Abstract
Control over the delivery of different functionalities and their synchronized activation in vivo is a challenging undertaking that requires careful design and implementation. The goal of the research highlighted herein was to develop a platform allowing the simultaneous activation of multiple RNA interference pathways and other functionalities inside cells. Our team has developed several RNA, RNA/DNA and DNA/RNA nanoparticles able to successfully complete such tasks. The reported designs can potentially be used to target myriad of different diseases.
Keywords: RNA nanotechnology, RNA/DNA hybrids, RNA interference, siRNAs, FRET
Along with DNA and proteins, RNA is one of the three major macromolecules that are essential for all known forms of life. However, RNA also shares the functional definition of DNA (informational storage) and proteins (catalytic, structural, transport, and defense). This functional versatility makes RNA a perfect material for building biologically active and meaningful nanostructures. Consequently, RNA nanotechnology has emerged as a significant modality in recent years due to the numerous advantages that it offers pertaining to precise control over the composition and stoichiometry of the delivered RNA-based functionalities as well as other functional moieties [1–8].
RNA interference (RNAi) is the biological process of specific gene silencing through a natural cellular post-transcriptional regulation process that involves short double stranded-RNAs [9–11]. The use of RNAi is showing significant potential for various therapeutic applications [12]. Simultaneous delivery of multiple therapeutic RNAi inducers (siRNAs, miRNAs, shRNAs, etc) to diseased cells is expected to have significant synergistic effects[13]. The precise controlled delivery of various RNAi-based therapeutics could be achieved by building programmable RNA scaffolds that can be further functionalized and assembled into RNA nanoparticles of various shapes and compositions [2,3,14,15].
Recently, we introduced a technique that allows the conditional activation of RNAi in vivo [16]. The basic idea lies in splitting the functional units into non-functional fragments, followed by their designed conditional re-association and complete restoration of the original function. Using this mechanism, we split the functionality of Dicer substrate RNAs (DS RNAs) [17] into two RNA-DNA hybrids, which when presented together inside the cell, recognize each other through toehold interactions embedded into the DNA portion of each hybrid, re-associate, and release DS RNAs. Cellular Dicer, an RNaseIII-like enzyme, is further employed to process DS RNAs into short interfering RNAs or siRNAs, which are then utilized by the RNA-induced Silencing Complex, called RISC, to activate RNAi. In order to deliver and conditionally activate split functionalities such as DS RNAs, FRET, or RNA aptamers, the inactive hybrids are decorated with complementary ssDNA toeholds that will interact and trigger the re-association process when both of the hybrids get close together within the same cell.
By simply elongating the hybrids, we then demonstrated the ability to simultaneously activate multiple (up to seven) DS RNAs, aptamers and FRET [18,19]. However, this approach was limited to the maximum lengths of the single-stranded DNA comprising the hybrids; the results from re-association of long hybrid double DNAs showed some immune-stimulatory effects [19]. To partially overcome these problems and to diversify the approach, we designed and tested various RNA-DNA and DNA-RNA hybrid nanoparticles consisting of either RNA[2,20] or DNA[20] cores decorated with six RNA-DNA hybrids (Figure 1). Two different previously extensively characterized nanodesigns – nanocubes [21,22] and nanorings [23,24] - were used as RNA cores (Figure 1a–b). The resulting functional RNA nanoparticles can be produced either by one-pot assembly[23] or co-transcriptionally [25]. However, due to the limitations of the nanoring design strategy [23], only the nanocubes can be used as the DNA core in the DNA/RNA nanoparticles and only the one-pot assembly protocol is available for their production (Figure 1c). The single-stranded DNA toeholds appending each nanoparticle were designed to initiate the re-association after the addition of the cognate hybrids (Figure 1b–c). The regulated displacement of the DNA partner induced the assembly of the RNA duplexes, which were further processed by the human Dicer enzyme, thus activating RNAi. Various experimental results [2,20] revealed significant cellular uptake of functionalized nanoparticles through endocytosis. Extensive levels of silencing of the targeted genes were observed and the silencing remained significant throughout the experiment even on the twelfth day post-transfection. Results showed that the silencing only occurred when the nanoparticles and the cognate hybrids were simultaneously delivered into the cells. Overall, the comprehensive cell culture experiments demonstrated FRET and RNAi activation by conditional triggering of the split functionalities in the cells. The use of RNA nanoparticles functionalized with six different siRNAs targeting different parts of the HIV genome[26] confirmed the successful down-regulation of viral production in HIV infected cells. Another important result presented in the highlighted research [20] revealed that the DNA-RNA nanoconstructs are potentially better suited for certain therapeutic purposes due to reduced cytokine release.
Figure 1. Application of RNA, RNA/DNA and DNA/RNA for RNAi activation.
