Abstract
More than 50% of the world’s population is infected with Helicobacter pylori. H. pylori is the major causative agent of gastric ulcers and gastric cancer. H. pylori eradication using antibiotics either alone or together with a proton pump inhibitor is the primary strategy to decrease the incidence of gastric cancer. Although eradication therapy is effective, there are significant adverse effects and more importantly, resistance to antibiotics occurs, which represents a major therapeutic challenge. Multiple natural products have been shown to suppress H. pylori both in vitro and in animal model systems. However, only a handful of natural products have been evaluated in human clinical trials. The focus of this review is to summarize the results of published human clinical trials to assess the ability of natural products to reduce or eliminate H. pylori infections. Current evidence suggests that these products appear to have great potential to be developed as pharmaceutical candidates for eradication of H. pylori, hopefully both antibiotic-sensitive and antibiotic-resistant strains. Frequent consumption of locally produced foodstuff for controlling H. pylori infection in different countries around the world may well be a feasible long-term solution to fight against this worldwide prevalent pathogen.
Keywords: Helicobacter pylori, natural products, resistant, gastric diseases, humans
1. INTRODUCTION
It was estimated that more than 50% of the world’s population is infected with Helicobacter pylori [1]. The bacterium highly links to gastric ulcers as well as gastric cancer and in 1994, it was classified as a group I carcinogen by the World Health Organization (WHO) [1]. H. pylori can induce gastric inflammation which could progress to superficial gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and ultimately to gastric cancer [2]. Eradication therapy using antibiotics and Proton Pump Inhibitors (PPIs) is the standard therapy, but in some regions of the world it fails due to emergence of antibiotic-resistant strains of the organism [3].
Numerous plant-derived compounds have been reported to inhibit H. pylori such as various polyphenols, flavonoids, quinones, coumarins, terpenoids, and alkaloids [2]. For example, quercetin, tea products, garlic extract, apple peel polyphenols, ginger-root extract, and many others have been studied. Mechanisms by which these compounds exert their anti-H. pylori effects are as follows: (1) inhibition of H. pylori enzymes such as urease which decrease the acidity of gastric juice; (2) inhibition of adhesion of the organism to gastric mucosa; and (3) reduction in oxidative radicals that damage cellular macromolecules. Several reviews have summarized the use of natural products in suppressing H. pylori infection and their mechanisms of action using in vitro cell culture and in vivo animal models [2–8]. Readers are referred to these comprehensive reviews for details. The present review summarizes the results of published human clinical trials and discusses current evidence of the ability of natural products to inhibit H. pylori in humans. Multiple medicinal plant products inhibit H. pylori growth and H. pylori-induced gastric inflammation in humans. These natural products appear to have significant potential to be developed as pharmaceutical lead candidates for H. pylori eradication and for prevention of gastric diseases induced by H. pylori infection.
2. GASTRIC CANCER AND H. PYLORI INFECTION
Gastric cancer is the third leading cause of cancer-related mortality worldwide [9]. Drs J.R. Warren and B.J. Marshall were awarded for the Nobel Prize for Physiology and Medicine for the isolation and culture of H. pylori, and demonstrating its relevance to peptic ulcer disease [10]. Several case-controlled studies have shown that H. pylori seropositivity is associated with a significantly increased risk for gastric cancer (2.1–16.7-fold greater than the seronegative individuals) [11]. Encouragingly, epidemiologic studies indicate that there was a progressive decline in H. pylori infection during the last century in developed countries [1]. Nevertheless, more than 50% of the world’s population remains infected with this organism, with the highest infection rates and gastric cancer incidence rates reported in lower socioeconomic countries [12].
To establish persistent infection, H. pylori escapes the immune system by mimicking host antigens [12]. This pathogen has the ability to escape the immune system and persist within host cells of the stomach leading to immune tolerance. Although establishment of H. pylori as a risk factor permits an approach to identify persons at increased risk for gastric adenocarcinoma, only a fraction of H. pylori-colonized persons develop the disease [12]. Recent data suggest that microbial–host interactions governed by differences in genotype acting in combination with permissive environmental conditions determine H. pylori-induced gastric cancer risk [11]. Therefore, it is not surprising that reducing an environmental factor such as salt intake may decrease the risk of gastric cancer irrespective of the presence of H. pylori [13].
