Abstract
Background
The EANO ESMO guidelines have proposed a classification of leptomeningeal metastases (LM) from solid cancers based on clinical, magnetic resonance imaging (MRI), and cerebrospinal fluid (CSF) cytology presentation. MRI patterns are classified as linear, nodular, both, or neither. Type I LM is defined by positive CSF cytology (confirmed LM) whereas type II LM is defined by typical clinical and MRI signs (probable or possible LM). Here we explored the clinical utility of these LM subtypes.
Patients and methods
We retrospectively assembled data from 254 patients with newly diagnosed LM from solid tumors. Survival curves were derived using the Kaplan–Meier method and compared by Log-rank test.
Results
Median age at LM diagnosis was 56 years. Typical clinical LM features were noted in 225 patients (89%); 13 patients (5%) were clinically asymptomatic. Tumor cells in the CSF were observed in 186 patients (73%) whereas the CSF was equivocal in 24 patients (9.5%) and negative in 44 patients (17.5%). Patients with confirmed LM had inferior outcome compared with patients with probable or possible LM (P = 0.006). Type I patients had inferior outcome than type II patients (P = 0.002). Nodular disease on MRI was a negative prognostic factor in type II LM (P = 0.014), but not in type I LM. On multivariate analysis, administration of either intrathecal pharmacotherapy (P = 0.012) or systemic pharmacotherapy (P = 0.0003) was associated with improved outcome in type I LM, but not in type II LM.
Conclusion
The EANO ESMO LM subtypes are highly prognostic and should be considered for stratification and overall design of clinical trials.
Keywords: cerebrospinal, fluid, intrathecal, meningitis, neoplastic
Key Points.
1. The EANO ESMO subtypes of leptomeningeal metastasis (LM) are highly prognostic.
2. Positive cytology in the cerebrospinal fluid is a negative prognostic factor.
3. Type I LM patients may benefit from intrathecal or systemic pharmacotherapy.
Importance of the Study.
The recently proposed EANO ESMO LM subtypes of leptomeningeal metastasis (LM) from solid tumors are highly prognostic. Type I LM, with positive cytology in the cerebrospinal fluid (CSF), has a poorer outcome than type II LM, without positive CSF cytology. Nodular disease is a negative prognostic factor in patients with negative CSF cytology. Administration of intrathecal or systemic pharmacotherapy is associated with better survival in Type I LM patients, but not or less so in Type II LM patients. EANO ESMO LM subtypes should be considered for stratification and overall design of clinical trials.
The lifetime risk of patients with metastatic solid tumors to develop leptomeningeal metastasis (LM) approaches 10%.1,2 The prognosis remains poor with an estimated median survival of 2–6 months.3–15 Only few randomized clinical trials in patients with LM have been completed16–21 and response assessments and endpoints in such trials have remained controversial.22 Moreover, most of these trials have been published long ago, compared various strategies of intrathecal pharmacotherapy and failed to demonstrate the superiority of one specific regimen in a tumor-specific manner.
The first European Association of Neuro-Oncology (EANO) and European Society for Medical Oncology (ESMO) Guidelines on LM proposed a novel classification of LM to guide therapeutic decision making according to clinical, neuroimaging, and CSF findings.23 The aim of the guidelines was to standardize diagnostic criteria of LM and to derive treatment recommendations based on the presentation of LM. However, the recommendations were based on expert consensus rather than high-level evidence because of a lack of results from controlled clinical trials.
Although the diagnosis of LM is based on clinical, MRI, and CSF findings,24 the identification of malignant cells in the CSF remains the gold standard for diagnosis. However, the sensitivity of CSF analysis has been estimated in the range of 34–90% in recent large cohorts of LM patients.3–12,25 Thus, clinical and MRI findings have also to be taken into account for the diagnosis of LM and may allow a diagnosis of probable LM in the absence of tumor cell detection in the CSF.23
The EANO ESMO guidelines sought to define standards for the clinical, imaging and CSF analysis in case of suspected LM. Typical clinical signs include headache, nausea and vomiting, mental changes, gait difficulties, cranial nerve palsies, for example, with diplopia or visual disturbance (cranial nerves II, III, IV, VI) and hearing loss (cranial nerve VIII), radicular signs including weakness, voiding and cauda equina problems, and focal or radiating (radicular) neck and back pain. Characteristic MRI findings include sulcal and folial enhancement or obliteration, linear ependymal enhancement, cranial nerve root enhancement and leptomeningeal enhancing nodules, notably of the cauda equina. The EANO ESMO recommendations propose to classify LM by neuroimaging findings based on MRI without and with contrast enhancement into: linear leptomeningeal disease (type A), nodular leptomeningeal disease (type B), both, requiring an estimated minimum of 20% of each pattern to the disease burden (type C), or neither nor, for example, no neuroimaging evidence of LM except possibly hydrocephalus (type D). CSF analysis shall be reported as positive, defined as the presence of malignant cells in the CSF; equivocal, corresponding to the detection of “suspicious” or “atypical” cells in the CSF; or negative, defined as the absence of malignant or potentially malignant (“equivocal”) cells in the CSF. According to the EANO ESMO criteria, the diagnosis of LM can be verified (or not) only cytologically or histologically: yes (type I) or equivocal/no (type II). Based on the disease characteristics, the diagnosis of LM is considered confirmed (type I), probable (clinical plus neuroradiological evidence) or possible (clinical or neuroradiological evidence only) (Supplementary Table 1). Here we explored the potential clinical utility of these newly proposed EANO ESMO LM subtypes to guide clinical decision making and to estimate course and outcome.
Patients and Methods
Patients
The LM patient cohort was assembled by the University Hospital Zurich, the University Hospital Lille, the University Hospital Bonn, the University Hospital of Turin, the Netherlands Cancer Institute Amsterdam, the Medical University of Vienna, and the Erasmus MC Cancer Institute, University Medical Center Rotterdam. Individual patient data were collected at the respective sites in an anonymized Excel file that captured data on cancer history, diagnosis of LM, response to treatment of LM per local assessment, and outcome; moreover, raters were instructed to apply the EANO ESMO classification to document clinical, MRI and CSF cytology findings (Supplementary Table 2). Patients had histologically confirmed solid cancer except primary brain tumors and a diagnosis of LM according to the treating physicians, a CSF analysis report at LM diagnosis available, contrast-enhanced cerebral (ideally cerebrospinal) MRI at LM diagnosis available, and had died or had a follow-up of one year or more. LM-related progression-free survival (LM-PFS) was defined as the time interval from diagnosis of LM to date of LM progression or date of death from any cause. Global PFS was defined as the time interval from diagnosis of LM to date of progression at any site or date of death from any cause. Overall survival was defined as the time interval from LM diagnosis to date of death from any cause. Observations were censored at the last follow-up date for patients alive at last follow-up.
Study Design
The main objective of this retrospective multicenter study was to evaluate the applicability, clinical correlations, tumor-specific disease characteristics, and prognostic value of the EANO ESMO LM classification.23
Ethics Statement
The sponsor of the study was the University Hospital Zurich. The Cantonal Ethics Committee of the Canton of Zurich approved the project (2018-00192). Appropriate ethics approvals as required were obtained at all participating centers.
