Abstract
Lipoprotein lipase (LPL) plays a major role in the lipid homeostasis mainly by mediating the intravascular lipolysis of triglyceride rich lipoproteins. Impaired LPL activity leads to the accumulation of chylomicrons and very low-density lipoproteins (VLDL) in plasma, resulting in hypertriglyceridemia. While low-density lipoprotein cholesterol (LDL-C) is recognized as a primary risk factor for atherosclerosis, hypertriglyceridemia has been shown to be an independent risk factor for cardiovascular disease (CVD) and a residual risk factor in atherosclerosis development. In this review, we focus on the lipolysis machinery and discuss the potential role of triglycerides, remnant particles, and lipolysis mediators in the onset and progression of atherosclerotic cardiovascular disease (ASCVD). This review details a number of important factors involved in the maturation and transportation of LPL to the capillaries, where the triglycerides are hydrolyzed, generating remnant lipoproteins. Moreover, LPL and other factors involved in intravascular lipolysis are also reported to impact the clearance of remnant lipoproteins from plasma and promote lipoprotein retention in capillaries. Apolipoproteins (Apo) and angiopoietin-like proteins (ANGPTLs) play a crucial role in regulating LPL activity and recent insights into LPL regulation may elucidate new pharmacological means to address the challenge of hypertriglyceridemia in atherosclerosis development.
Keywords: lipoprotein lipase, ANGPTL, atherosclerosis
1. Introduction
There are three main lipids in the blood: cholesterol, phospholipids, and triglycerides. Cholesterol is important for the synthesis of bile acids and steroids and for maintaining the integrity of cell membranes, while phospholipids are a major component of all cell membranes. Triglycerides (TGs) serve as a storable high-energy fuel. In humans, almost 80% of the energy requirements of the heart and liver are fulfilled by oxidation of the long fatty acyl chains present in TGs [1].
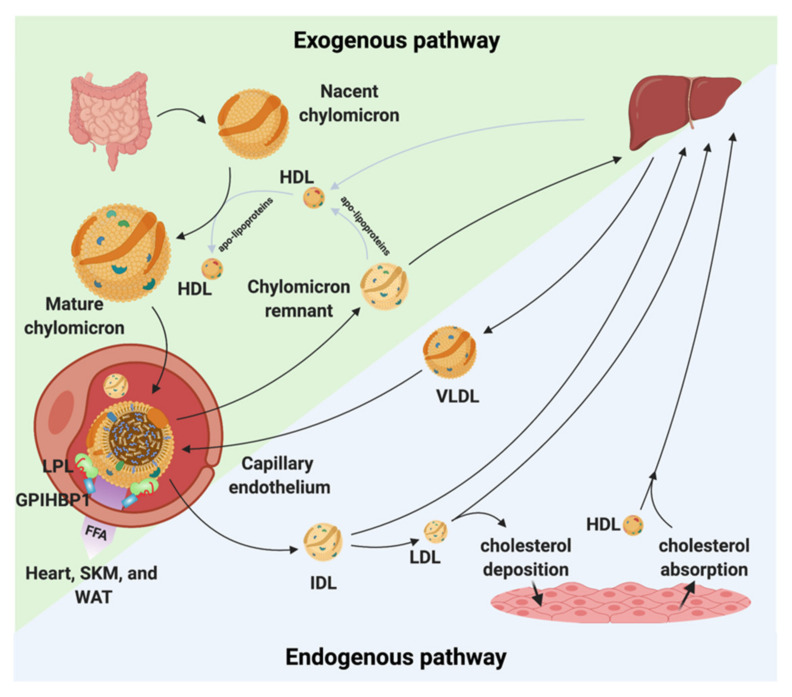
Because lipids are poorly soluble in blood plasma, they need to be transported as lipoproteins. Lipoproteins are composed of cholesteryl esters and TGs surrounded by a hydrophilic shell made of phospholipids, unesterified cholesterol, and apolipoproteins. Depending upon the origin of the lipoproteins they differ in size, density, and composition [2,3]. The lipoproteins are generally divided into five major classes. These classes are chylomicrons, VLDL, LDL, intermediate-density lipoprotein (IDL), and high-density lipoproteins (HDL). Each class of lipoprotein serves distinct roles in the lipid metabolism (Figure 1); (i) the large chylomicrons and VLDLs are responsible for the transport and delivery of energy rich TGs, (ii) LDL deposits cholesterol in tissues, and (iii) HDL absorbs cholesterol and transports it back to the liver for degradation and redistribution (Figure 1). The lipoproteins constantly undergo enzymatic processing, exchange of lipids or apolipoproteins, and progressive degradation by lipid unloading. Combined, this network is crucial for lipid homeostasis, and even minor alternations in either of the lipoproteins classes potentially perturbs the entire lipoprotein processing network. LDL and HDL have been found to be important for the development of arteriosclerosis due to their cholesterol cargo. However, studies have shown that elevated plasma triglyceride levels known as hypertriglyceridemia (HTG) is associated with an increased risk of atherosclerosis and CVD [4,5,6]. LPL is responsible for the hydrolysis of the triglyceride in capillaries and thereby transform chylomicrons and VLDL to smaller lipoproteins particles. This process is tightly regulated by a number of proteins that either display a stabilizing, activating, or inhibitory effect of LPL and perturbations of this homeostasis may have anti-atherogenic effects [7,8,9,10,11]. On the other hand, data have shown that when LPL is expressed by macrophages in the vessel wall it displays pro-atherogenic effects [12]. With this in mind, there is a great need to understand the underlining molecular mechanism(s) causing increased triglyceride levels in hypertriglyceridemia and atherosclerosis and map the complex regulation of LPL. This knowledge will likely identify new avenues for lipid lowering strategies that could serve as supplements to the existing interventions (e.g., statins) to help further restraining ASCVD progression.
Figure 1.
Chylomicron and VLDL life cycle. Chylomicrons are secreted from the intestines to the circulation where they extract apolipoproteins from HDL. The mature chylomicrons are arrested at the capillary endothelium by LPL that is in complex with glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPIHBP1). LPL performs lipolysis on the encapsulated TGs, which releases free fatty acids that are taken up by the underlying tissues (white adipose tissue (WAT), skeletal muscle (SKM), and the heart). Upon complete hydrolysis, a chylomicron remnant particle is released to the circulation. The chylomicron remnant particle back-exchanges apolipoproteins to circulating HDL, before final catabolism in the liver. A similar life cycle is found for VLDL. VLDL is secreted from the liver and is arrested and hydrolyzed by the LPL•GPIHBP1 complex. This releases IDL, which can either be taken up by the liver or be hydrolyzed further into LDL. LDL deposits cholesterol esters in various tissues. HDL absorbs cholesterol and transports it to the liver.