3. CURRENT THERAPIES, EFFECTIVENESS, AND RESISTANCE
Helicobacter pylori is known to induce chronic inflammation in the gastric mucosa through its production of agents such as superoxide that induce DNA damage [14]. H. pylori eradication using antibiotic treatment in combination with a PPI is the major chemoprevention strategy to decrease the incidence of gastric cancer [11]. Eradication therapy is effective, but there is a steady increase in the emergence of antibiotic-resistant strains of the organism. A meta-analysis used data from 20 nationwide trials (3624 men and women) of H. pylori eradication in the United States to estimate the prevalence of H. pylori resistance to antibiotics and to characterize factors associated with resistance [15]. These factors included geographic region, age, sex, study year, ethnicity, ulcer status, and test method. The overall resistance to clarithromycin, metronidazole, and amoxicillin was 10.1% [95% Confidence Interval (CI), 9.1–11.1% (360 of 3571 patients)], 36.9% [95% CI, 35.1–38.7% (1063 of 2883 patients)], and 1.4% (95% CI, 1.0–1.8% (48 of 3486 patients)], respectively. Factors associated with resistance to different antibiotics varied. For example, clarithromycin resistance was associated with geographic region, older age, female sex, and inactive ulcer disease. Metronidazole resistance was associated with female sex, earlier year of study enrollment, Asian ethnicity, and use of an Epsilometer test. Amoxicillin resistance was low and was not significantly associated with any risk factor. These data are valuable for clinicians to consider when they decide which patients should have susceptibility testing when choosing appropriate anti-H. pylori treatments.
An important area of controversy in H. pylori eradication is the apparent difference in eradication rates seen in different countries. A recent meta-analysis showed that several factors may affect the outcome of therapy in different countries [16]. For example, individuals residing in northeast Asia had higher eradication rates than those residing in Europe or other areas of Asia. In Japan, eradication therapy significantly diminished the prevalence of gastric cancer by around one-third [14,17]; however, studies from China [18] and the United States [19] reported different negative results [17]. Subgroup analysis suggests that eradication of H. pylori decreases the risk for development of gastric cancer and is most effective in H. pylori carriers who do not have atrophic gastritis or precancerous lesions [18].
It should be noted that other strains of Helicobacter such as Helicobacter suis, Helicobacter heilmannii sensu stricto, and Helicobacter felis may reside in the stomach of humans and animals [20]. Recent advancements in in vitro cultivation of these strains and recent reports from in vivo preclinical studies suggest that these strains may have more clinical significance than thought in the past. Additional studies of the health effects of these strains are warranted.
In addition to eradication therapy, vaccines against H. pylori have been developed in animals and tested in phase 3 clinical trials in humans. These trials have been largely unsuccessful, although H. pylori-specific immune responses have been induced [21]. New insights into promoting immunity and overcoming the immunosuppressive nature of H. pylori infections are required to improve the efficacy of the vaccines [21].
Recurrence of H. pylori infection after successful eradication occurs and is associated with relapse of gastroduodenal diseases [22]. Individual susceptibility and re-exposure to H. pylori are suggested as two major causes of reinfection. This represents a major concern that should be addressed by the research community.
4. HUMAN CLINICAL TRIALS OF NATURAL COMPOUNDS AGAINST H. PYLORI
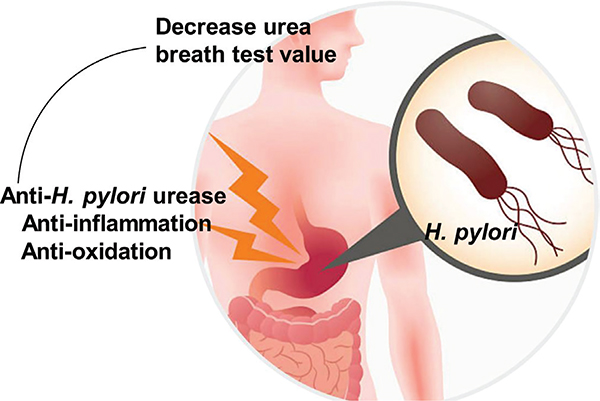
There are multiple reports from around the world of the ability of natural products to inhibit H. pylori [7]. In the following sections, we summarize the ability of natural products to suppress H. pylori as evidenced from human clinical trials (Table 1), and we discuss their mechanisms of action (Figure 1).
Table 1.