Statistical Analyses
Descriptive analyses were performed to present the data in tables. Survival curves were generated using the Kaplan–Meier method. Comparisons of subgroups were performed with the log-rank test. Hazard ratios (HRs) and adjusted HR were computed using the Cox proportional hazard model. Prognostic factor analysis included sex, age at LM diagnosis and age with a cut-off at 60 years, cancer type, LM level of evidence, administration of any systemic treatment, and administration of any intrathecal treatment. Karnofsky performance status (KPS) was omitted because of its largely retrospective assessment. Statistical analyzes were performed using the SAS V9.4 software (Cary, NC).
Results
Patient Characteristics
We assembled data of 254 LM patients diagnosed between 1996 and 2019 at seven different sites, 245 patients were treated in 2005 or later. Hundred-sixty six patients (65%) were female, median age at LM diagnosis was 56 years (interquartile range, IQR: 48–66). Breast cancer (41%), lung cancer (26%), and melanoma (20%) were the most common primary tumors. The median KPS at LM diagnosis was estimated at 70 (IQR: 60–80). The median number of lines of systemic treatment prior to the diagnosis of LM was one (IQR: 1–3). Hundred-two of 254 patients (40%) had a history of parenchymal brain metastases prior to the diagnosis of LM; 77 of 102 patients (75%) had received treatment for parenchymal brain metastases prior to LM diagnosis, including surgery in 30 patients (29%), stereotactic radiotherapy in 33 patients (32%), whole brain radiotherapy in 32 patients (31%) and systemic pharmacotherapy in 47 patients (46%); 92 of 254 patients (36%) had progressing or new brain parenchymal metastases at the time of LM diagnosis (Supplementary Table 3).
Classification According to EANO ESMO Guidelines
At LM diagnosis, 225 of 254 patients (89%) presented with typical clinical symptoms and signs whereas 13 patients (5%) were asymptomatic. Hundred-seventeen patients (46%) had an EANO ESMO type A MRI imaging subtype with linear meningeal disease only, 32 patients (12%) had type B with nodular disease only, 55 patients (21%) had a type C imaging subtype combining linear and nodular meningeal disease. MRI was interpreted as normal or as showing hydrocephalus only in 50 patients (20%) (type D). Tumor cells were identified in the CSF in 186 patients (73%), the CSF was equivocal in 24 patients (9%); it was negative in 44 patients (17%). A leptomeningeal biopsy was positive in 4 of 5 patients. Distribution among the eight EANO ESMO classes and disease characteristics by subtype are compiled in Supplementary Table 3.
Treatment of LM and Outcome
In response to LM diagnosis, 104 patients (41%) received systemic pharmacotherapy, 117 patients (46%) received intrathecal therapy and 104 patients (41%) received radiotherapy. Forty-three patients (17%) were not treated. First-line treatment of LM was radiotherapy alone for 42 patients (16%), intrathecal treatment alone for 41 patients (16%), a combination of systemic and intrathecal pharmacotherapy for 38 patients (15%), and a combination of systemic treatment and radiotherapy for 29 patients (11%); 171 patients (70%) were on steroids at the time of LM treatment initiation (Table 1).
Table 1.
Treatment for LM Diagnosis and Outcome
| MRI Subtype | All | Type I | Type II | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | A | B | C | D | all I | A | B | C | D | all | |
| n = 254 | n = 88 | n = 19 | n = 39 | n = 43 | n = 189 | n = 29 | n = 13 | n = 16 | n = 7 | n = 65 | |
| 100% | 35% | 7% | 15% | 17% | 74% | 11% | 5% | 6% | 3% | 26% | |
| Systemic treatment | |||||||||||
| Any: n (%) | |||||||||||
| Yes | 104 (41) | 38 (43) | 6 (32) | 18 (46) | 14 (33) | 76 (40) | 12 (41) | 6 (46) | 9 (56) | 1 (14) | 28 (43) |
| No | 145 (57) | 46 (52) | 13 (69) | 21 (54) | 29 (67) | 109 (58) | 17 (59) | 6 (46) | 7 (44) | 6 (86) | 36 (55) |
| unknown | 5 (2) | 4 (5) | 0 | 0 | 0 | 4 (2) | 0 | 1 (8) | 0 | 0 | 1 (2) |
| Intrathecal treatment | |||||||||||
| Intrathecal therapy: n (%) | |||||||||||
| Yes | 117 (46) | 44 (50) | 4 (21) | 23 (59) | 21 (49) | 92 (49) | 13 (45) | 3 (23) | 6 (37.5) | 3 (43) | 25 (38) |
| No | 137 (54) | 44 (50) | 15 (79) | 16 (41) | 22 (51) | 97 (51) | 16 (55) | 10 (77) | 10 (62.5) | 4 (57) | 40 (62) |
| Agent: n (%) | |||||||||||
| Liposomal cytarabine | 71 (60) | 25 (56) | 2 (50) | 17 (71) | 12 (57) | 56 (30) | 8 (62) | 2 (67) | 3 (50) | 2 (67) | 15 (23) |