2. The Lipoprotein Lipase
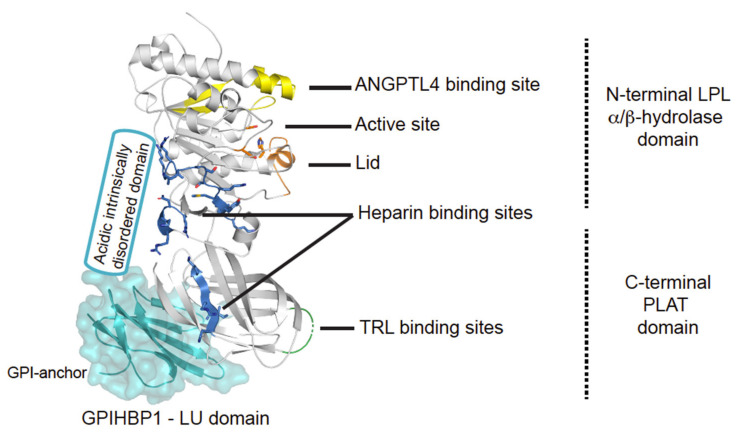
The central role of LPL in triglyceride hydrolysis was recognized over 60 years ago by the discovery of the enzyme in 1955 [13]. The expression level of LPL is robust in cells and organs with a high oxidative metabolism, but it is also expressed in other tissue types, not related to intravascular lipolysis such as the spleen, testis, lung, kidneys, and brain as well as in macrophages [14]. LPL is a 55 kDa glycoprotein consisting of an N-terminal α/β-hydrolase domain and a C-terminal Polycystin-1, Lipoxygenase, Alpha-Toxin (PLAT) domain (Figure 2). The α/β-hydrolase domain harbors the active site that primarily hydrolyses the sn-1/sn-3 ester bonds of triglycerides, thereby releasing two unesterified fatty acids and a sn-2 monoacylglycerol [15]. The hydrolysis takes place at the luminal face of the capillary endothelium where LPL hydrolyzes the triglycerides within the neutral cores of chylomicrons and VLDLs producing chylomicron remnants and IDLs, respectively (Figure 1) [16]. The released free fatty acids (FFA) can either be used as an energy source or be re-esterified and stored in adipose tissues as triglycerides.
Figure 2.
Structural elements in LPL. Cartoon representation of the human LPL•GPIHBP1 complex and with the molecular surface of the LU domain (Ly6/uPAR protein domain family) of GPIHBP1 shown as a transparent light blue envelope (generated with The PyMol Molecular Graphic System (Version 2.0 Schrödinger, LLC) using coordinates from the LPL•GPIHBP1 crystal structure; Protein Data Bank ID code 6E7K). LPL elements implicated in ANGPTL4 binding are highlighted in yellow [17]. GPIHBP1 binds to the C-terminal PLAT domain of LPL, and the location of GPIHBP1’s membrane-tethering site (GPI anchor) is indicated [18]. GPIHBP1’s acidic intrinsically disordered domain stabilizes LPL’s α/β-hydrolase domain and is assumed to interact with a large basic patch on the surface of LPL [19,20]. The active site containing the catalytic triad (Ser134, Asp158, and His243) is represented with orange residues [21] and the orange helix is the lid covering the active site responsible for substrate specificity [22]. The triglyceride-rich lipoprotein (TRL) binding site is indicated in green and residues important for heparin binding is shown in blue [23,24,25].
2.1. Maturation and Transportation of LPL
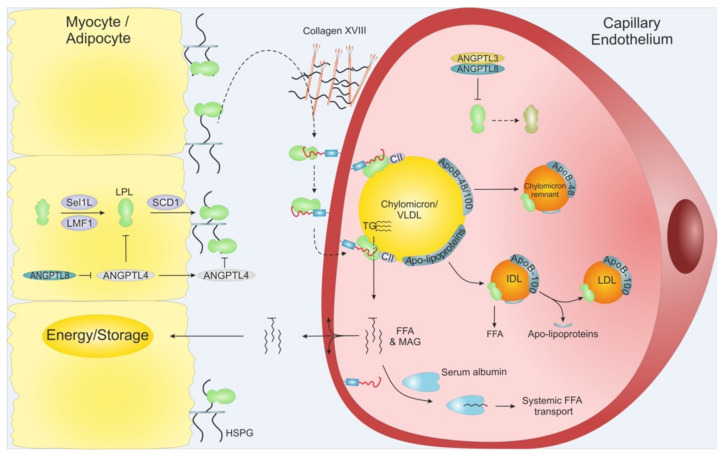
Adipocytes and myocytes are the prime sources of LPL production and because these cells are located distantly from the capillary lumen, where LPL acts on marginated lipoproteins, it needs to be trafficked across the subendothelial space and trancytosed across the endothelial cells [14,26]. Accordingly, LPL secretion requires several chaperones and transporter proteins to ensure correct shuttling and folding of LPL [27,28,29,30]. One such protein is the lipase maturation factor (LMF1), which is essential for LPL processing since loss-of-function variants in LMF1 cause hypertriglyceridemia due to inefficient LPL secretion [28,31]. Besides LMF1, the suppressor of lin-12-like protein 1 (Sel1L) and syndecan-1 (SCD1) have been shown to be vital for LPL secretion (Figure 3) [29,30].
Figure 3.
Schematic overview of LPL translocation. LPL is secreted from adipose tissue and myocytes from skeletal muscles and heart. Intracellular LPL folding and secretion is assisted by LMF1, Sel1L and SCD1. After secretion, active LPL is bound to cell surface HSPG before being translocated to GPIHBP1, potentially via Collagen XVIII. GPIHBP1 captures LPL in the subendothelial space and mediates LPL trancytosis across the capillary endothelium, into the lumen. Within the lumen, the LPL•GPIHBP1 complex is responsible for the arrest of VLDLs and chylomicrons. After final hydrolysis, either a chylomicron remnant particle or an IDL are released to be taken up by the liver or turned into LDL via further TG hydrolysis, respectively. ANGPTL4 act as a potent LPL inhibitor in the adipose tissue, and ANGPTL3•ANGPTL8 act on LPL mainly in the capillaries of the muscles and heart. The inhibition of the ANGPTLs is driven by nutritional status and exercise. MAG: Monoacylglycerol.
Upon successful secretion, LPL becomes tethered to cell surface heparans sulfate proteoglycans (HSPGs) via its heparin binding sites (Figure 2 and Figure 3) [23,24,25]. HSPG-bound LPL provides a storage pool that can be rapidly translocated to the capillary lumen via the endothelial cell surface bound protein GPIHBP1 (Figure 3). The movement of LPL towards GPIHBP1 is currently controversial and has been suggested to include random diffusion [32], directed diffusion on HSPG sulfation-gradients [19], and heparanase mediated release [33]. Interestingly, collagen XVIII with its heparin sulfate modifications may act as the proximal reservoir of LPL on the abluminal face of the capillary endothelium since Col18a1−/− mice have increased plasma TGs levels and decreased plasma LPL mass [34].