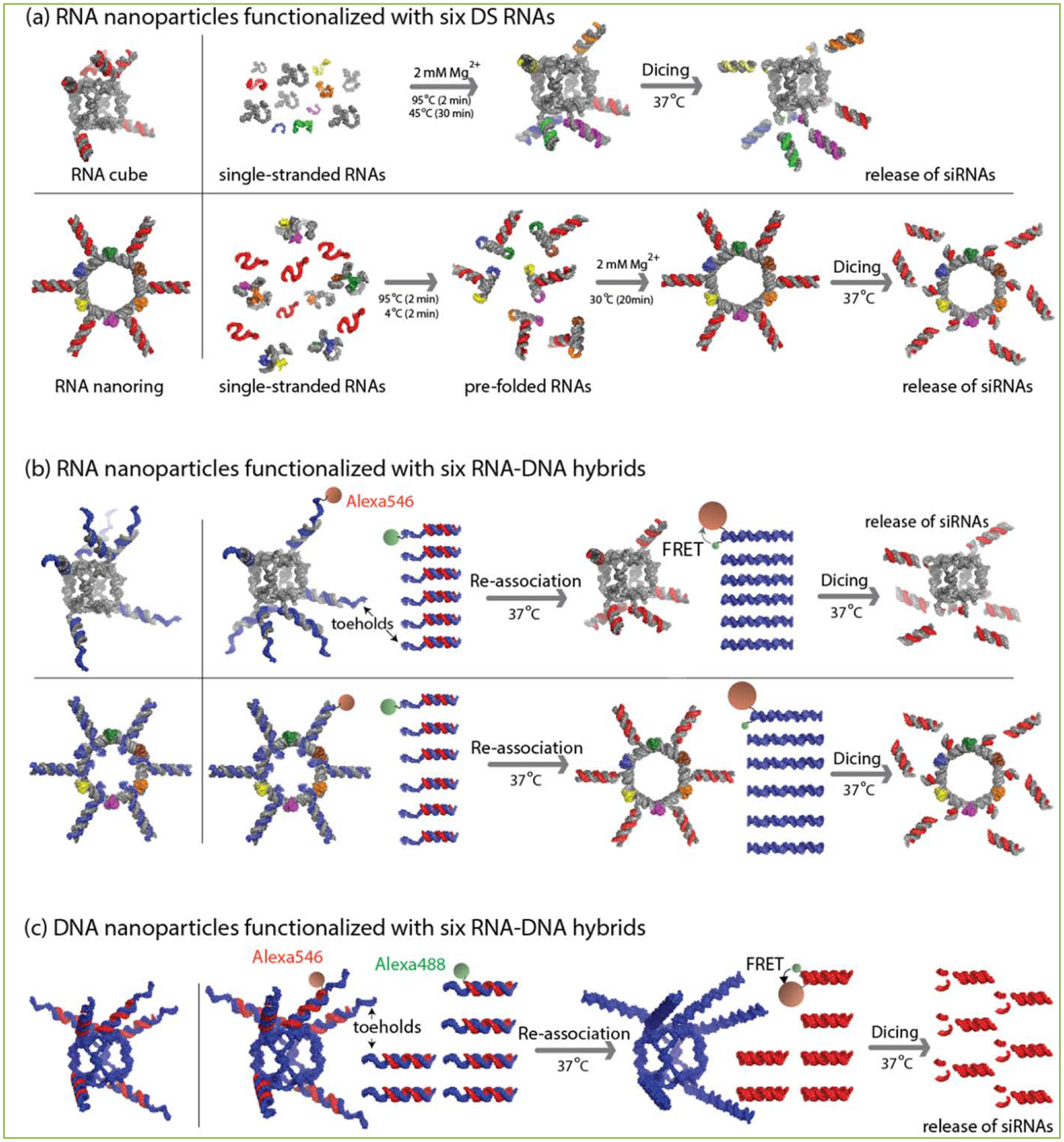
(a) Schematic representation of the assemblies leading to the formation of RNA nanocubes and nanorings functionalized with DS RNAs. (b) Schematic representation of the re-association of the RNA/DNA nanocubes and nanorings initiated by toehold interaction and further release of the siRNAs from the RNA nanorings. (c) Re-association of DNA/RNA nanocubes and hybrids trigger the release of DS RNA further diced into the siRNAs.
In conclusion, the novel technique highlighted here could be used to exploit the multiple existing three-dimensional shapes formed by DNAs and RNA-DNA hybrid structures [27–32].