Effects of natural products against Helicobacter pylori in humans
| Natural product | Human patient | Treatment duration | Location | Effects | References |
|---|---|---|---|---|---|
| Burdock complex | Adults (19 treated, 17 placebo) who were asymptomatic but with H. pylori infection | 8 weeks | Taiwan | Decreased UBT value and inflammatory markers, increased antioxidant activity. | [23] |
| Conifer green needle complex | Adults (26 treated, 24 no treatment) with precancerous gastric lesions | 6 months | Russia | Partial regression of dyspeptic symptoms, eradication of H. pylori infection, reduction in endoscopic signs of gastritis, an increase of pepsinogen-pepsin in gastric juice, and total regression or reduction in the degree of intestinal metaplasia and lymphoplasmacytic infiltration. | [24] |
| Bovine lactoferrin | 24 Adults and 25 children (aged 4–13 yr) who were asymptomatic and with minimal upper gastrointestinal symptoms, positive for H. pylori infection | 12 weeks | Japan | Decreased UBT value at week 12, but it increased 4 weeks after stopping intervention. | [25] |
| Bovine lactoferrin | 74 Adult H. pylori-positive patients with symptomatic dyspepsia, with or without active ulcer | Group A: 24 patients received triple therapy (rabeprazole, clarithromycin, and tinidazole) plus lactoferrin for 7 days; Group B: 26 patients received triple therapy (rabeprazole, clarithromycin, and tinidazole) for 7 days; Group C: 24 patients received triple therapy (rabeprazole, clarithromycin, and tinidazole) for 10 days | Italy | The eradication rates in groups A, B, and C were 100% (24/24), 76.9% (20/26; 95% Cl, 61–93%), and 70.8% (17/24; 95% Cl, 53–89%), respectively. Significant differences in eradication rates were found between group A and group B, and group A and group C, but not between group B and group C. |
[26] |
| Bovine lactoferrin | 402 Adult H. pylori-positive patients | Group A: 136 patients received triple therapy (esomeprazole, clarithromycin, and tinidazole) for 7 days; Group B: 132 patients received lactoferrin for 7 d followed by triple therapy (esomeprazole, clarithromycin, and tinidazole) for 7 days; Group C: 134 patients received triple therapy (esomeprazole, clarithromycin and tinidazole) plus lactoferrin for 7 days | Italy | The eradication rate was 77% (105/136) in group A, 73% (97/132) in group B, and 90% (120/134) in group C. The incidence of side effects was 9.5%, 9%, and 8.2% in groups A, B, and C, respectively. |
[28] |
| Cranberries | 295 Asymptomatic children (aged 6–16 years) | Cranberry juice and the probiotic Lactobacillus johnsonii La1 (La1) alone or in combination for 3 weeks | Chile | The combination group showed a slight but nonsignificant increase in suppression of H. pylori when compared with either intervention alone. When treatment was stopped, H. pylori was positive again in 80% of the negative patients who received cranberry and/or La1 previously. |
[30] |
| Cranberries | 189 Adults with H. pylori infection: n = 97 for the cranberry juice group and n = 92 for the placebo group | 90 days | China | Negative test results: 14/97 in the cranberry juice group versus 5/92 in the placebo group. | [31] |
| Probiotics | A meta-analysis from 33 randomized, controlled trials involving a total of 4459 patients | Multiple durations | Multiple countries | Supplementation of specific strains of probiotics with eradication therapy may be considered as an addition for increasing eradication rates. These probiotics are Lactobacillus acidophilus, Lactobacillus casei DN-114001, Lactobacillus gasseri, and Bifidobacterium infantis 2036. |
[32] |
| Vitamins and garlic | 3365 Adult patients (2258 H. pylori positive, 1107 H. pylori negative) | Vitamin supplement: vitamin C, vitamin E, and selenium for 7.3 years Garlic supplement: aged garlic extract and steam-distilled garlic oil for 7.3 years |
China | Long-term vitamin or garlic supplementation alone or given after eradication therapy did not have beneficial effects on the prevalence of precancerous gastric lesions or incidence of gastric cancer. | [33] |
| Garlic | A meta-analysis of 18 studies (142,921 patients) | Multiple durations | Multiple countries | Current epidemiological evidence is insufficient to reach a definitive conclusion regarding the association of garlic consumption with H. pylori infection. | [36] |
| β-Carotene, ascorbic acid | 852 Adult patients with confirmed histologic diagnoses of multifocal nonmetaplastic atrophy, intestinal metaplasia, or dysplasia | Ascorbic acid or β-carotene for 6 years | USA | Both ascorbic acid and /Ucarotene increase lesion regression rates. Combinations of triple therapy with ascorbic acid or /Ucarotene did not significantly increase the rates of lesion regression. By contrast, the combination treatment was worse than placebo. |
[37] |
| β-Carotene, ascorbic acid | 795 Adult patients with confirmed histologic diagnoses of multifocal nonmetaplastic atrophy, intestinal metaplasia, or dysplasia | Ascorbic acid or β-carotene for 6 years and follow-up for another 6 years | USA | Ascorbic acid and β-carotene were effective to increase the regression rates of lesions at the first 6 years. However, this effect disappeared during the 6 years of follow-up of no supplementation, and no residual effect was observed. | [19] |
CI, confidence interval; UBT, urea breath test.
Figure 1 |.
Mechanisms by which natural compounds suppress Helicobacter pylori in humans. Adapted from https://centrefordigestivediseases.com/for-patients/tests/urea-breath-test/.