| Cytarabine | 12 (10) | 8 (18) | 0 | 1 (5) | 1 (5) | 10 (5) | 1 (8) | 1 (33) | 0 | 0 | 2 (3) |
| Methotrexate | 25 (21) | 10 (22) | 1 (25) | 3 (12.5) | 7 (33) | 21 (11) | 2 (15) | 0 | 1 (17) | 1 (33) | 4 (6) |
| Thiotepa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trastuzumab | 6 (5) | 1 (2) | 0 | 3 (12.5) | 0 | 4 (2) | 0 | 0 | 2 (33) | 0 | 2 (3) |
| Liposomal cytarabine + MTX | 1 (1) | 0 | 1 (25) | 0 | 0 | 1 (<0.5) | 0 | 0 | 0 | 0 | 0 |
| Cytarabine + MTX | 4 (3) | 1 (2) | 0 | 0 | 1 (5) | 2 (1) | 2 (15) | 0 | 0 | 0 | 2 (3) |
| Route of administration: n (%) | |||||||||||
| Lumbar only | 92 (79) | 36 (82) | 4 (100) | 17 (74) | 17 (81) | 74 (39) | 8 (62) | 2 (67) | 5 (83) | 3 (100) | 18 (28) |
| Ventricular only | 14 (12) | 5 (11) | 0 | 2 (9) | 4 (19) | 11 (6) | 2 (16) | 1 (33) | 0 | 0 | 3 (5) |
| Both | 10 (9) | 3 (7) | 0 | 4 (17) | 0 | 7 (4) | 2 (15) | 0 | 1 (17) | 0 | 3 (5) |
| Unknown | 1 (1) | 0 | 0 | 0 | 0 | 0 | 1 (8) | 0 | 0 | 0 | 1 (2) |
| Radiotherapy | |||||||||||
| Radiotherapy for LM: n (%) | |||||||||||
| Local radiotherapy, including radiosurgery | 12 (5) | 3 (3) | 1 (5) | 3 (8) | 0 | 7 (4) | 3 (10) | 1 (8) | 1 (6) | 0 | 5 (8) |
| Whole brain radiotherapy | 91 (36) | 27 (31) | 5 (26) | 20 (51) | 14 (33) | 66 (35) | 11 (38) | 5 (38) | 6 (37.5) | 3 (43) | 25 (38) |
| Whole spine RT | 1 (0.5) | 1 (1) | 0 | 0 | 9 | 1 (<0.5) | 0 | 0 | 0 | 0 | 0 |
| None | 149 (59) | 56 (64) | 13 (68) | 16 (41) | 29 (67) | 114 (60) | 15 (52) | 7 (54) | 9 (56) | 4 (57) | 35 (54) |
| Unknown | 1 (0.5) | 1 (1) | 0 | 0 | 0 | 1 (<0.5) | 0 | 0 | 0 | 0 | 0 |
| Radiotherapy for concomitant progressive brain metastases: n (%) | |||||||||||
| Local radiotherapy, including radiosurgery | 8 (3) | 0 | 1 (5) | 1 (3) | 2 (5) | 4 (2) | 2 (7) | 2 (15) | 0 | 0 | 4 (6) |
| Whole brain radiotherapy | 47 (18.5) | 10 (11) | 4 (1) | 14 (36) | 3 (7) | 31 (16) | 7 (24) | 4 (31) | 4 (25) | 1 (14) | 16 (25) |
| None | 183 (72) | 72 (82) | 13 (68) | 23 (59) | 34 (79) | 142 (75) | 19 (66) | 5 (38) | 12 (75) | 5 (71) | 41 (63) |
| Unknown | 16 (6.5) | 6 (7) | 1 (5) | 1 (3) | 4 (9) | 12 (6) | 1 (3) | 2 (15) | 0 | 1 (14) | 4 (6) |
| Radiotherapy for LM or BM after LM diagnosis: n (%) | |||||||||||
| Local radiotherapy, including radiosurgery | 14 (5.5) | 3 (3) | 1 (5) | 3 (8) | 1 (2) | 8 (4) | 3 (10) | 2 (15) | 1 (6) | 0 | 6 (9) |
| Whole brain radiotherapy | 91 (36) | 26 (30) | 5 (26) | 21 (54) | 13 (30) | 65 (34) | 11 (38) | 5 (38) | 6 (37.5) | 4 (57) | 6 (40) |
| Whole spine RT | 1 (0.5) | 1 (1) | 0 | 0 | 0 | 1 (<0.5) | 0 | 0 | 0 | 0 | 0 |
| WBRT + SRT | 3 (1) | 0 | 0 | 0 | 1 (2) | 1 (<0.5) | 1 (3) | 1 (8) | 0 | 0 | 2 (3) |
| None | 129 (51) | 52 (59) | 12 (63) | 14 (36) | 24 (56) | 102 (54) | 13 (45) | 3 (23) | 9 (56) | 2 (29) | 27 (42) |
| Unknown | 16 (6) | 6 (7) | 1 (5) | 1 (3) | 4 (9) | 12 (6) | 1 (3) | 2 (15) | 0 | 1 (14) | 4 (6) |
| Combination of treatment | |||||||||||
| Modalities: n (%) | |||||||||||
| Systemic alone | 23 (9) | 8 (9) | 4 (21) | 2 (5) | 4 (9) | 18 (9) | 1 (3) | 3 (23) | 1 (6) | 0 | 5 (8) |
| Intrathecal alone | 41 (16) | 13 (15) | 2 (10.5) | 7 (18) | 12 (28) | 34 (18) | 5 (17) | 1 (8) | 0 | 1 (14) | 7 (11) |
| Radiotherapy alone | 42 (16) | 11 (12.5) | 4 (21) | 5 (26) | 9 (21) | 29 (15) | 5 (17) | 3 (23) | 3 (19) | 2 (29) | 13 (20) |
| Systemic + intrathecal | 38 (15) | 16 (18) | 1 (5) | 4 (10) | 6 (14) | 27 (14) | 5 (17) | 0 | 5 (31) | 1 (14) | 11 (17) |
| Systemic + RT | 29 (11) | 9 (10) | 1 (5) | 6 (15) | 2 (5) | 18 (9) | 6 (21) | 2 (15) | 3 (19) | 0 | 11 (17) |
| Intrathecal + RT | 19 (7) | 6 (7) | 1 (5) | 6 (15) | 1 (2) | 14 (7) | 3 (10) | 0 | 1 (6) | 1 (14) | 5 (8) |
| Systemic + intrathecal + RT | 14 (5.5) | 5 (6) | 0 | 6 (15) | 2 (5) | 13 (7) | 0 | 1 (8) | 0 | 0 | 1 (1.5) |
| No treatment | 43 (17) | 16 (18) | 6 (31.5) | 3 (8) | 7 (18) | 32 (17) | 4 (14) | 2 (15) | 3 (19) | 2 (29) | 11 (17) |
| Unknown | 5 (2) | 4 (4) | 0 | 0 | 0 | 4 (2) | 0 | 1 (8) | 0 | 0 | 1 (1.5) |
| Outcome from diagnosis of LM | |||||||||||
| Type of any first progression | |||||||||||
| LM | 134 (53) | 61 (69) | 9 (47) | 23 (74) | 21 (49) | 114 (60) | 6 (21) | 6 (46) | 5 (31) | 3 (43) | 20 (31) |
| BM | 6 (2) | 1 (1) | 0 | 0 | 0 | 1 (<0.5) | 1 (3) | 1 (7) | 2 (12.5) | 1 (14) | 5 (8) |
| Extra-CNS | 24 (9) | 5 (6) | 2 (10.5) | 2 (5) | 6 (14) | 15 (8) | 5 (17) | 1 (7) | 2 (12.5) | 1 (14) | 9 (14) |
| LM + BM | 13 (5) | 1 (1) | 2 (10.5) | 3 (8) | 2 (4) | 8 (4) | 4 (14) | 0 | 1 (6) | 0 | 5 (8) |