Once bound to GPIHBP1, LPL can move bidirectionally between the luminal and abluminal surfaces of the endothelial cells by caveolin1-independent transcytosis [35,36]. The expression of GPIHBP1 is restricted to capillary endothelial cells—it is absent in larger vessels—rendering the capillaries the sole relevant site for intravascular lipolysis [26,37]. GPIHBP1 contains a folded LU domain with ten consensus cysteines and a disordered acidic domain (Figure 2) [20,38]. The pivotal role of GPIHBP1 in intravascular lipolysis is emphasized by the fact that a fraction of patients suffering from chylomicronemia are homozygous or combined biallelic heterozygous for GPIHBP1 missense variants that encode a non-functional GPIHBP1 protein [39,40,41,42,43]. In these patients, the binding between GPIHBP1 and LPL is impaired, and hence LPL activity is absent in the lumen of the capillary. Further underscoring the importance of GPIHBP1, is a subset of patients with hitherto unexplained, late-onset acquired chylomicronemia that had a new disease etiology—auto-immunity against GPIHBP1 with auto-antibodies preventing LPL-translocation into capillary lumen [44]. Recently, it has been shown that this disease severity can be ameliorated in some patients with immunosuppressive therapy [45].
2.2. Enzymatic Function of LPL
Once translocated to the luminal face of the capillary endothelium, the LPL•GPIHBP1 complex is responsible for the margination (arrest) of the circulating triglyceride-rich lipoprotein (TRL) particles [46]. In Gpihbp1−/− mice, the TRLs do not marginate if LPL is absent from the luminal endothelium. The systemic administration of bovine LPL intravenously or the transgenic expression of human LPL in endothelial cells do not induce TRL magination in Gpihbp1−/− mice [46]. The binding to TRLs is exclusively mediated by LPL, not GPIHBP1 (Figure 2) [46,47]. Although the molecular mechanism(s) of LPL-mediated TRL processing is not completely understood, several regions of the lipase molecule are known to be essential for its activation, TRL binding and substrate specificity [18,48,49,50,51,52,53].
The LPL-mediated hydrolysis of TGs in the core of TRLs is extremely efficient. In vivo imaging studies with 2H-triglycerides showed that margination, TG hydrolysis, and the subsequent uptake of free fatty acids occur within a few minutes in perfused hearts [54]. These observations are in line with kinetic data from in-vitro studies on TRL processing in solution, which estimate that on average 44 LPL molecules engage simultaneously in the lipolytic processing each TRL particle [15]. Electron microscopy of cardiomyocytes show that GPIHBP1•LPL complexes are located on small membrane protrusions (“nanovilli”) lining the TRL particle. This anatomical structure allows for a larger contact area with the TRLs and enables the concomitant engagement of a larger number of LPL molecules in the lipolytic processing [46].
It is currently debated exactly how the released FFA are taken up by the underlying tissues. Observing 2H-free fatty acids in the cytoplasma of cardiomyocytes a few minutes after margination indicates that they rapidly diffuses across the plasma membrane into the underlying cells [54]. It was suggested previously that CD36 actively transports FFAs. However, the genetic ablation of CD36 did not change the fast kinetics of 2H-FFA uptake in cardiomyocytes [54]. In addition to the local FFA uptake, binding to serum albumin allows the FFA to be transported and used at sites distant from the site of hydrolysis [15]. If the generated FFAs are not removed locally they will auto-inhibit LPL and slow down the lipolysis [15]. Release of high amount of FFAs by systemic lipolysis may cause inflammation and lipotoxicity [55].
2.3. LPL Is Active as a Monomer
Despite intensive research efforts, the structural insights into LPL function remained elusive for decades primarily due to the low stability of the enzyme. Perceptions of the structure–function relationships in LPL relied largely on homology modeling using the crystal structure of pancreatic lipase (PL) as a template [50]. Although these homology considerations provided some useful information, the low sequence identity between PL and LPL (27%) paired with PL’s need for a cofactor (co-lipase) limits the confidence of such homology models [56]. From a structural perspective, the discovery of GPIHBP1 and the realization that GPIHBP1 stabilizes LPL was decisive for solving the crystal structure of LPL [20,36,57]. In short succession, two independent groups published virtually identical X-ray structures of LPL in complex with GPIHBP1 [18,58]. In both structures, LPL formed the expected head-to-tail homodimer. That dimeric LPL configuration was widely accepted to represent the active conformation of LPL, and the individual monomers thought to be unstable and inactive [59,60]. In the crystal structure, the reciprocal dimer interface was, however, formed by the burial of the TRL substrate binding tryptophans of one protomer in the active site cleft of the partner protomer [18,58]. That observation raised the question: How does the dimeric LPL allows substrate entry when the active site is blocked by the TRL binding loop? This conundrum was, however, quickly resolved as LPL monomers rather than LPL dimers were found to represent the active LPL conformation [61]. This shift in the paradigm on the active LPL conformation was subsequently supported by biophysical measurements showing that LPL monomers and LPL dimers had equal stability, esterase activity and inhibitory profile when exposed to ANGPTL4 [62].
2.4. GPIHBP1-Binding Render LPL Stable at Body Temperature
In recent years, it has become increasingly clear that LPL’s inherent protein instability plays a key role for the regulation of intravascular lipolysis. Early studies reported the progressive loss of LPL activity at 37 °C, and that the presence of heparin preserved enzymatic activity [60,63]. Studies with hydrogen-deuterium exchange coupled to mass spectrometry (HDX-MS) provided the first details on the molecular mechanism(s) underlying this instability. The Achille’s heel of the LPL protein weakening its thermal stability is found in the α/β-hydrolase domain, which unfolds spontaneously [17,20,53,62]. In contrast, the C-terminal PLAT domain is stable. The global unfolding observed with HDX-MS was confirmed by differential scanning fluorimetry (DSF) showing that the catalytic α/β-hydrolase domain unfolds with a Tm of 34.8 °C, which is well below normal body temperature [17]. Binding of either GPIHBP1 or HSPGs stabilize the α/β-hydrolase domain profoundly (Tm = 57.6 °C or 42.2 °C, respectively). Importantly, ANGTPL4 has the opposite effect on LPL stability decreasing Tm for unfolding of the α/β-hydrolase domain to less than 15 °C for LPL alone and to 36.6 °C for LPL•GPIHBP1 complexes. Another event stabilizing LPL is the binding to lipoproteins, which also mitigates ANGPTL4 and ANGPTL3 mediated inhibition [64]. In aggregate, these studies show that LPL auto-inactivates by spontaneous unfolding, unless its intrinsic instability is counteracted by binding to GPIHBP1 or HSPG. This weakness is further exploited by ANGPTL3 and ANGPTL4 that inhibit LPL by catalyzing the unfolding of its catalytic domain [65,66].