4.1. Burdock Complex
Burdock complex contains burdock (Arctium lappa), angelica (Angelica sinensis), gromwell (Lithospermum erythrorhizon), and sesame (Sesamum indicum) oil commonly used in traditional Chinese medicine for treating various disorders [23]. Burdock complex was tested on asymptomatic H. pylori-infected patients in a randomized, double-blind, placebo-controlled clinical trial in Taiwan [23]. H. pylori-positive patients [Urea Breath Test (UBT) > 10%, n = 36] were enrolled and consumed burdock complex (n = 19) or placebo (n = 17) for 8 weeks. Antioxidant capacity, total phenol, UBT, and various inflammatory markers were analyzed at baseline and on the 4th, 8th, and 10th weeks after intervention. In addition, endoscopic examinations were carried out at baseline and at the 10th week. H. pylori-infected patients who had consumed burdock complex for 8 weeks had significantly decreased UBT values. In addition, the inflammatory markers (interleukin-8 and tumor necrosis factor-α) were suppressed, antioxidant activity was increased, and phenolic levels were increased when compared with placebo. Endoscopic examination indicated that consumption of burdock complex considerably reduced the stomach ulcers.
4.2. Conifer Green Needle Complex
In an observational pilot study conducted in Russia, a tablet form of Conifer Green Needle Complex (CGNC) extracted from Pinus sylvestris and Picea abies (L) Karst was prescribed to 26 patients with precancerous gastric lesions for 6 months [24]. Another 24 patients received no treatment (control/no placebo). Compared with control patients, CGNC-treated patients showed total or partial regression of dyspeptic symptoms, a decrease in H. pylori infection, a decrease in endoscopic signs of gastritis, an increase of pepsinogen–pepsin in the gastric juice, and total regression or reduction in the degree of intestinal metaplasia and lymphoplasmacytic infiltration.
4.3. Bovine Lactoferrin
Bovine lactoferrin, a protein with antimicrobial activity, was evaluated for its efficacy to suppress H. pylori colonization in a randomized, double-blind, placebo-controlled study in Japan [25]. Fifty-nine healthy individuals (34 adults and 25 children, aged 4–13) positive for H. pylori infection were recruited and received bovine lactoferrin or placebo for 12 weeks. UBT was performed before, during, and at the end of administration, and again at 4 weeks after administration (week 16). Positive response was defined as a >50% decrease in the UBT value at the end of administration. At week 12, a significantly higher positive response was observed in 10 of 31 lactoferrin-treated individuals versus one of 28 placebo-treated cases, combining data from both adults and children. There was no significant difference in positive response rates between adults and children. However, at week 16, the responders in both adults and children had increased UBT, suggesting that continuous consumption of lactoferrin is required to eradicate H. pylori.
The efficacy of a triple antibiotic therapy regimen in combination with bovine lactoferrin for the eradication of H. pylori infection was investigated in an open, randomized, single-center study in Italy [26]. This trial included 74 H. pylori-positive patients with symptomatic dyspepsia, with or without active ulcer. There were three groups: 24 patients received triple therapy (rabeprazole, clarithromycin, and tinidazole) + lactoferrin for 7 days (group A); 26 patients received triple therapy (rabeprazole, clarithromycin, and tinidazole) for 7 days (group B); and 24 patients received triple therapy (rabeprazole, clarithromycin, and tinidazole) for 10 days (group C). Eight weeks after the end of treatment, the H. pylori status was assessed using UBT or H. pylori stool antigen test. The results showed that the eradication rates in groups A, B, and C were 100% (24/24), 76.9% (20/26; 95% CI, 61–93%) and 70.8% (17/24; 95% CI, 53–89%), respectively. Significant differences were found between groups A and B and groups A and C, but not between groups B and C. These data suggest that combination of lactoferrin and triple therapy is more effective in H. pylori eradication. One limitation of this trial was that resistance to antibiotics was not determined. Although clarithromycin resistance is low in Italy (<2%), it is about 10% in the United States and metronidazole resistance is about 37% in the United States [15]. Therefore, it will be important to determine the ability of lactoferrin to effectively eradicate clarithromycin resistance in countries such as the United States where resistance is higher than in Italy [27]. Of importance in this study is that the incidence of adverse effects was not increased by the addition of lactoferrin to the antibiotic therapy. However, the study was too small to assess whether lactoferrin diminishes antibiotic-related side effects.