| LM + extra-CNS | 17 (7) | 6 | 0 | 5 (13) | 2 (4) | 13 (7) | 2 (7) | 2 (15) | 0 | 0 | 4 (6) |
| BM + extra-CNS | 1 (<0.5) | 0 | 0 | 0 | 1 (<0.5) | 1 (<0.5) | 0 | 0 | 0 | 0 | 0 |
| LM + BM + extra-CNS | 2 (<0.5) | 0 | 1 (0.5) | 0 | 0 | 1 (<0.5) | 0 | 0 | 1 (6) | 0 | 1 (1) |
| None | 17 (7) | 3 (3) | 3 (16) | 2 (5) | 4 (9) | 12 (6) | 4 (14) | 0 | 1 (6) | 0 | 5 (8) |
| Unknown | 40 (16) | 11 (12) | 2 (10.5) | 4 (10) | 7 (16) | 24 (13) | 7 (24) | 3 (23) | 4 (25) | 2 (29) | 16 (25) |
| LM progression: n (%) | |||||||||||
| No | 30 (12) | 5 (6) | 4 (21) | 2 (5) | 10 (23) | 21 (11) | 6 (21) | 0 | 2 (12.5) | 1 (14) | 9 (14) |
| Yes | 186 (73) | 74 (84) | 13 (68) | 32 (82) | 27 (63) | 146 (77) | 16 (55) | 9 (69) | 11 (69) | 4 (57) | 40 (62) |
| Unknown | 38 (15) | 9 (10) | 2 (11) | 5 (13) | 6 (14) | 22 (12) | 7 (24) | 4 (31) | 3 (19) | 2 (29) | 16 (25) |
| LM PFS, in months: median (IQR) | 1.75 (0.7–3.7) (n = 156) | 1.7 (0.6–3.7) (n = 60) | 1.2 (0.2–1.6) (n = 12) | 2.0 (0.7–3.0) (n = 29) | 1.0 (0.5–2.3) (n = 23) | 1.5 (0.6–3.0) (n = 124) | 5.4 (2.4–9.5) (n = 14) | 2.8 (2.6–5.1) (n = 5) | 1.4 (1.0–4.2) (n = 10) | 3.2 (1.8–5.5) (n = 3) | 3.0 (1.5–7.8) (n = 32) |
| BM progression: n (%) | |||||||||||
| No | 96 (38) | 33 (37.5) | 11 (58) | 16 (41) | 17 (40) | 77 (41) | 9 (31) | 3 (23) | 4 (25) | 3 (43) | 19 (29) |
| Yes | 32 (13) | 3 (3) | 3 (16) | 7 (18) | 4 (9) | 17 9) | 9 (8) | 1 (8) | 4 (25) | 1 (14) | 15 (23) |
| Unknown | 107 (42) | 52 (59) | 5 (26) | 16 (41) | 2 (51) | 75 (40) | 12 (41) | 9 (69) | 8 (50) | 3 (43) | 32 (49) |
| Median BM PFS, in months: median (IQR) | 3.4 (1.8–8.5) (n = 26) | 2.1 (1.3–5.0) (n = 3) | 3.7 (3.7-3.7) (n = 1) | 10.5 (4–13.7) (n = 6) | 4.0 (2.7–6.1) (n = 3) | 4.0 (2.1–8.5) (n = 13) | 5.5 (1.9–11.7) (n = 8) | 2.8 (2.8-2.8) (n = 1) | 0.9 (0.7–1.2) (n = 3) | 3.1 (3.1-3.1) (n = 1) | 2.8 (1.7–7.4) (n = 13) |
| Extra CNS progression: n (%) | |||||||||||
| No | 77 (30) | 24 (27) | 10 (53) | 12 (31) | 11 (26) | 57 (30) | 9 (31) | 4 (31) | 6 (37.5) | 3 (43) | 22 (34) |
| Yes | 56 (22) | 17 (19) | 5 (26) | 8 (20) | 11 (26) | 41 (22) | 9 (31) | 3 (23) | 2 (12.5) | 1 (14) | 15 (23) |
| Unknown | 119 (47) | 47 (53) | 4 (21) | 19 (49) | 21 (49) | 91 (48) | 11 (38) | 6 (46) | 8 (50) | 3 (43) | 28 (43) |
| Median extra-CNS PFS, in months: median (IQR) | 3.3 (2.2–5.0) (n = 48) | 2.6 (1.8–3.6) (n = 13) | 3.3 (2.4–3.8) (n = 4) | 3.1 (2.7–3.7) (n = 8) | 2.5 (1.7–4.0) (n = 10) | 2.8 (1.9–3.8) (n = 35) | 8.0 (5.6–11.0) (n = 8) | 3.9 (3.3–4.5) (n = 2) | 2.4 (1.8–3.0) (n = 2) | 1.6 (1.6-1.6) (n = 1) | 5.1 (3.8–9.7) (n = 13) |
| Overall progression: n (%) | |||||||||||
| No | 12 (5) | 3 (3) | 2 (11) | 1 (3) | 4 (9) | 10 (5) | 1 (3) | 0 | 1 (6) | 0 | 2 (3) |
| Yes | 206 (81) | 76 (86) | 16 (84) | 33 (85) | 34 (79) | 159 (84) | 20 (69) | 10 (77) | 12 (75) | 5 (71) | 47 (72) |
| Unknown | 36 (14) | 9 (10) | 1 (5) | 5 (13) | 5 (12) | 20 (11) | 8 (28) | 3 (23) | 3 (19) | 2 (29) | 16 (25) |
| Median overall PFS, in months: median (IQR) | 1.7 (0.7–3.5) (n = 182) | 1.5 (0.5–3.1) (n = 62) | 1.4 (0.5–2.2) (n = 15) | 1.95 (0.8–3.3) (n = 34) | 1.4 (0.7–2.3) (n = 30) | 1.5 (0.6–2.8) (n = 141) | 4.8 (1.7–8) (n = 20) | 2.7 (2.6–5.1) (n = 6) | 1.22 (0.8–2.6) (n = 11) | 2.3 (1.3–3.1) (n = 4) | 2.6 (1.3–5.9) (n = 41) |
| Death: n (%) | |||||||||||
| Alive | 8 (3) | 2 (2) | 1 (5) | 0 | 1 (2) | 4 (2) | 2 (7) | 0 | 1 (6) | 1 (14) | 4 (6) |
| Dead | 240 (95) | 84 (95) | 18 (95) | 39 (100) | 40 (93) | 181 (96) | 25 (86) | 13 (100) | 15 (94) | 6 (86) | 59 (91) |
| Lost of follow-up | 6 (2) | 2 (2) | 0 | 0 | 2 (5) | 4 (2) | 2 (7) | 0 | 0 | 0 | 2 (3) |
| Overall survival in months: median (IQR) | 2.8 (1.0–6.0) (n = 254) | 2.2 (0.8–4.7) (n = 88) | 2.2 (0.8–4.9) (n = 19) | 3.3 (1.1–7) (n = 39) | 2 (0.6–3.8) (n = 43) | 2.3 (0.82–4.9) (n = 189) | 6.5 (1.9-15) (n = 29) | 3.4 (2.1–4.7) (n = 13) | 2.1 (1.5–6.5) (n = 16) | 3.5 (2.7–5.1) (n = 7) | 3.5 (1.6–9.4) (n = 65) |
| Overall survival breast cancer patients: median (IQR) | 3.4 (1.3–7.5) (n = 104) | 2.2 (0.8–5.5) (n = 29) | 3.1 (2.1–6.4) (n = 7) | 5.8 (3.5–9.8) (n = 12) | 2.1 (1.1–3.9) (n = 19) | 22.4 (1.0–6.6) (n = 68) | 19.7 (8.3–28.6) (n = 10) | 2.8 (1.8–4.0) (n = 7) | 1.5 (1.5–1.6) (n = 2) | 3.5 (3.3–4.3) (n = 3) | 4.5 (2.5–10.5) (n = 36) |
| Overall survival lung cancer patients: median (IQR) | 2.2 (0.9–4.6) (n = 65) | 2.9 (0.9–4.7) (n = 32) | 2 (0.4–2.2) (n = 5) | 1.1 (1.1–3.3) (n = 5) | 1.9 (0.4–4.7) (n = 11) | 2 (0.8–4.4) (n = 55) | 8.4 (5–11.7) (n = 2) | 4.3 (3.9–4.9) (n = 4) | 9.2 (6.5–10.2) (n = 6) | n.a. | 2.9 (1.7–4.5) (n = 10) |