References
- 1.Afonin KA, Lindsay B, Shapiro BA. Engineered RNA Nanodesigns for Applications in RNA Nanotechnology. RNA Nanotechnology 2013; 1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonin KA, Viard M, Koyfman AY, Martins AN, Kasprzak WK, Panigaj M, et al. Multifunctional RNA nanoparticles. Nano letters 2014; 14:5662–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khisamutdinov EF, Li H, Jasinski DL, Chen J, Fu J, Guo P. Enhancing immunomodulation on innate immunity by shape transition among RNA triangle, square and pentagon nanovehicles. Nucleic acids research 2014; 42:9996–10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu Y, Pi F, Sharma A, Rajabi M, Haque F, Shu D, Leggas M, et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Advanced drug delivery reviews 2014; 66:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla GC, Haque F, Tor Y, Wilhelmsson LM, Toulme JJ, Isambert H, Guo P, et al. A boost for the emerging field of RNA nanotechnology. ACS nano 2011; 5:3405–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afonin KA, Kasprzak WK, Bindewald E, Kireeva M, Viard M, Kashlev M, at al. In silico design and enzymatic synthesis of functional RNA nanoparticles. Accounts of chemical research 2014; 47:1731–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo P The emerging field of RNA nanotechnology. Nature nanotechnology 2010; 5:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno H, Kobayashi T, Kabata R, Endo K, Iwasa T, Yoshimura SH, et al. Synthetic RNA-protein complex shaped like an equilateral triangle. Nature nanotechnology 2011; 6:116–120. [DOI] [PubMed] [Google Scholar]
- 9.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 2001; 15:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. Embo J 2001; 20:6877–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806–811. [DOI] [PubMed] [Google Scholar]
- 12.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol 2012: 19:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YP, von Eije KJ, Schopman NC, Westerink JT, ter Brake O, Haasnoot J, Berkhout B. Combinatorial RNAi against HIV-1 using extended short hairpin RNAs. Mol Ther 2009; 17:1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khaled A, Guo S, Li F, Guo P. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano letters 2005; 5:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabow WW, Jaeger L. RNA self-assembly and RNA nanotechnology. Accounts of chemical research 2014; 47:1871–1880. [DOI] [PubMed] [Google Scholar]
- 16.Afonin KA, Viard M, Martins AN, Lockett SJ, Maciag AE, Freed EO, et al. Activation of different split functionalities on re-association of RNA-DNA hybrids. Nature nanotechnology 2013; 8:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME, et al. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic acids research 2005; 33:4140–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afonin KA, Bindewald E, Kireeva M, Shapiro BA. Computational and Experimental Studies of Reassociating RNA/DNA Hybrids Containing Split Functionalities. Methods in enzymology 2015; 553:313–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afonin KA, Desai R, Viard M, Kireeva ML, Bindewald E, Case CL, et al. Co-transcriptional production of RNA-DNA hybrids for simultaneous release of multiple split functionalities. Nucleic acids research 2014; 42:2085–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afonin KA, Viard M, Kagiampakis I, Case CL, Dobrovolskaia MA, Hofmann J, et al. Triggering of RNA Interference with RNA-RNA, RNA-DNA, and DNA-RNA Nanoparticles. ACS nano 2015; 9:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afonin KA, Bindewald E, Yaghoubian AJ, Voss N, Jacovetty E, Shapiro B, et al. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nature nanotechnology 2010; 5:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afonin KA, Kasprzak W, Bindewald E, Puppala PS, Diehl AR, Hall KT, et al. Computational and experimental characterization of RNA cubic nanoscaffolds. Methods 2014; 67:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afonin KA, Grabow WW, Walker FM, Bindewald E, Dobrovolskaia MA, Shapiro BA, et al. Design and self-assembly of siRNA-functionalized RNA nanoparticles for use in automated nanomedicine. Nature protocols 2011; 6:2022–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabow WW, Zakrevsky P, Afonin KA, Chworos A, Shapiro BA Jaeger L. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano letters 2011; 11: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afonin KA, Kireeva M, Grabow WW. Kashlev M, Jaeger L, Shapiro BA Co-transcriptional assembly of chemically modified RNA nanoparticles functionalized with siRNAs. Nano letters 2012; 12:5192–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low JT, Knoepfel SA, Watts JM, ter Brake O, Berkhout B, Weeks KM. SHAPE-directed discovery of potent shRNA inhibitors of HIV-1. Mol Ther 2012; 20:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Ye T, Su M, Zhang C, Ribbe AE, Jiang W, Mao C. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 2008; 452:198–201. [DOI] [PubMed] [Google Scholar]
- 28.Ko SH, Su M, Zhang C, Ribbe AE, Jiang W, Mao C. Synergistic self-assembly of RNA and DNA molecules. Nat Chem 2010; 2:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen ES, Dong M, Nielsen MM, Jahn K, Lind-Thomsen A, Mamdouh W, et al. DNA origami design of dolphin-shaped structures with flexible tails. ACS nano 2008; 2:1213–1218. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro AV, Han D, Shih WM, Yan H. Challenges and opportunities for structural DNA nanotechnology. Nature nanotechnology 2011; 6:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers TA, Andrews GE, Jaeger L, Grabow WW. Fluorescent monitoring of RNA assembly and processing using the split-spinach aptamer. ACS synthetic biology 2015; 4:162–166. [DOI] [PubMed] [Google Scholar]
- 32.Endo M, Takeuchi Y, Emura T, Hidaka K, Sugiyama H. Preparation of chemically modified RNA origami nanostructures. Chemistry 2014; 20:15330–15333. [DOI] [PubMed] [Google Scholar]