Results from the aforementioned study were confirmed by another study conducted by the same group of scientists in Italy [28]. In a multicentered prospective study [28], 402 H. pylori- positive adult patients were assigned to one of three following regimens: 7 days of triple therapy (esomeprazole, clarithromycin, and tinidazole; group A); 7 days of lactoferrin followed by 7 days of triple therapy (esomeprazole, clarithromycin, and tinidazole; group B); 7 days of triple therapy (esomeprazole, clarithromycin, and tinidazole) followed by 7 days of lactoferrin (group C). The results showed that the eradication rate was 77% (105/136) in group A, 73% (97/132) in group B, and 90% (120/134) in group C. The incidence of side effects was 9.5%, 9%, and 8.2% in groups A, B, and C, respectively. This study demonstrated that bovine lactoferrin is an effective addition to the triple therapy (7 days) for eradication of H. pylori infection. However, the adverse effects were not significantly decreased by adding lactoferrin [28].
4.4. Cranberries
The potential of cranberries to inhibit H. pylori infection in humans has been summarized in a recent review [29]. The effects of regular consumption of cranberry juice and the probiotic Lactobacillus johnsonii La1 (La1), either alone or in combination, on inhibition of H. pylori colonization in children were investigated in a study in Chile [30]. The study was a randomized, controlled, double-blind multicentric trial carried out in 295 asymptomatic children (aged 6–16) positive for H. pylori, as determined using UBT [30]. There were four groups: cranberry juice + La1 (CB/La1), placebo juice + La1 (La1), cranberry juice + heat-killed La1 (CB), and placebo juice + heat-killed La1 (control). The children consumed cranberry juice and La1 product for 3 weeks and then a second UBT was carried out. After a 1-month washout, a third UBT was performed in children who were negative in the second UBT. H. pylori eradication rates were 1.5% in the control and 14.9%, 16.9%, and 22.9% in the La1, CB, and CB/La1 groups, respectively. The increase in UBT in the CB/La1 group was not significant compared with the La1 and CB groups. In the third UBT, which was performed in 19 of the 38 children who were negative in the second UBT, H. pylori was positive in 80% of the children. These results suggest that regular and/or frequent intake of cranberry juice or La1 could be a useful management in asymptomatic children with H. pylori infection. The results also suggest that there are no apparent synergistic inhibitory effects on H. pylori infection when patients consumed both cranberry juice and the probiotic L. johnsonii La1 (La1) together.
A trial conducted in China reported similar results. In Linqu County of Shandong Province in China, a prospective, randomized, double-blind, placebo-controlled trial suggested that cranberry juice suppresses H. pylori infection [31]. A total of 189 adults with positive H. pylori were randomly assigned into two groups to receive either cranberry juice (n = 97) or placebo juice (n = 92) for 90 days. H. pylori infection was determined using UBT. The study concluded that 14 of the 97 adults in the cranberry juice treatment group versus five of the 92 in the placebo group yielded negative results in UBT.
4.5. Probiotics
A meta-analysis from 33 randomized controlled trials involving a total of 4459 patients suggested that supplementation of specific strains of probiotics (Lactobacillus acidophilus, Lactobacillus casei DN-114001, Lactobacillus gasseri, and Bifidobacterium infantis 2036) with H. pylori eradication therapy may be considered as an option for increasing eradication rates, particularly when antibiotic therapies are relatively ineffective [32].
4.6. Vitamins and Garlic
In China, a randomized trial was set out to test the eradication effects of one-time antibiotic treatment for H. pylori infection followed by a long-term vitamin or garlic supplementation in decreasing gastric lesion prevalence [33]. A total of 3365 eligible individuals selected from 13 villages in Linqu County, Shandong Province, China who were positive for H. pylori were randomly assigned to three interventions or placebo in a factorial design [33]. These groups were H. pylori treatment (amoxicillin and omeprazole) for 2 weeks; vitamin supplement (vitamin C, vitamin E, and selenium) for 7.3 years; and garlic supplement (aged garlic extract and steam-distilled garlic oil) for 7.3 years. Patients were treated for H. pylori eradication and with vitamin or garlic supplement, either alone or in combination. Histopathologic examination was performed on biopsies collected by endoscopies to determine the prevalence of precancerous gastric lesions. Long-term vitamin or garlic supplementation alone or in combination with eradication therapy did not have beneficial effects on either the precancerous gastric lesion prevalence or gastric cancer incidence [33] despite the fact that antioxidants were shown to reduce the risk of gastric cancer in nutritional intervention trials in China [34]. The lack of response in this patient group might have been due to their consumption of adequate amounts of vitamin and garlic before treatment; therefore, there were no added benefits of supplementation of these nutrients [35]. Individuals at the highest risk should be monitored endoscopically to detect dysplasia and early cancer.
The association of garlic with H. pylori infection and gastric cancer risk has been investigated in multiple human clinical trials around the world. A meta-analysis of 18 studies (142,921 patients) demonstrated that high garlic consumption (comparing the highest category with the lowest) was associated with a reduced gastric cancer risk [Odds Ratio (OR), 0.51; 95% CI, 0.44–0.57] [36]. However, this association became nonsignificant if prospective studies were included in the analysis (OR, 0.95; 95% CI, 0.66–1.24).