| Overall survival melanoma patients: median (IQR) | 1.7 (0.9–5.2) (n = 51) | 3.1 (0.8–3.9) (n = 15) | 0.6 (0.6–7.2) (n = 6) | 3.4 (1.3–10.5) (n = 11) | 1.5 (0.9–2.2) (n = 6) | 1.5 (0.8–4.3) (n = 39) | 2.9 (1.9–5.7) (n = 6) | 13.3 (7.3–19.2) (n = 2) | 1.5 (1.4–1.6) (n = 3) | 29.7 (29.7-29.7) (n = 1) | 2.2 (1.5–8.1) (n = 12) |
| Overall survival other primaries: median (IQR) | 2.0 (1.0–5.3) (n = 34) | 1.0 (0.5–4.6) (n = 12) | 2.7 (2.2–3.3) (n = 2) | 3.0 (1.4–9.2) (n = 6) | 2.3 (0.7–3.2) (n = 7) | 2.3 (0.6–4.1) (n = 27) | 5.7 (3.6–8.3) (n = 3) | n.a. | 1.3 (1.3-1.3) (n = 1) | 29 (29-29) (n = 1) | 1.5 (1.2–8.3) (n = 7) |
| Cause of death: n (%) | |||||||||||
| Neurological | 131 (55) | 51 (58) | 10 (53) | 23 (59) | 22 (55) | 106 (56) | 8 (28) | 6 (46) | 7 (44) | 4 (57) | 25 (38) |
| Extra-CNS | 17 (7) | 4 (5) | 3 (16) | 1 (3) | 2 (5) | 10 (5) | 5 (17) | 1 (8) | 1 (6) | 0 | 7 (11) |
| CNS and extra-CNS | 2 (1) | 0 | 0 | 1 (3) | 1 (2) | 2 (1) | 0 | 0 | 0 | 0 | 0 |
| Complication of treatment | 4 (2) | 2 (2) | 1 (5) | 0 | 0 | 3 (2) | 1 (3) | 0 | 0 | 0 | 1 (2) |
| Other | 10 (4) | 5 (6) | 0 | 1 (3) | 2 (5) | 8 (4) | 1 (3) | 1 (8) | 0 | 0 | 2 (3) |
| Unknown | 75 (31) | 26 (30) | 5 (26) | 13 (33) | 16 (37) | 60 (32) | 14 (48) | 5 (38) | 8 (50) | 3 (43) | 30 (46) |
Abbreviations: BM, brain metastases; CNS, central nervous system; CSF, cerebrospinal fluid; IQR, interquartile ranges; LM, leptomeningeal metastases; MTX, methotrexate; n, number; n.a., not applicable; PFS, progression free survival; RT, radiotherapy; SRT, stereotactic radiotherapy; WBRT, whole brain radiotherapy.
Clinical response was documented in 41 patients (16%), a stable status for a minimum of 4 weeks in another 64 patients (25%), and early progression in 99 patients (39%); 50 patients (20%) were not evaluable and their median survival was 1.4 months, neurological death was documented for 19 of these patients.
The best MRI response was response in 19 patients (7%), stability in 30 patients (12%) and early progression in 41 patients (16%). The imaging response assessment was not evaluable for 164 patients (64%) who had a median overall survival of 1.6 months, neurological death was documented for 88 of these patients.
The best CSF response was response in 19 patients (7%), stability in 54 patients (21%) and early progression in 6 patients (2%). CSF response assessment was not evaluable for 171 patients (67%). Their median overall survival was 1.9 months; neurological death was documented for 93 of these patients (54%) (Supplementary Table 4).
First progression was LM progression alone in 134 patients (53%) and progression of extra-CNS disease alone in 24 patients (9%) (Table 1). LM progression was documented in 186 patients (73%) overall. The median LM PFS was 1.75 months (IQR: 0.7–3.7). Parenchymal brain metastasis progression was observed in 32 patients (13%) with a median brain metastasis PFS after LM diagnosis of 3.4 months. Progression of extra-CNS disease was noted in 56 patients (22%), with a median extra-CNS PFS of 3.3 months (IQR: 2.2–5.0). The median global PFS after LM diagnosis was 1.7 months (IQR: 0.9–5.2). At the time of the analysis, 240 patients (95%) had died. The median overall survival was 2.8 months (IQR: 1.0–6.0). Cause of death was mainly neurological in 131 patients (55%).
Univariate prognostic factor analysis showed that female gender (P = 0.003) and lower age at LM diagnosis (P = 0.022), lower diagnostic level of evidence for LM diagnosis (confirmed, probable, possible, no evidence) (P = 0.005) and administration of any systemic treatment were associated with longer overall survival (P = 0.001). A trend was also noted for the cancer subtype (P = 0.047), with a longer survival for breast cancer patients than for patients with other cancer. In contrast, there was no association with outcome for administration of any intrathecal treatment (P = 0.226).
To specifically explore interactions between sex, age and cancer type, we performed a multivariate analysis (backward method) including these factors with the age cut-off at 60 years. In this analysis, only sex remained significant (P = 0.003). On subsequent multivariate analysis including only sex (P = 0.035), level of LM diagnostic evidence (P = 0.006) and administration of any systemic treatment (P = 0.001), all three factors were confirmed to be prognostic.