In normal subjects, plasma LPL mass does not correlate with LPL activity since most plasma LPL is inactive. Upon heparin administration, active LPL is efficiently released causing mass and activity to correlate [67,68]. As mentioned, inactive LPL was widely accepted to represent LPL monomers, but now we understand that the inactive form most likely represents partly unfolded LPL monomers, with a folded PLAT domain [20,61]. This scenario has implications such as, (i) antibodies recognizing that active LPL is unlikely to bind the unfolded catalytic domain of inactive species in plasma and (ii) interactions with inactive LPL in circulation would be expected to involve the relatively thermostable PLAT domain. In fact, this is in line with evidence suggesting that the PLAT domain of inactive LPL is responsible for remnant uptake via LDL receptor related protein 1 (LRP1) and LDL-receptor (LDL-R) [69,70,71]. Lipoprotein profiling suggests that both inactive and active LPL associates mainly with LDL and HDL in both pre- and post-heparin plasma, whereas active LPL associates predominantly with TRLs when it is released with heparin in the post-prandial state [67]. Furthermore it has been suggested that inactive LPL preferably binds native LDL, while active LPL preferentially binds oxidized LDL [72]. How these differences in binding preferences between active and inactive LPL relate to the conformational stability of LPL’s α/β hydrolase domain is currently unexplored.
3. LPL’s Activity Is Regulated by Apolipoproteins
Apolipoproteins are integrated in the maintenance of lipid homeostasis. They constitute a class of amphipathic proteins having a hydrophobic region that interacts with lipids in the lipoprotein particles, while the hydrophilic region allows interaction with soluble proteins. They provide structure-defining elements for lipoprotein particles and serve as ligands for specific receptors. Both ApoC-II and ApoA-V act as positive regulators of LPL in intravascular lipolysis, while ApoC-I and ApoC-III display an inhibitory role towards LPL. Apolipoproteins may promote atherogenic or anti-atherogenic effects depending on their expression levels and microenvironment.
3.1. Apolipoprotein C-II Enhances LPL Activity
ApoC-II is the only known and essential cofactor for LPL in mediating the hydrolysis of TRLs [73,74]. In plasma, ApoC-II is found associated to VLDL, chylomicrons and HDL particles [75,76]. It contains 79 amino acids arranged in three α-helices and the third C-terminal helix interacts with LPL [77,78,79]. Chemical cross-linking and site directed mutagenesis studies have provided some insights into how ApoC-II interacts with LPL. Four specific amino acid residues (Tyr63, Ile66, Asp69, and Gln70) in the C-terminal helix of ApoC-II have been shown to be important for interaction with LPL [80], but the amino acid residues of LPL that are supposed to interact with ApoC-II have not been fully resolved yet [81,82]. Recently, it was proposed that ApoC-II regulates LPL activity in a pressure dependent model [83]. In this model, ApoC-II remains attached to the VLDL and chylomicron surfaces during LPL mediated TG hydrolysis. As LPL consumes the neutral core TGs, the surface pressure on TRLs increases. Once this surface pressure is greater than a given ApoC-II retention pressure, it leads to the extrusion of ApoC-II from the TRL. An unresolved paradox in Apo-CII biology is that over-expression of ApoC-II leads to HTG, due to decreased lipolysis [84]. Using the pressure dependent model, we can therefore hypothesize that high levels of ApoC-II on the TRL surface would increase the surface pressure preventing LPL binding, thereby attenuating lipolysis, when ApoC-II is overexpressed.
One of the pathogenic properties of ApoC-II is its ability to form amyloid fibrils by undergoing self-association in the absence of lipids [85]. In vivo studies have demonstrated the accumulation of these fibrils in atherosclerotic plaques in the arteries [86]. These amyloid fibrils were reported to initiate CD-36 dependent signaling resulting in macrophage-mediated inflammatory response such as production of tumor necrosis factor-α and reactive oxygen species (ROS)—a crucial event in the progression of atherosclerosis [86]. Accordingly, ApoC-II was shown to co-localize with macrophages in murine arterial lesions [87]. Due to its pivotal role in maintaining lipid homeostasis, ApoC-II has attracted considerable attention as a potential therapeutic strategy in CVD [88]. Although the mechanistic insights of ApoC-II and its association with atherosclerosis are not yet fully elucidated, further investigations in this field may lead to new therapeutic potentials aimed at ASCVD.
3.2. Apolipoprotein A-V as Positive Regulator of LPL Mediated Lipolysis
ApoA-V was first identified by Vliet and colleagues [89]. In vivo studies showed that ApoA-V−/− mice had four times higher serum TG levels compared to littermate controls, indicating a possible role in TG metabolism [90]. An independent study on ApoA-V−/− mice confirmed the beneficial impact of ApoA-V on TG hydrolysis and clearance of lipoprotein remnants [91]. This impact was underscored by lowered TG levels in transgenic mice overexpressing ApoA-V [92]. In humans, genome-wide association studies (GWAS) found associations between common variants of APOA5 and HTG [93]. In aggregate, ApoA-V has a positive impact on intravascular lipid homeostasis.
ApoA-V is only expressed in the liver [89] and is found integrated in circulating VLDL, chylomicrons, and HDL particles [94]. It is highly hydrophobic and its mature form (343 amino acids) is predicted to have a high α-helix propensity [95]. With a view to its pronounced impact on intravascular lipolysis homeostasis, the plasma concentration of ApoA-V is intriguingly low (24–400 ng/mL), which equals one ApoA-V molecule per 1000 VLDL [94].
Circumstantial biochemical evidence suggests that ApoA-V may interact with LDL-R family receptors and GPIHBP1, but the functional impact of such interactions remains unclear primarily due to the vast molar excess of these potential binding partners [36,96]. In vitro assays suggest that ApoA-V at concentration >3 µM inhibits LPL, which is supraphysiologic concentration and in vivo effect is therefore questionable [97]. A more plausible mode of action for ApoA-V in intravascular lipolysis has recently been formulated by Chen and coworkers, who took the low concentrations of plasma ApoA-V into account in their model [98]. They reported that ApoA-V interacts specifically and with very high affinity with ANGPTL3/ANGPTL8 complexes and in doing so it abrogates the LPL inhibitory effect of that complex [98].