Pooled evidence from epidemiologic studies, mainly case-control studies, suggested a significant inverse association of garlic intake with gastric cancer risk. However, when analyzing data from all epidemiologic studies, the evidence is insufficient to reach a definitive conclusion concerning the association between garlic consumption and H. pylori infection.
4.7. Beta-Carotene and Ascorbic Acid
A total of 852 adult patients with confirmed histologic diagnoses of multifocal nonmetaplastic gastric atrophy (n = 194), intestinal metaplasia (n = 579), or dysplasia (n = 79) were recruited in a randomized, controlled chemoprevention trial conducted in the United States [37]. Patients were assigned to receive anti-H. pylori triple antibiotic therapy and/or dietary supplementation with ascorbic acid or β-carotene [37]. Gastric biopsy specimens were collected at baseline and 6 years for histopathological examination. The results showed that the rates of regression were significantly increased by all three basic interventions. For anti-H. pylori treatment, relative risk was 4.8 (95% CI, 1.6–14.2); for β-carotene treatment, the relative risk was 5.1 (95% CI, 1.7–15.0); and for ascorbic acid treatment, the relative risk was 5.0 (95% CI, 1.7–14.4) in patients with atrophy. Among patients with intestinal metaplasia, relative risks of regressions were 3.1 (95% CI, 1.0–9.3), 3.4 (95% CI, 1.1–9.8), and 3.3 (95% CI, 1.1–9.5) for anti-H. pylori, β-carotene, and ascorbic acid treatment, respectively. However, the regression rates were not increased by combination treatments. Importantly, 74% of the treated cases had negative H. pylori infection and they also had significantly decreased precursor lesions; relative risks for patients with atrophy were 8.7 (95% CI, 2.7–28.2) and for those with intestinal metaplasia were 5.4 (95% CI, 1.7–17.6). Accordingly, this study suggested that in the very high-risk population, anti-H. pylori antibiotic treatment and antioxidant micronutrient dietary supplementation may delay the precancer lesion progression. This approach may be useful to prevent gastric cancer.
At the 12-year follow-up of the aforementioned trial [19], 795 adults with preneoplastic gastric lesions were randomized to receive anti-H. pylori antibiotic treatment and/or antioxidants. At baseline, and at 3, 6, and 12 years, gastric biopsies were collected for histopathological examination. At baseline and 12 years, 97% and 53% of patients were H. pylori positive, respectively. More rapid healing was observed in patients with years free of H. pylori infection and those with less advanced lesions. In other words, regression rate of preneoplastic gastric lesions is equal to the square of time that patients were H. pylori infection free. These findings suggest that patients who have preneoplastic gastric lesions and H. pylori infection should be treated by antibiotic therapy. However, patients treated with antioxidant supplements did not have an additional benefit compared with those treated with antibiotics only [19]. Although an antioxidant effect was observed at 6 years, it disappeared during the 6 years of no supplementation, and no residual effect was observed. This study also showed that acute gastric inflammation disappeared soon after H. pylori treatment, and chronic inflammation responded at a slower pace but was still reduced significantly after 12 years [19]. Accordingly, gastric cancer chemoprevention via eradication of H. pylori infection is a viable option, but the greatest beneficial effects might not be evident in the first 3–6 years of observation [19]. It should be noted that analysis at 6 years showed that there was a significant negative interaction between H. pylori treatment and β-carotene supplementation; some patients who received antibiotic treatment for H. pylori showed improvement, but those who received H. pylori treatment and β-carotene supplementation did worse than those on placebo [37]. This finding may suggest that both interventions likely have a final common pathway—decreasing oxidative damage to the gastric epithelial cell.
5. NATURAL COMPOUNDS FIGHT AGAINST RESISTANT H. PYLORI
Antibiotic treatment combined with a PPI is effective in eradication of H. pylori [11]. However, the occurrence of drug-resistant H. pylori and the adverse effect of antibiotics are significant problems of this therapy [7]. The adverse effects include the disturbance of human gastrointestinal microflora, severe complications such as liver and kidney dysfunction, and rejection of or allergy to antibiotics [7]. Natural products that have been shown to suppress clinical isolates of resistant H. pylori strains are Epigallocatechin-3-Gallate (EGCG) and Epicatechin Gallate (ECG) [38], aqueous and ethanol extracts of propolis or Zingiber officinale [39], sulforaphane [40], and paeonol and benzoic acid identified in Paeonia lactiflora root [41]. Although they were tested in in vitro assays, together with several natural products that have been shown to suppress H. pylori in human clinical trials, it is now important to test these natural products in patients with resistant H. pylori.