Outcome by EANO ESMO Classification in the Overall Cohort
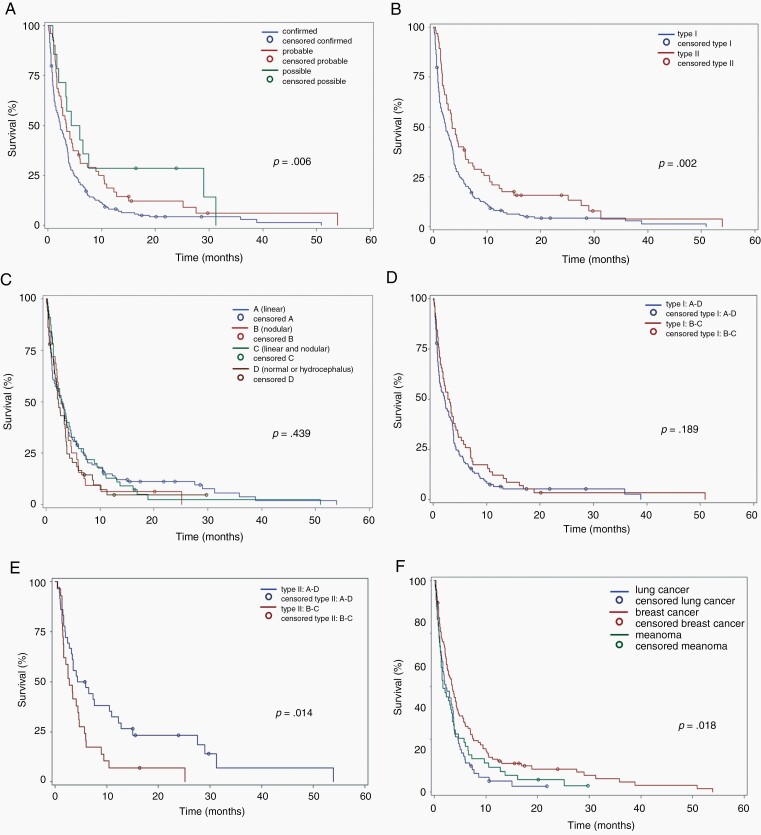
Survival differed significantly by level of evidence for the diagnosis of LM: (confirmed, probable, possible): possible LM had the longest survival (5.1 months, IQR: 2.4–14.2) and confirmed LM had the shortest survival (2.3 months, IQR: 0.8–4.9) (Fig. 1A, Table 2). Accordingly, the prognosis was also different between type I, with cytological or histological confirmation, and type II, with diagnosis based on the combination of clinical and imaging signs (Fig. 1B, Table 2). When comparing the four different MRI patterns, no significant survival difference between groups became apparent (Fig. 1C, Table 2). Yet, the survival curves for A and C patients and the curves for B and D patients looked similar, respectively, and B and D patients had numerically inferior outcome than A and C patients. Of note, most patients presenting with a D MRI subtype were classified as EANO ESMO type I (with tumor cells in the CSF) (n = 43) whereas only 7 D patients were classified as EANO ESMO type II (without tumor cells in the CSF). In these 7 patients, clinical features without or with MRI evidence of hydrocephalus led to the diagnosis of LM, and the diagnosis is least certain among all patients reported here. To estimate the prognostic impact of nodular disease, we compared imaging types A and D pooled with types B and C pooled, but there was no difference in the overall population (P = 0.999) (data not shown). When the same analysis was done in types I and II separately, there was still no difference in outcome for type I patients (Fig. 1D, Table 2), but a strong association of nodular disease with inferior survival in type II patients (Fig. 1E, Table 2).
Fig. 1.
Survival correlates of the EANO ESMO classification. Survival stratified by level of evidence of the LM diagnosis (confirmed, probable, possible) (A), by presence vs absence of tumor cells in the CSF (B), by pattern of neuroimaging (C), by absence vs presence of nodular disease in type I (D) and type II (E) patients, and by the most common primary tumor (F).
Table 2.
Prognosis Among the EANO ESMO Classification of LM at Baseline
| Items Among All Patients | Survival (Mo) | P |
|---|---|---|
| Median (IQR) | ||
| Level of evidence of LM | ||
| Type 1 (confirmed LM): n = 189 (74%) | 2.3 (0.8–4.9) | 0.006 |
| Type 2 (probable LM): n = 51 (20%) | 3.4 (1.6–9.2) | |
| Type 2 (possible LM): n = 14 (5%) | 5.1 (2.4–14.2) | |
| Type 1 (confirmed LM): n = 189 (74%) | 2.3 (0.8–4.9) | 0.002 |
| Type 2 (diagnosis based on clinical and MRI signs): n = 65 (26%) | 3.5 (1.7–9.4) | |
| MRI subtype | ||
| A (linear LM presentation): n = 117 (46%) | 2.9 (0.8–6.5) | 0.439 |
| B (nodular LM presentation): n = 32 (13%) | 2.9 (1.4–4.9) | |
| C (linear and nodular LM presentation): n = 55 (22%) | 2.7 (1.3–6.9) | |
| D (normal MRI or hydrocephalus (n = 50) (20%) | 2.2 (0.8–3.8) | |
| Nodular vs non-nodular MRI presentation | ||
| A plus D: n = 167 (66%) | 2.1 (0.7–4.3) | 0.999 |
| B plus C: n = 87 (34%) | 2.9 (1.1–6.7) | |
| Nodular vs non-nodular MRI presentation among type 1 patients | ||
| A plus D: n = 131 (69%) | 2.1 (0.7–4.3) | 0.189 |
| B plus C: n = 58 (31%) | 2.9 (1.1–6.7) | |
| Nodular vs non-nodular MRI presentation among type 2 patients | ||
| A plus D: n = 36 (55%) | 5.0 (2.0–13.3) | 0.014 |
| B plus C: n = 29 (45%) | 2.7 (1.5–5.7) |
Abbreviations: IQR, interquartile ranges; LM, leptomeningeal metastases.
Outcome by EANO ESMO Classification and Treatment
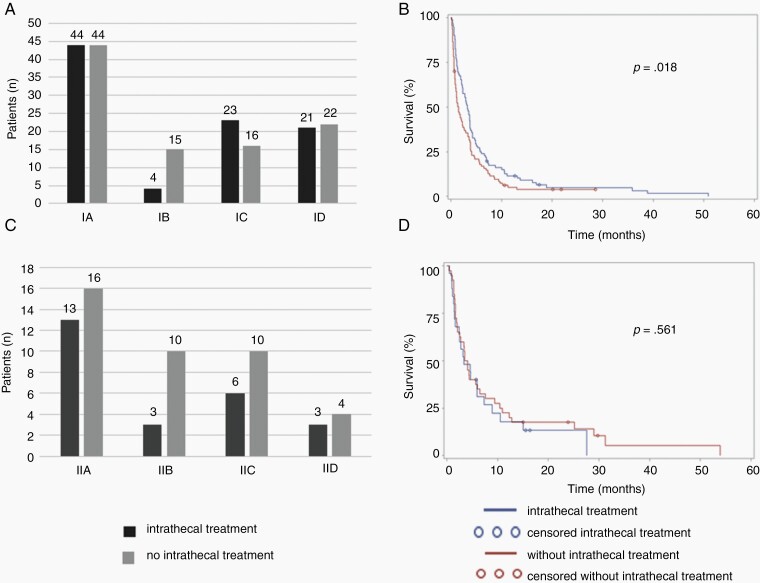
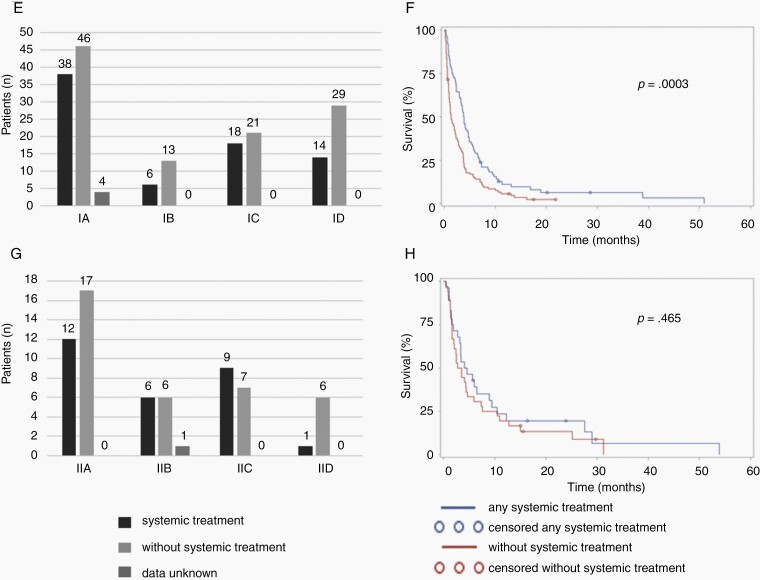
We next asked whether the absence or presence of tumor cells in the CSF would correlate with the relative efficacy of treatment as administered (Supplementary Tables 5 and 6). Among the whole population, administration of intrathecal treatment was not associated with survival (Supplementary Figure 1A) whereas patients treated with systemic pharmacotherapy had longer survival than those who were not (P = 0.0007, log-rank analysis) (Supplementary Figure 1B). Half (49%) of all patients with tumor cells in the CSF (type I) received intrathecal therapy whereas 38% of patients without cells in the CSF (type II) received intrathecal treatment (Fig. 2A and C). There was a significant association with improved outcome in type I patients receiving intrathecal therapy (P = 0.018), but not in type II patients (P = 0.561) (Fig. 2B and D). An analysis of the association of outcome with systemic treatment revealed similar results in that the administration of systemic treatment was linked to survival in type I patients (P = 0.0003) to a greater extent than in type II patients (P = 0.465) (Fig. 2E–H).