The impact of ApoA-V on TG metabolism and atherosclerosis is also evident from in vivo mice studies [99,100]. In a hypercholesterolemic mouse model (ApoE−/−), the presence of ApoA-V reduced both TG and cholesterol levels in plasma when they were fed a regular chow diet and the clearance rate of remnant particles was increased two-fold higher [100,101]. HSPGs, most notably SCD1, are prominent endocytic receptors in liver involved in the clearance of TRL remnants [102,103]. Recently, it has been reported that ApoA-V binds to HSPGs and mediates the clearance of TRL remnant particles [101] and thus likely reducing the risk of atherosclerosis.
Given its important role in lipid metabolism, ApoA-V holds a strong therapeutic potential. In that setting, increasing plasma levels of ApoA-V by an intravenous injection of recombinant ApoA-V or by adeno-associated virus mediated gene transfer was beneficial in treating HTG [104,105,106].
3.3. Overexpression of Apolipoprotein C-I Leads to Severe HTG in Both LPL Dependent and LPL-Independent Manner
ApoC-I is the smallest and most basic of all the apolipoproteins [107] containing 57 amino acids in its mature form [108,109]. In plasma, ApoC-I is found associated to HDL during fasting, while in the postprandial state it moves from HDL to VLDLs and chylomicrons [110,111]. Despite being a small protein with two short α-helices, ApoC-I has a pleiotropic effect on lipoprotein metabolism [112]. It inhibits multiple enzymes involved in lipid metabolism such as LPL, hepatic lipase, phospholipase A2, and cholesteryl ester transfer protein (CETP) [113,114,115,116]. It also mediates the efflux of cholesterol from macrophages [117]. In vivo studies suggest that LPL inhibition by ApoC-I causes TG levels to increase in the setting of ApoC-I overexpression [113,118]. Another mechanism by which ApoC-I increases TG levels is by inhibiting ApoE-mediated hepatic uptake of IDL and VLDL via LRP1 and LDL-R [119,120]. ApoC-I also regulates HDL levels as it inhibits HDL uptake by scavenger receptor class B type 1 (SRB1) [121]. Finally, ApoC-I is a relatively poor activator of lecithin-cholesterol acyltransferase (LCAT), which is the enzyme responsible for converting cholesterol to cholesteryl ester [122]. Due to this pleiotropic effect on TGs and cholesterol regulation, investigators have tried to dissect ApoC-I’s potential role in atherosclerosis, which appears to be highly context dependent. A number of observations indicate that ApoC-I levels are elevated in atherosclerotic lesions which is predictive for disease progression and has a bearing on foam cell formation [87,123]. Increased ApoC-I content in remnant particles has been proposed as an independent marker for estimating risk of coronary heart disease (CHD) [124,125]. In postprandial TRLs, increased ApoC-I level is also associated with increased cholesterol content, further bolstering its role in atherosclerosis [125]. Another study reports reduction in LDL uptake by macrophages upon silencing of ApoC-I mRNA strengthening the pro-atherosclerotic role of ApoC-I [126]. Paradoxically, systemic ApoC-I levels are positively correlated to risk of atherosclerosis [117], while decreased level of ApoC-I in HDL particles is observed in patients with CHD [127].
Transgenic rabbits expressing human ApoC-I displayed a 22% reduction in atherosclerotic lesions and increased plasma HDL cholesterol when fed a diet high in cholesterol [128]. Rabbits on chow diet also displayed increased levels of HDL cholesterol (HDL-C) and 14% decrease in specific CETP activity [128]. Due to these deviating results, it is difficult to unambiguously assign the impact of ApoC-I as pro- or anti-atherosclerotic.
3.4. Apolipoprotein C-III Inhibits LPL and Blocks Hepatic Uptake of Remnant Particles
ApoC-III is one of the key regulators inhibiting triglyceride metabolism. Studies combining human genetics and epidemiology reveal that heterozygous missense variants in APOC3 are associated with a 44% decrease in non-fasting TGs levels and a significant reduction in the risk of CHD and ischemic vascular disease incidents [129]. Further consolidating this association, life-long deficiency of ApoC-III has a cardioprotective and anti-atherosclerotic profile [130], while increased ApoC-III level is associated with a higher risk of CHD [131]. ApoC-III contains 79 amino acids and associates mainly to VLDL, chylomicrons, HDL particles, and to a lower degree with LDL [132]. It inhibits LPL activity [133,134,135] and blocks the hepatic uptake of remnant particles [136]. Accordingly, elevated levels of ApoC-III in mice and humans are correlated to increased TG levels [131,137]. ApoC-III assists in the intracellular synthesis, assembly, and secretion of VLDLs [138]. In hypertriglyceridemia caused by elevated ApoC-III levels, the VLDL concentration in plasma increases due to a dual impact of ApoC-III: (i) decreased LPL activity and (ii) enhanced synthesis and hepatic secretion of VLDL. Although the molecular mechanism of LPL inhibition by ApoC-III are not fully elucidated, it may involve the competitive displacement of other apolipoproteins from the surface on TRL particles [139]. The involvement of ApoC-III in key steps of lipoprotein metabolism makes it a potential target for therapeutics aimed at treating residual CVD and atherosclerosis. Volanesorsen, is one such second-generation drug, targeting ApoC-III expression with antisense probes. As a supplement to dietary restrictions, it has proven highly beneficial in patients with familial chylomicronemia syndrome (FCS) lowering TGs levels by 70–80% [140,141] and this represents a promising start for future drugs targeting ApoC-III.
4. Regulation of LPL Lipolytic Activity by ANGPTLs
Intravascular lipolysis is strictly regulated by specific moderators of LPL activity. A tight temporal and spatial regulation of LPL ensures that the flux of FFA arrives at the right time in target tissues in need of energy supply. This anatomical regulation is mediated by members of the angiopoietin-like (ANGPTL) family, ANGPTL3, ANGPTL4, and ANGPTL8 (Figure 3) [142]. ANGPTL3 and ANGPTL4 are both composed by an N-terminal coiled-coil domain (CCD), a linker region, and a C-terminal fibrinogen-like domain (FLD), while ANGPTL8 lacks a FLD. ANGPTL3 and ANGPLT4 both inhibit LPL, but with vastly different efficacy, and recent studies have expanded the complexity of LPL regulation as ANGPTL8 binding increased the potency of ANGPTL3 and reduces that of ANGPLT4. Like other key players in TG metabolism, the atherosclerotic profiles for ANGPTLs are context dependent, highlighting the importance of understanding their biological and biochemistry.