6. FUTURE PERSPECTIVES
Many foodstuff such as burdock complex, CGNC, bovine lactoferrin, cranberries, probiotics have exhibited inhibitory activity against the growth of H. pylori in humans. It is encouraging that natural compounds such as EGCG and ECG from tea, the aqueous and ethanol extracts of Propolis or Z. officinale, sulforaphane, and paeonol and benzoic acid identified in P. lactiflora root suppressed clinical isolates of resistant H. pylori strains. The crucial next step is to test these promising natural products for their ability to suppress resistant H. pylori in humans. Although these natural products may reduce the bacterial load of H. pylori, the effects are usually transient and H. pylori is not totally eradicated in the majority of the patients. Considering the feasibility of consuming foodstuffs for controlling H. pylori infection in different countries around the world, it is important to find locally consumed foods that are anti-H. pylori as long term solutions to fight against this world-wide prevalent pathogen.
Footnotes
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
REFERENCES
- [1].Peek RM Jr. Eradication of Helicobacter pylori as a means towards eliminating gastric adenocarcinoma. Gastroenterology 2004;127:344–6. [DOI] [PubMed] [Google Scholar]
- [2].Wang YC. Medicinal plant activity on Helicobacter pylori related diseases. World J Gastroenterol 2014;20:10368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vale FF, Oleastro M. Overview of the phytomedicine approaches against Helicobacter pylori. World J Gastroenterol 2014;20:5594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ghasemian A, Fattahi A, Shokouhi Mostafavi SK, Almarzoqi AH, Memariani M, Ben Braiek O, et al. Herbal medicine as an auspicious therapeutic approach for the eradication of Helicobacter pylori infection: a concise review. J Cell Physiol 2019;234:16847–60. [DOI] [PubMed] [Google Scholar]
- [5].Sharifi-Rad M, Fokou PVT, Sharopov F, Martorell M, Ademiluyi AO, Rajkovic J, et al. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules 2018;23:pii: E1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Salehi B, Sharopov F, Martorell M, Rajkovic J, Ademiluyi AO, Sharifi-Rad M, et al. Phytochemicals in Helicobacter pylori infections: what are we doing now? Int J Mol Sci 2018;19:pii: E2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Takeuchi H, Trang VT, Morimoto N, Nishida Y, Matsumura Y, Sugiura T. Natural products and food components with anti- Helicobacter pylori activities. World J Gastroenterol 2014;20:8971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harsha C, Banik K, Bordoloi D, Kunnumakkara AB. Antiulcer properties of fruits and vegetables: a mechanism based perspective. Food Chem Toxicol 2017;108:104–19. [DOI] [PubMed] [Google Scholar]
- [9].Huang RJ, Choi AY, Truong CD, Yeh MM, Hwang JH. Diagnosis and management of gastric intestinal metaplasia: current status and future directions. Gut Liver 2019;13:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Konturek SJ, Konturek PC, Brzozowski T, Konturek JW, Pawlik WW. From nerves and hormones to bacteria in the stomach; Nobel prize for achievements in gastrology during last century. J Physiol Pharmacol 2005;56:507–30. [PubMed] [Google Scholar]
- [11].Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2002;2:28–37. [DOI] [PubMed] [Google Scholar]
- [12].Gupta N, Maurya S, Verma H, Verma VK. Unraveling the factors and mechanism involved in persistence: host-pathogen interactions in Helicobacter pylori. J Cell Biochem 2019;120:18572–87. [DOI] [PubMed] [Google Scholar]
- [13].Weisburger JH. Dietary risk factors for gastric carcinoma. JAMA 2004;291:1564; author reply 1564. [DOI] [PubMed] [Google Scholar]
- [14].Ito M, Tanaka S, Kamada T, Haruma K, Chayama K. Causal role of Helicobacter pylori infection and eradication therapy in gastric carcinogenesis. World J Gastroenterol 2006;12:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meyer JM, Silliman NP, Wang W, Siepman NY, Sugg JE, Morris D, et al. Risk factors for Helicobacter pylori resistance in the United States: the Surveillance of H. pylori Antimicrobial Resistance Partnership (SHARP) study, 1993–1999. Ann Intern Med 2002;136:13–24. [DOI] [PubMed] [Google Scholar]
- [16].Vakil N Are there geographical and regional differences in Helicobacter pylori eradication? Can J Gastroenterol 2003;17:30B–2B. [DOI] [PubMed] [Google Scholar]