Fig. 2.
Survival associations with intrathecal and systemic pharmacotherapy per LM subtype. Numbers of patients treated with intrathecal (A,C) or systemic (E,G) pharmacotherapy per LM subtype and corresponding survival (B,D,F,H) (Type I: A, B, E, F; Type II: C, D, G, H).
We then further dissected potential contributions to outcome of these two treatments in type I LM patients. On univariate analysis, a survival benefit was observed among patients treated with intrathecal therapy vs patients not receiving intrathecal therapy (HR = 0.703, 95% CI 0.524–0.945, P = 0.020). A longer survival was also noted for patients treated with systemic pharmacotherapy relative to those who were not (HR = 0.578, 95% CI 0.426–0.784, P = 0.0004). When combining the two treatment modalities in a multivariate analysis, a significant effect remained for both therapeutic interventions (P = 0.001 and P = 0.0003) (Table 3), but there was no difference in outcome of patients receiving both modalities compared with either modality alone (data not shown).
Table 3.
Univariate and Multivariate Analysis of Survival Associations with Intrathecal and Systemic Pharmacotherapy in Patients with Type I LM (with Tumor Cells in the CSF)
| Parameter | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Pr > ChiSq | Hazard Ratio | 95% CI | Pr > ChiSq | Hazard Ratio | 95% CI | |||
| Intrathecal pharmacotherapy | 0.018 | 0.703 | 0.524 | 0.945 | 0.012 | 0.684 | 0.507 | 0.921 |
| Systemic pharmacotherapy | 0.0004 | 0.578 | 0.426 | 0.784 | 0.0003 | 0.565 | 0.416 | 0.768 |
Abbreviation: CSF, cerebrospinal fluid.
LM Presentation, Treatment, and Outcome by Primary Tumor
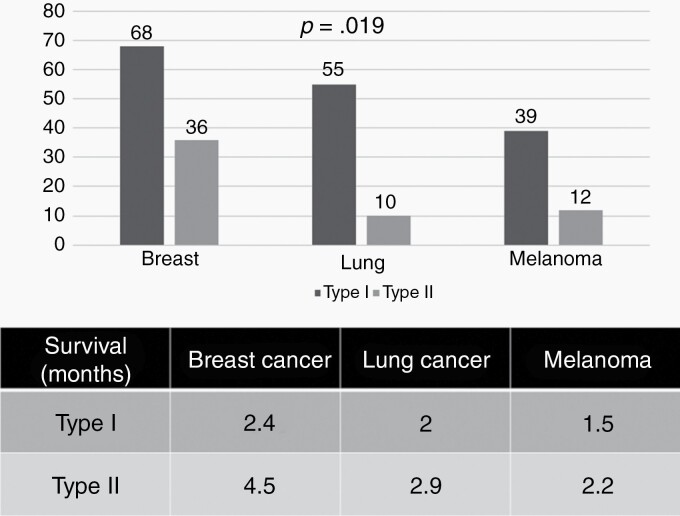
Detailed disease-specific characteristics including molecular marker status for the major tumor entities are provided in Supplementary Tables 7–9. Breast cancers were mainly ductal carcinomas (n = 67, 64%) and lobular (n = 22, 21%). They were HER2-positive in 26 patients (25%) and triple negative in 25 patients (24%). Lung cancers were mainly non-small cell lung cancer (89%), an EGFR mutation was found in 16 patients (25%) and an ALK fusion in 2 patients (3%). Melanomas had BRAF V600E mutations in 30 patients (59%) and another BRAF mutation in 8 patients (17%). Survival was best for breast cancer, intermediate for lung cancer and lowest in melanoma (Fig. 1F). Fig. 3 shows the distribution of EANO ESMO types and type-specific outcome in the main cancer types. Type I was more common in lung cancer whereas type II was most common in breast cancer. Type D was most frequent in breast cancer where nodular disease was most common in melanoma. Type I LM was associated with worse outcome than type II LM across entities. The strongest survival difference between type I and type II LM was seen in patients with breast cancer and linear disease only (type A) (Supplementary Figure 2).
Fig. 3.
Outcome per LM type and primary tumor. Distribution per EANO ESMO subtype (A) and survival per subtype (B) in patients with LM from breast cancer, lung cancer or melanoma. Comparisons of subgroups were performed with the log-rank test.
Discussion
The first EANO ESMO guidelines on the diagnosis and treatment of LM were largely based on expert consensus.23 One urgent need identified during guideline preparation was the need to better classify LM patients clinically to derive recommendations for interventions and to facilitate the design of more meaningful clinical trials. As a result of this, a provisional classification based on clinical findings, MRI pattern and CSF cytology has been proposed (Supplementary Table 1).
The present study aimed at exploring clinical correlates and prognostic value and thus the clinical utility of this new classifier. Several observations support the value of the EANO ESMO classification. The level of evidence by which the LM diagnosis is supported is prognostic (Fig. 1A). Positive CSF cytology is not only the gold standard of LM diagnosis (type I), but also highly prognostic (Fig. 1B), suggesting that it may be a surrogate marker of disease burden. Future studies should explore whether actually quantifying CSF tumor cells could refine prognostication.11,26 Conversely, nodular disease was a strong prognostic factor in the absence of positive CSF cytology (type II) (Fig. 1D and E). Failure to detect tumor cells in type II patients with MRI patterns A and D could indicate that these patients have low tumor burden in the CSF. Alternatively, notably with MRI pattern D, that is, lack of linear enhancement, it cannot be ruled out that some of these patients did not have LM at all. This would bias the survival analyses against type I patients where tumor cells were detected in the CSF. Conversely, the poor outcome of MRI type D patients with type I LM was remarkable, and future studies should explore whether hydrocephalus might be prognostic in this patient group. That there were no apparent differences in outcome between type A and C patients suggests that the absence or presence of nodular leptomeningeal disease is of little relevance in patients with positive CSF cytology (type I) (Fig. 1C). The administration notably of intrathecal, but also of systemic pharmacotherapy was associated with survival significantly only in type I LM, although patient numbers with type II were lower and there was a trend towards better survival with systemic pharmacotherapy, too (Fig. 2).