4.1. ANGPTL4 Inhibits LPL Activity in WAT in the Fasting State
Human ANGPTL4 is a 50 kDa glycoprotein highly expressed in WAT, but also in other tissues/cell types such as liver and in macrophages [143]. The expression of ANGPTL4 is induced by exercise or low serum lipid levels [144,145,146,147]. This ensures that LPL activity in WAT is suppressed when serum TGs levels are low (fasting), prioritizing energy uptake in oxidative tissues. Conversely, in the fed state, where serum lipid levels are abundant, ANGPTL4 expression is decreased in WAT, allowing replenishing of the energy storage [147,148]. ANGPTL4 levels directly affect plasma TGs levels as shown in Angptl4−/− mice that have low serum TG levels as a consequence of unchecked LPL activity in WAT [147,148]. The observation that levels of circulating ANGPTL4 rarely correlate with plasma TG levels appeared at first sight enigmatic [10,149,150]. This paradox was, however, rooted in the intracellular action of ANGPTL4 in WAT where it primes LPL to degradation by promoting PCSK3 cleavage and subsequent degradation [146,151,152]. Recently, it was suggested that ANGPTL4-mediated unfolding of LPL sensitized it to cleavage by PCSK3 [65]. Notwithstanding this intracellular effect, freshly secreted LPL bound to cell surfaces in the subendothelial space is also targeted by ANGPTL4 [57,153].
The molecular mechanism of ANGPTL4-mediated LPL inhibition represents a novel mode of action for enzyme inhibition. ANGPTL4 binds close to the active site in LPL and this binding event triggers the progressive and irreversible unfolding of LPL’s α/β hydrolase domain [17,59,62,154]. Accordingly, ANGPTL4 was dubbed a “molecular unfolding chaperone” and its catalytic function is evident by the fact that sub-stoichiometric amounts of ANGPTL4 over time causes a complete LPL inhibition in vitro [59,154]. Importantly, this irreversible inactivation by unfolding is mitigated by binding to GPIHBP1 [17,57,154,155].
In human genetic and epidemiologic studies, ANGPTL4 was shown to affect plasma TGs levels. Function impairing missense variations in ANGPTL4 (e.g., ANGPTL4 E40K) thus correlated with increased plasma levels of TGs and HDL-C and furthermore decreased the risk of CVD [9,10,156,157]. As the corresponding ANGPTL4-E15K protein variant has impaired inhibition of LPL [154,157], these studies advance the proposition that a systemic lowering of the plasma TGs, by boosting LPL activity via a reduced ANGPTL4 inhibition, has therapeutic potential. Unfortunately, Angptl4−/− mice kept on a high fat diet becomes moribund due to excessive intestinal inflammation and fibrosis [158]. This unexpected pathology arose, since ANGPTL4 prevents lipid overload in mesenteric macrophages during feeding and in the absence of ANGPTL4 protection these macrophages transform into foam cells associated with chronic inflammatory lesions [158,159,160]. This case story underscores the pivotal importance of understanding the normal biology of the protein to be targeted by a given intervention strategy.
4.2. ANGPTL3 Inhibits Lipoprotein Lipase and Endothelial Lipase
Human ANGPTL3 is a 62 kDa glycoprotein expressed in the liver [143,161,162]. ANGPTL3 regulates VLDL levels by inhibiting LPL [163] and plasma HDL levels by inhibiting endothelial lipase (EL) [164]. EL hydrolyses HDL-bound phospholipids and promotes HDL clearance [165,166]. The impact of ANGPTL3 was further studied in a mouse model of atherosclerosis (ApoE−/− background), where the Angptl3−/− resulted in a significant reduction in the atherosclerotic lesions [7]. This combined effect of ANGPTL3 loss-of-function on LPL and EL regulation was underscored by other investigations finding low circulating concentrations of LDL-C, HDL-C and TGs [162,167,168,169]. Furthermore, human subjects with circulating ANGPTL3 concentrations in the lowest tertile had reduced risk of myocardial infarction compared to subjects in the highest tertile [8].
ANGPTL3 is sensitive to cleavage by proprotein convertases between its N-terminal and C-terminal domain [170], which reduces its potency towards LPL inhibition [171]. ANGPTL3 inhibition of LPL sensitizes LPL to cleavage by proprotein convertases [172] as found with ANGPTL4-mediated LPL inhibition (Dijk 2016; Lund Winther 2021). Exactly how ANGPTL3 inhibits LPL and EL still needs to be clarified, but the first α-helix in ANGPTL3 contains an inhibitory LPL motif [173] proximal to a putative heparin binding motif [164,170,172].
Besides inhibiting LPL and EL, ANGPTL3 may have unrelated pro-atherogenic effects [174] such as arterial wall thickening [175], and promoting angiogenesis via integrin αVβ3 [176].
4.3. ANGPTL8 Forms Complexes with ANGPTL3 and ANGPTL4
ANGPTL8 is a 22 kDa protein that has very limited inhibitory effect towards LPL on its own [177,178] and overexpression of ANGPTL8 does not decrease TGs levels in Angptl3−/− mice as it does in wt mice [179]. The reason for this difference is the obligate requirement of ANGPTL8 to enter a 1:3 complex with ANGPTL3 to become secreted and gain a high potency in LPL inhibition. ANGPTL8 can also forms a 1:1 complex with ANGPTL4 [180]. While ANGPTL3/8 is a potent inhibitor of LPL, ANGPTL4/8 is a very poor inhibitor and the ANGPTL3/8 and ANGPTL4/8 levels are positively correlated with circulating TGs [180]. In the fed state, ANGPTL4 expression is downregulated in WAT and hepatic secretion of the ANGPTL3/ANGPTL8 complex is increased [146,147,181]. This promotes LPL activity in WAT, and ANGPTL3/ANGPTL8 mediated LPL suppression in heart and skeletal muscle. In the fasted state, this scenario is reversed as ANGPTL8 is downregulated and ANGPTL4 upregulated, which provides an increased flux of FA into adipose tissue [148,182].
5. Dysfunctional LPL and Hypertriglyceridemia
Homozygous or compound heterozygous individuals with loss-of-function mutations in LPL, ApoC-II, ApoA-V, LMF1, and GPIHBP1 develop a rare monogenic disorder known as familial chylomicronaemia syndrome (FCS). The disorder is defined by an excessive accumulation of chylomicrons with clinical symptoms such as eruptive xanthomas and acute pancreatitis that are associated with very high serum TGs concentrations (>2000 mg/dL) [183,184,185]. The more common polygenic variant of HTG is caused by genetic factors in combination with secondary factors such as type 2 diabetes, obesity, renal disease, pregnancy, and alcohol intake and is characterized by increased plasma levels of TRLs and reduced levels of HDL-C [186]. GWAS identified an association between 32 common gene variants and elevated plasma TGs concentrations and in many cases increased risk of CVD. These variants include ApoA-V, LPL, and ApoB, which are all strong determinants of polygenic HTG [187]. Of the more than 100 LPL variants that have been identified, a subset has been classified as pathogenic. The more common LPL variants (LPL-N291A, LPL-D9N, and LPL-G188E) are associated with mildly increased TGs levels [188] and generate an atherogenic lipoprotein profile [5]. On the contrary, another common LPL variant with gain-of-function characteristics (LPL-S447X), is associated with decreased plasma TGs levels compared to non-carriers [188] and lower risk of CHD [189]. The beneficial properties of this variant were exploited in a LPL gene therapy strategy (Alipogene tiparvovec) for treatment of patients with LPL deficiency. The trials demonstrated transiently reduced fasting plasma triglyceride levels compared to baseline after 12 weeks of administration, and reduction in pancreatitis incidences [190]. Despite these promising results, Alipogene tiparvovec was discontinued as therapy due to excessive costs. Currently, first line of treatment for HTG is dietary and lifestyle interventions and control of secondary factors. Recent developments have nonetheless provided new treatment options for patients with monogenic HTG as exemplified by the approval of Volanesorsen, an ApoC-III targeting drug, for the treatment of adults with FCS [141].
