Li, Wang, Wang, Hu, & Chen, 2016). Alternatively, if FMT in animal studies is not available, in vitro gastrointestinal digestion can be considered as a useful approach to investigate the interaction between gut microbiota and dietary nutrients (Danila et al., 2019; Zhang et al., 2020).
4 |. CONCLUSIONS
Our experimental design mimics fecal microbiota transplantation in humans, as it does not use antibiotics to first remove the recipients’ gut bacteria. Furthermore, instead of using normal gut microbiota for transplantation, we used fecal material from wild-type mice fed BRBs. This material contains both fecal microorganisms and their metabolites, a combination that better maintains the gut microbiota and can favorably modulate the immune system. We therefore propose transplantation of fecal material from BRB-fed humans as an approach to managing certain physical or mental disorders and this approach warrants further investigation.
ACKNOWLEDGMENTS
This work was supported by NIH grants CA148818 and USDA/NIFA 2020-67017-30843 (to L.-S.W.), and CA185301, AI129582, and NS106170 (to J.Y.).
Funding information
NIH; National Institute of Food and Agriculture, Grant/Award Numbers: CA148818, USDA/NIFA 2020-67017-30843, CA185301, AI129582, NS106170
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Aroniadis OC, & Brandt LJ (2013). Fecal microbiota transplantation: Past, present and future. Current Opinion in Gastroenterology, 29(1), 79–84. [DOI] [PubMed] [Google Scholar]
- Aron-Wisnewsky J, & Clément K (2016). The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nature Reviews Nephrology, 12(3), 169–181. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, & ... Chan, A. (2018). Long-term use of antibiotics and risk of colorectal adenoma. Gut, 67(4), 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wu J, Jin D, Wang B, & Cao H (2019). Fecal microbiota transplantation in cancer management: Current status and perspectives. International Journal of Cancer, 145(8), 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HH, & Cho YS (2016). Fecal microbiota transplantation: Current applications, effectiveness, and future perspectives. Clinical Endoscopy, 49(3), 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila C, Tamara Yuliett F-H, Francesca G, Jiaojiao Z, Johura A, Mattia P, & Battino M (2019). Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds and antioxidant activity of manuka honey. eFood, 1(1), 85–93. [Google Scholar]
- De Luca F, & Shoenfeld Y (2019). The microbiome in autoimmune diseases. Clinical and Experimental Immunology, 195(1), 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Odorico I, Di Bella S, Monticelli J, Giacobbe DR, Boldock E, & Luzzati R(2018).Role of fecal microbiotatransplantation in inflammatorybowel disease. Journal of Digestive Diseases, 19(6), 322–334. [DOI] [PubMed] [Google Scholar]
- Evrensel A, & Ceylan ME (2016). Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clinical Psychopharmacology and Neuroscience, 14(3), 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganusov VV, & De Boer RJ (2007). Do most lymphocytes in humans really reside in the gut? Trends Immunology, 28(12), 514–518. [DOI] [PubMed] [Google Scholar]
- Goldin A, Venditti JM, & Geran R (1985). The effectiveness of the anthracycline analog 4’-epidoxorubicin in the treatment of experimental tumors: A review. Investigational New Drugs, 3(1), 3–21. [DOI] [PubMed] [Google Scholar]
- Grasa L, Abecia L, Forcén R, Castro M, de Jalón JA, Latorre E, & Murillo MD (2015). Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in toll-like receptor patterns and intestinal motility. Microbial Ecology, 70(3), 835–848. [DOI] [PubMed] [Google Scholar]
- Holleran G, Scaldaferri F, Ianiro G, Lopetuso L, Mc Namara D, Mele MC, ... Cammarota G (2018). Fecal microbiota transplantation for the treatment of patients with ulcerative colitis and other gastrointestinal conditions beyond Clostridium difficile infection: An update. Drugs of Today (Barcelona, Spain: 1998), 54(2), 123–136. [DOI] [PubMed] [Google Scholar]
- Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, GomezDuarte OG, Beaulieu DB, & Acra S (2018). Fecal transplantation for treatment of inflammatory bowel disease. The Cochrane Database of Systematic Reviews, 11(11), Cd012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Li G, Huang P, Liu Z, & Zhao B (2017). The gut microbiota and Alzheimer’s disease. Journal of Alzheimers Disease, 58(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Kang Y, & Cai Y (2017). Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones (Athens), 16(3), 223–234. [DOI] [PubMed] [Google Scholar]