This contemporary cohort of LM patients confirms breast cancer, lung cancer and melanoma as the main primary tumors14,15 and shows that the prognosis remains best in breast cancer (Fig. 1F),14 despite recent advances in systemic therapy for lung cancer and melanoma. We assume that too few lung cancer and melanoma patients of our cohort may have been diagnosed and treated in recent years where such novel treatments have become widely available. That type II and pattern D were most common in breast cancer (Fig. 3) may indicate that the diagnosis of LM could be more challenging in patients with this cancer than in patients with lung cancer or melanoma.
The limitations of the study include its retrospective and uncontrolled design, the lack of validation of the neuroimaging classifier of the EANO ESMO proposal, and small patient numbers for some subgroup analyses. Further, the lack of central review of source data, notably of MRI imaging, is a weakness although central imaging review of patients with LM has recently been shown to be very challenging, for example, even using RANO criteria.27 Yet, compared with other contemporary series, the cohort is large and clinically well annotated, and we have demonstrated that EANO ESMO LM subtypes may be highly prognostic and should be considered in the design of future clinical trials of LM.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement.
E.L.R. has received honoraria for lectures or advisory board from Tocagen, Abbvie, Daiichi Sankyo. P.D. has nothing to disclose. J.W. has nothing to disclose. K.S. has received honoraria for advisory board participation from Roche and financial support from the “Filling-The-Gap” Program (University of Zurich) and “Walter und Gertrud Siegenthaler” Foundation. F.M. has nothing to disclose. A.C. has nothing to disclose. A.S.B. has research support from Daiichi Sankyo (≤10 000€), Roche (>10 000€) and honoraria for lectures, consultation or advisory board participation from Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo (all <5000€) as well as travel support from Roche, Amgen and AbbVie. J.J. has nothing to disclose. F.W. has received travel support from Roche. R.R. has received honoraria for lectures or consultation from UCB, Novocure. D.B. has nothing to disclose. M.V.D.B. has received honoraria for consultation from Abbvie, Agios, Carthera, Celgene, Bayer, Nerviano, Karyopharm, Boehringer. M.P. has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie. U.H. reports grants and personal fees from Roche, personal fees and non-financial support from Medac and Bristol-Myers Squibb, and personal fees from Novocure, Novartis, Daichii-Sankyo, Riemser, and Noxxon. M.W. has received research grants from Abbvie, Adastra, Dracen, Merck, Sharp & Dohme (MSD), Merck (EMD) and Novocure, and honoraria for lectures or advisory board participation or consulting from Abbvie, Basilea, Bristol Meyer Squibb (BMS), Celgene, Medac, Merck, Sharp & Dohme (MSD), Merck (EMD), Nerviano Medical Sciences, Orbus, Philogen, Roche and Tocagen.
Authorship statement. Experimental design and implementation: E.L.R., M.W. Acquisition, analysis, or interpretation of data: all authors. Statistical analysis: P.D. Manuscript preparation: E.L.R., M.W. Manuscript approval: all authors.
References
- 1. Brufsky AM, Mayer M, Rugo HS, et al. . Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 2. Seute T, Leffers P, ten Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer. 2005;104(8):1700–1705. [DOI] [PubMed] [Google Scholar]
- 3. Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis – the role of multimodality treatment. J Neurooncol. 2007;84(1):57–62. [DOI] [PubMed] [Google Scholar]
- 4. Gauthier H, Guilhaume MN, Bidard FC, et al. . Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21(11):2183–2187. [DOI] [PubMed] [Google Scholar]
- 5. Lee S, Ahn HK, Park YH, et al. . Leptomeningeal metastases from breast cancer: intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat. 2011;129(3):809–817. [DOI] [PubMed] [Google Scholar]
- 6. de Azevedo CR, Cruz MR, Chinen LT, et al. . Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565–572. [DOI] [PubMed] [Google Scholar]
- 7. Le Rhun E, Taillibert S, Zairi F, et al. . A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. [DOI] [PubMed] [Google Scholar]
- 8. Yust-Katz S, Garciarena P, Liu D, et al. . Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol. 2013;114(2):229–235. [DOI] [PubMed] [Google Scholar]
- 9. Morikawa A, Jordan L, Rozner R, et al. . Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gwak HS, Joo J, Kim S, et al. . Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol. 2013;8(5):599–605. [DOI] [PubMed] [Google Scholar]
- 11. Nevel KS, DiStefano N, Lin X, et al. . A retrospective, quantitative assessment of disease burden in patients with leptomeningeal metastases from non-small-cell lung cancer [Published online November 1, 2019]. Neuro Oncol. 2021;23:592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro Oncol. 2008;10(6):1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geukes Foppen MH, Brandsma D, Blank CU, van Thienen JV, Haanen JB, Boogerd W. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann Oncol. 2016;27(6): 1138–1142. [DOI] [PubMed] [Google Scholar]
- 14. Herrlinger U, Förschler H, Küker W, et al. . Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223(2):167–178. [DOI] [PubMed] [Google Scholar]
- 15. Brower JV, Saha S, Rosenberg SA, Hullett CR, Ian Robins H. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 2016;27:130–137. [DOI] [PubMed] [Google Scholar]
- 16. Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11(3):561–569. [DOI] [PubMed] [Google Scholar]
- 17. Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5(10):1655–1662. [DOI] [PubMed] [Google Scholar]
- 18. Glantz MJ, Jaeckle KA, Chamberlain MC, et al. . A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 19. Boogerd W, van den Bent MJ, Koehler PJ, et al. . The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18): 2726–2733. [DOI] [PubMed] [Google Scholar]
- 20. Shapiro WR, Schmid M, Glantz M, Miller JJ. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis. J Clin Oncol. 2006;24(18_suppl):1528–1528. [Google Scholar]
- 21. Le Rhun E, Wallet J, Mailliez A, et al. . Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol. 2020;22(4):524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chamberlain M, Soffietti R, Raizer J, et al. . Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Rhun E, Weller M, Brandsma D, et al. . EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl_4):iv84–iv99. [DOI] [PubMed] [Google Scholar]
- 24. Chamberlain M, Junck L, Brandsma D, et al. . Leptomeningeal metastases: a RANO proposal for response criteria [Published online December 29, 2016]. Neuro Oncol. 2017;19:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glitza IC, Rohlfs M, Guha-Thakurta N, et al. . Retrospective review of metastatic melanoma patients with leptomeningeal disease treated with intrathecal interleukin-2. ESMO Open. 2018;3(1): e000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malani R, Fleisher M, Kumthekar P, et al. . Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer [Published online June 6, 2020]. J Neurooncol. doi: 10.1007/s11060-020-03555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Rhun E, Devos P, Boulanger T, et al. . The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility, and a revised proposal [Published online January 23, 2019]. Neuro Oncol. 2019;21:648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.