- [17].Ito M, Takata S, Tatsugami M, Wada Y, Imagawa S, Matsumoto Y, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol 2009;44:365–71. [DOI] [PubMed] [Google Scholar]
- [18].Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–94. [DOI] [PubMed] [Google Scholar]
- [19].Mera R, Fontham ETH, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54:1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Øverby A, Yamagata Murayama S, Matsui H, Nakamura M. In the aftermath of Helicobacter pylori: other helicobacters rising up to become the next gastric epidemic? Digestion 2016;93:260–5. [DOI] [PubMed] [Google Scholar]
- [21].Blanchard TG, Czinn SJ. Current status and prospects for a Helicobacter pylori vaccine. Gastroenterol Clin North Am 2015;44:677–89. [DOI] [PubMed] [Google Scholar]
- [22].Xia HX, Talley NJ, Keane CT, O’Morain CA. Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Dig Dis Sci 1997;42:1821–34. [DOI] [PubMed] [Google Scholar]
- [23].Yen CH, Chiu HF, Huang SY, Lu YY, Han YC, Shen YC, et al. Beneficial effect of burdock complex on asymptomatic Helicobacter pylori-infected subjects: a randomized, double-blind placebo- controlled clinical trial. Helicobacter 2018;23:e12469. [DOI] [PubMed] [Google Scholar]
- [24].Bespalov V, Sherbakov A, Novik V, Kalinovsky V, Shamsi K, Soultanov V. Conifer green needle complex in patients with precancerous gastric lesions: an observational pilot study. Evid Based Complement Alternat Med 2016;2016:3848409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Okuda M, Nakazawa T, Yamauchi K, Miyashiro E, Koizumi R, Booka M, et al. Bovine lactoferrin is effective to suppress Helicobacter pylori colonization in the human stomach: a randomized, double-blind, placebo-controlled study. J Infect Chemother 2005;11:265–9. [DOI] [PubMed] [Google Scholar]
- [26].Di Mario F, Aragona G, Bò ND, Ingegnoli A, Cavestro GM, Moussa AM, et al. Use of lactoferrin for Helicobacter pylori eradication. Preliminary results. J Clin Gastroenterol 2003;36:396–8. [DOI] [PubMed] [Google Scholar]
- [27].Meyer JM. Use of lactoferrin for Helicobacter pylori eradication. J Clin Gastroenterol 2003;36:384–5. [DOI] [PubMed] [Google Scholar]
- [28].Di Mario F, Aragona G, Dal Bó N, Cavallaro L, Marcon V, Olivieri P, et al. Bovine lactoferrin for Helicobacter pylori eradication: an open, randomized, multicentre study. Aliment Pharmacol Ther 2006;23:1235–40. [DOI] [PubMed] [Google Scholar]
- [29].Howell AB. Potential of cranberry for suppressing Helicobacter pylori: a risk factor for gastric cancer. J Berry Res 2019;1:1–9. [Google Scholar]
- [30].Gotteland M, Andrews M, Toledo M, Muñoz L, Caceres P, Anziani A, et al. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition 2008;24:421–6. [DOI] [PubMed] [Google Scholar]
- [31].Zhang L, Ma J, Pan K, Go VL, Chen J, You WC. Efficacy of cranberry juice on Helicobacter pylori infection: a double-blind, randomized placebo-controlled trial. Helicobacter 2005;10: 139–45. [DOI] [PubMed] [Google Scholar]
- [32].Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One 2014;9:e111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 2006;98:974–83. [DOI] [PubMed] [Google Scholar]
- [34].Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 1993;85:1483–92. [DOI] [PubMed] [Google Scholar]
- [35].Baron JA. (Nutritional) chemoprevention of cancer: what’s up? J Natl Cancer Inst 2006;98:945–6. [DOI] [PubMed] [Google Scholar]
- [36].Li Z, Ying X, Shan F, Ji J. The association of garlic with Helicobacter pylori infection and gastric cancer risk: a systematic review and meta-analysis. Helicobacter 2018;23:e12532. [DOI] [PubMed] [Google Scholar]
- [37].Correa P, Fontham ETH, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881–8. [DOI] [PubMed] [Google Scholar]
- [38].Yanagawa Y, Yamamoto Y, Hara Y, Shimamura T. A combination effect of epigallocatechin gallate, a major compound of green tea catechins, with antibiotics on Helicobacter pylori growth in vitro. Curr Microbiol 2003;47:244–9. [DOI] [PubMed] [Google Scholar]
- [39].Nostro A, Cellini L, Di Bartolomeo S, Cannatelli MA, Di Campli E, Procopio F, et al. Effects of combining extracts (from propolis or Zingiber officinale) with clarithromycin on Helicobacter pylori. Phytother Res 2006;20:187–90. [DOI] [PubMed] [Google Scholar]
- [40].Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA 2002;99:7610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].My Ngan LT, Moon JK, Shibamoto T, Ahn YJ. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J Agric Food Chem 2012;60:9062–73. [DOI] [PubMed] [Google Scholar]