- Kokuina E, Breff-Fonseca MC, Villegas-Valverde CA, & Mora-Díaz I (2019). Normal values of T, B and NK lymphocyte subpopulations in peripheral blood of healthy Cuban adults. MEDICC Review, 21(2–3), 16–21. [DOI] [PubMed] [Google Scholar]
- Kumar BV,Connors TJ,& Farber DL(2018).Human Tcell development, localization, and function throughout life. Immunity, 48(2), 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang P, Wang P, Hu X, & Chen F (2016). The gut microbiota: A treasure for human health. Biotechnology Advances, 34(7), 1210–1224. [DOI] [PubMed] [Google Scholar]
- Li D, Wang P, Wang P, Hu X, & Chen F (2019). Targeting the gut microbiota by dietary nutrients: A new avenue for human health. Critical Reviews in Food Science and Nutrition, 59(2), 181–195. [DOI] [PubMed] [Google Scholar]
- Li Q, Han Y, Dy ABC, & Hagerman RJ (2017). The gut microbiota and utism spectrum disorders. Frontiers in Cellular Neuroscience, 11, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, & Gasbarrini A (2016). Gut microbiota in autism and mood disorders. World Journal of Gastroenterology, 22(1), 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, ... Lee C (2015). Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology, 149(1), 102–109.e6. [DOI] [PubMed] [Google Scholar]
- Nagao-Kitamoto H, Shreiner AB, Gillilland MG 3rd, Kitamoto S, Ishii C, Hirayama A, & Kamada N (2016). Functional characterization of inflammatory bowel disease-associated gut dysbiosis in gnotobiotic mice. Cellular and Molecular Gastroenterology and Hepatology, 2(4), 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BH (2008). The impact of T-cell immunity on ovarian cancer outcomes. Immunological Reviews, 222, 101–116. [DOI] [PubMed] [Google Scholar]
- Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, & Andoh A (2018). Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical Journal of Gastroenterology, 11(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Pan P, Huang YW, Oshima K, Yearsley M, Zhang J, Arnold M, ... Wang L-S (2019). The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Critical Reviews in Food Science and Nutrition, 59(6), 992–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Huang YW, Oshima K, Yearsley M, Zhang J, Yu J, ... Wang L-S (2018). An immunological perspective for preventing cancer with berries. Journal of Berry Research, 8(3), 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Kang S, Wang Y, Liu K, Oshima K, Huang YW, ... Wang L-S (2017). Black raspberries enhance natural killer cell infiltration into the colon and suppress the progression of colorectal cancer. Frontiers in Immunology, 8, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, & Wang L-S (2017). Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutrition and Cancer, 69(6), 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Oshima K, Huang YW, Agle KA, Drobyski WR, Chen X, ... Wang L-S (2018). Loss of FFAR2 promotes colon cancer by epigenetic dysregulation of inflammation suppressors. International Journal of Cancer, 143(4), 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Oshima K, Huang YW, Yearsley M, Zhang J, Arnold M, ... Wang L-S (2018). Gut bacteria are required for the benefits of black raspberries in Apc (Min/+) mice. Journal of Berry Research, 8(4), 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer CW, Stirdivant SM, Young MR, Stoner GD, Lechner JF, ... Wang L-S (2015). Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prevention Research, 8(8), 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer CW, Wang HT, Kreiser MA, Stirdivant SM, Oshima K, ... Wang L-S (2017). Systemic metabolite changes in wild-type C57BL/6 mice fed black raspberries. Nutrition and Cancer, 69(2), 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer CW, Wang HT, Oshima K, Huang YW, Yu J, ... Wang L-S (2017). Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis, 38(1), 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer CW, Wang HT, Stirdivant SM, Young MR, Oshima K, ... Wang L-S (2015). Black raspberries suppress colonic adenoma development in ApcMin/+ mice: Relation to metabolite profiles. Carcinogenesis, 36(10), 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer C, Yu J, Zhao H, Ren H, Oshima K, ... Wang L-S (2017). Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. Journal of Berry Research, 7(3), 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Zhu Z, Oshima K, Aldakkak M, Tsai S, Huang YW, ... Wang L-S (2020). Black raspberries suppress pancreatic cancer through modulation of NKp46(+), CD8(+), and CD11b(+) immune cells. Food Frontiers, 1(1), 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, & Borody TJ (2017). Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet, 389(10075), 1218–1228. [DOI] [PubMed] [Google Scholar]
- Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, & Miller G (2018). The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discovery, 8(4), 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta G, Sanguinetti M, & Masucci L (2019). Fecal microbiota transplantation: A potential tool for treatment of human female reproductive tract diseases. Frontiers in Immunology, 10, 2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, & Santos J (2018). A review of microbiota and irritable bowel syndrome: Future in therapies. Advances in Therapy, 35(3), 289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, & Ponsioen CY (2015). Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology, 149(1), 110–118.e4. [DOI] [PubMed] [Google Scholar]
- Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, ... Ferrone S (2014). NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology, 3(8), e952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TD (2017). Diet and gut microbiota in health and disease. Nestlé Nutrition Institute Workshop Series, 88, 117–126. [DOI] [PubMed] [Google Scholar]
- Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, ... Wang X-Y (2018). Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World Journal of Gastroenterology, 24(1), 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo RA, Chen Z, Yan Teng RS, Tan HL, Iacopetta B, Tai BC, & Soong R (2018). Prognostic significance of immune cells in non-small cell lung cancer: Meta-analysis. Oncotarget, 9(37), 24801–24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley C, Kaiser T, Beura LK, Hamilton MJ, Weingarden AR, Bobr A, & Khoruts A (2017). Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome, 5(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JJ, Fan GL, Wang XG, & Xu K (2017). The research on the influences of hyperthermal perfusion chemotherapy combined with immunologic therapy on the immunologic function and levels of circulating tumor cells of the advanced colorectal cancer patients with liver metastasis. European Review for Medical and Pharmacological Sciences, 21(13), 3139–3145. [PubMed] [Google Scholar]
- Talavéra S, Felgines C, Texier O, Besson C, Manach C, Lamaison JL, & Rémésy C (2004). Anthocyanins are efficiently absorbed from the small intestine in rats. Journal of Nutrition, 134(9), 2275–2279. [DOI] [PubMed] [Google Scholar]
- Vaiserman AM, Koliada AK, & Marotta F (2017). Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Research Reviews, 35, 36–45. [DOI] [PubMed] [Google Scholar]
- Vivier E,Tomasello E,Baratin M,Walzer T,& Ugolini S(2008).Functions of natural killer cells. Nature Immunology, 9(5), 503–510. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang P, Li D, Hu X, & Chen F (2020). Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. European Journal of Nutrition, 59(2), 699–718. [DOI] [PubMed] [Google Scholar]
- Xiao J, Peng Z, Liao Y, Sun H, Chen W, Chen X, ... Yang W (2018). Organ transplantation and gut microbiota: Current reviews and future challenges. American Journal of Translational Research, 10(11), 3330–3344. [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sun X, Xin Q, Cheng Y, Zhan Z, Zhang J, & Wu J (2018). Effect of immunonutrition on colorectal cancer patients undergoing surgery: A meta-analysis. International Journal of Colorectal Disease, 33(3), 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, & Wang B (2015). MFecal microbiota transplantation broadening its application beyond intestinal disorders. World Journal of Gastroenterology, 21(1), 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tian J, & Yang B (2018). Targeting gut microbiome: A novel and potential therapy for autism. Life Sciences, 194, 111–119. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shi M, Tian M, Wang X, Ji J, Liao X, & Chen F (2020). Guidelines for absolute quantitative real-time PCR for microbial determination in in vitro gastrointestinal digestion. Food Frontiers, 1(2), 200–204. [Google Scholar]