6. Clearance of Processed Lipoproteins and Risk of Atherosclerosis
After intravascular processing of the marginated TRLs, the remaining lipoprotein (remnant particle) detaches from the endothelial cells along with some inactive LPL molecules still attached. That inactive LPL acts as a ligand for a number of internalization receptors present on hepatocytes, facilitating the hepatic uptake of remnant particles [191,192]. These receptors include LDL receptor, VLDL receptor, LRP1 and SCD1 [193,194,195,196,197,198,199]. A possible downside of this mechanism, is that, LPL may act to promote LDL and VLDL retention in the subendothelial cell matrix thus delaying their return to the circulation by endothelial transcytosis [200]. Such LPL-mediated LDL retention represents a possible pathogenic factor for the progression of the atherosclerotic lesions as it promotes lipoprotein uptake in macrophages increasing the risk of foam cell formation and inflammation [12]. In the arterial intima, LPL facilitates this temporal sequestering of lipoproteins by its strong ionic interactions with proteoglycans such as biglycan, versican, and collagen XVIII [201,202]. Excessive accumulation of lipoproteins in the subendothelial space leads to fatty streaks, which is a hallmark of early precursor lesions in atherogenesis leading to plaque formation and progression [203,204]. When macrophages are recruited to lipid-laden precursor lesions in the arterial intima, they start phagocytosing the dense lipid depositions and transform into foam cells. This elicits a number of pathogenic responses ultimately leading to chronic inflammation: (i) excessive lipid overload in macrophages impairs cholesterol efflux [205] and (ii) secretion of pro-inflammatory cytokines and ROS upon macrophage activation by oxLDL [206,207]. Furthermore, these foam cells secrete a number of proteases that degrade the extracellular matrix weakening the plaque structure provoking its rupture with severe cardiovascular damage and risk of thromboembolism [208,209]. Studies in mice show that macrophages display increased LPL expression in early atherosclerosis [210] and the enhanced LPL expression accelerates foam cell formation [211,212]. Importantly, mice with Lpl−/− confined to macrophages have reduced lesion sizes and lower diet-induced atherosclerosis [213,214].
7. Remnant Particles and Their Role in Atherosclerosis
Very severe HTG characterized by the accumulation of chylomicrons and large VLDLs is generally not considered pro-atherogenic since these particles are too large to be trancytosed across endothelial cells and enter the arterial intima. Remnant lipoproteins, however, with a diameter <70 nm can be transcytosed [215]. IDL and small VLDL particles readily enter the arterial intima and are hence considered atherogenic [129]. The high atherogenic impact of VLDLs and IDLs in arterial intima is underscored by the finding that the majority of ApoB isolated from human atherosclerotic plaques is derived from VLDL and IDL lipoproteins rather than LDL [216]. Studies on hyperlipidemic rabbits show that both VLDL and IDL particles enter and are retained in the arterial intima to the same extent as LDL particles [217]. Other studies claim remnant particles to be more atherogenic than LDL itself [218]. This proposition is underscored by two observations. First, remnant particles do not need to be oxidized before they are taken up by macrophages in the arterial intima [218,219]. Second, they carry approximately 40 times more cholesterol per remnant particle compared to LDL particles [215]. Previously, oxLDL and chylomicron remnants were considered the main atherogenic lipoproteins, but VLDL remnants may have an even greater atherogenic potential [220].
The formation of remnant particles is an integral part of intravascular lipolysis and represents a byproduct of normal lipid homeostasis. Therapeutic interventions preventing the retention of remnant particles in the arterial intima appear more straightforward than limiting the production of remnant particles. One such therapeutic approach is to block or reduce binding of ApoB to HSPGs in the subendothelial spaces. ApoB exists in two isoforms on lipoprotein particles, ApoB-48 and ApoB-100, both of which are encoded by the same gene and play a crucial role in lipid homeostasis [221]. ApoB-100 is a very large protein containing 4536 amino acids [222]. It is expressed in liver and all lipoproteins synthesized by hepatocytes (VLDL) contain ApoB-100 [222]. On the other hand, the shorter isoform ApoB-48 is found on lipoproteins of intestinal origin [223]. ApoB-100 is required for the synthesis, assembly and secretion of TRLs originating from liver. Loss-of-function variants in APOB cause hypobetalipoproteinemia, normotriglyceridemic hypobetalipoproteinemia, and hypercholesterolemia—diseases affecting plasma cholesterol and ApoB levels. In aggregate, these observations highlight the potential of ApoB as a clinical biomarker in diagnostics as well as therapeutic target. Mipomersen is an FDA-approved second generation antisense oligonucleotide, that has been designed to reduce plasma concentration of ApoB-100 [224]. Since ApoB-100 is an integrated component of all atherogenic lipoproteins (including LDL), use of Mipomersen may also reduce the risk of atherosclerosis.
Increase Triglyceride Levels—A Residual Risk in Atherosclerosis
Epidemiological studies suggest that triglycerides and remnant cholesterol are independent risk factors for atherosclerotic cardiovascular disease [225]. Therapeutic interventions have focused on lowering LDL-C levels with statins, cholesterol absorption inhibitors, bile acid sequestrants, and proprotein convertase subtilisin/kexin type-9 (PCSK-9) inhibitors [226]. Statins have proven remarkably efficient in lowering LDL-C [227]. Although, the impressive results from statin therapy are indisputable, they do not represent a universal remedy to patients with atherosclerosis and CVD. Even when treated with the recommended statin levels, some patients require additional lipid lowering therapies and others simply do not respond to statin treatment. One such scenario is patients suffering from familial hypercholesterolaemia, a common genetic disorder, where statin monotherapy does not reach target LDL-C levels [228]. In some cases, even after lowering LDL-C levels to desired level, residual cardiovascular risk still remains, leading to ASCVD [229]. This has led researchers to target atherosclerosis from other therapeutic angles by looking further into disease mechanism(s).
A prime focus has been on the role of HDL-C in atherosclerosis and as a potential therapeutic target for treatment of ASCVD. Low HDL-C has been shown to be a strong independent predictor of premature atherosclerosis, but very high levels of HDL-C were not athero-protective [230]. The role of HDL-C in atherosclerosis is still under debate and it has been suggested that the proportion of dysfunctional HDL versus functional HDL rather than total HDL-C levels may be of importance [231].
Currently, the aim of reducing plasma TGs levels gains momentum as it may have additional benefits in slowing atherosclerotic progression. The association between impaired LPL activity and risk of ASCVD were evaluated in a meta-analysis showing that increased risk of CHD was found in carriers of loss-of-function LPL variants [5]. The association between impaired intravascular lipolysis and atherosclerosis was further strengthened by meta-analysis showing that increased levels of plasma triglycerides (TGs) is a risk factor for cardiovascular disease [4] and for coronary artery disease [6]. Residual HTG persist in 20% of U.S. adults with diabetes, including those on statin therapy with well-controlled LDL-C and these patients are at moderate to high 10-year risk for ASCVD [232]. Elevated triglyceride levels are concordantly considered a residual risk factor for the atherosclerosis [225].
8. Conclusions
Several different strategies are being exploited for the treatment of severe HTG to reduce the risk of atherosclerosis. This includes monoclonal antibody therapy, antisense oligonucleotide therapy, and gene therapy. The antisense oligonucleotide therapy Mipomersen targeting APOB was approved by FDA in 2013 as lipid-lowering medication. Unfortunately, adverse effects combined with high withdrawal rate due to intolerance led to the withdrawal of Mipomersen in 2019. More promising are the APOC-III targeting antisense oligonucleotide therapies, such as Volanesorsen, which received approval in EU for the treatment of adult patients with FCS [141] and has paved the way for a next-generation of ligand-based antisense oligonucleotide targeting APOC-III (AKCEA-APOCIII-LRX). Recently, targeting ANGPTL3 and ANGPTL8 to lower circulating TGs levels in the treatment of dyslipidemia is also pursued. An explorative novel approach is to target dyslipidemia using a variation of the CRISPR-Cas9 platform permanently downregulating Angptl3 by introducing non-sense mutations [233]. More mature drug development programs targets ANGPTL3 utilizing monoclonal antibody (Evinacumab) or antisense oligonucleotide treatment (ANGPTL3-LRx) and are in clinical phase 2. These drugs retard progression of atherosclerosis in mice and reduce levels of atherogenic lipoproteins in mice, monkeys, and humans [11,234,235,236]. Conclusive data of phase 3 clinical trials are still needed to define their efficacy profiles. Targeting ANGPTL8 is also exploited in early pre-clinical studies with antibodies that neutralize ANGPTL8 and increase cardiac LPL activity and decrease plasma TGs levels in mice and monkeys with beneficial effects on body weight [237,238].
Targeting ANGPTL3 or ANGPTL8 represents promising strategies for the development of new treatment of dyslipidemia. This line of work was recently encouraged by data from combination therapies using statins, antibodies targeting PCSK9, and ANGPTL3 to treat mice with atherosclerotic cardiovascular disease. Statins and PCSK9 antibodies reduce LDL-C plasma levels, while the monoclonal antibody against ANGPTL3 increase VLDL and remnant particle hydrolysis by LPL thereby decreasing the circulating TGs levels in plasma. As only the triple-targeting regime regressed atherosclerotic lesion area and improved lesion composition, this approach seems promising for treating atherosclerosis [239]. These novel approaches may be a future treatment strategy for dyslipidemia when further developed.
Abbreviations
| ANGPTLs | Angiopoietin-like proteins |
| Apo | Apolipoproteins |
| ASCVD | Atherosclerotic cardiovascular disease |
| CCD | Coiled-coil domain |
| CETP | Cholesteryl ester transfer protein |
| CHD | Coronary heart disease |
| CVD | Cardiovascular disease |
| DSF | Differential scanning fluorimetry |
| EL | Endothelial lipase |
| FCS | Familial chylomicronemia syndrome |
| FFA | Free fatty acids |
| FLD | Fibrinogen-like domain |
| GPIHBP1 | Glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 |
| GWAS | Genome-wide association studies |
| HDL | High-density lipoproteins |
| HDL-C | High-density lipoproteins cholesterol |
| HDX-MS | Hydrogen-deuterium exchange coupled to mass spectrometry |
| HSPGs | Heparans sulfate proteoglycans |
| HTG | Hypertriglyceridemia |
| IDL | Intermediate-density lipoprotein |
| LCAT | Lecithin-cholesterol acyltransferase |
| LDL | Low density lipoprotein |
| LDL-C | Low density lipoprotein cholesterol |
| LDL-R | LDL-receptor |
| LMF1 | Lipase maturation factor |
| LPL | Lipoprotein lipase |
| LRP1 | LDL receptor related protein 1 |
| LU | Ly6/uPAR protein domain family |
| PLAT | Polycystin-1, Lipoxygenase, Alpha-Toxin |
| SCD1 | Syndecan-1 |
| Sel1L | Suppressor of lin-12-like protein 1 |
| SKM | Skeletal muscle |
| SRB1 | Scavenger receptor class B type 1 |
| TG | Triglyceride |
| TRL | Triglyceride-rich lipoprotein |
| VLDL | Very low-density lipoproteins |
| WAT | White adipose tissue |
Author Contributions
A.K., K.K.K., M.P., A.-M.L.W., writing—review and editing, K.K.K., A.-M.L.W., visualization, M.P., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the authors’ laboratories that was instrumental for writing this review was supported by grants from the Lundbeck Foundation (R230-2016-2930), NOVO Nordisk Foundation Grants (NNF17OC0026868, NNF18OC0033864, NNF20OC0063444), The John and Birthe Meyer Foundation, a Transatlantic Network grant (12CVD04) from the Leducq Foundation, the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 801481, The Capital Region (RegionH) grant no A6867).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cox M., Nelson D.L. Lehninger Principles of Biochemistry. 4th ed. W.H. Freeman; New York, NY, USA: 2005. [Google Scholar]
- 2.Bayly G.R. Clinical Biochemistry: Metabolic and Clinical Aspects. Elsevier; New York, NY, USA: 2014. Lipids and disorders of lipoprotein metabolism; pp. 702–736. [Google Scholar]
- 3.Nordestgaard B.G. A Test in Context: Lipid Profile, Fasting Versus Nonfasting. J. Am. Coll. Cardiol. 2017;70:1637–1646. doi: 10.1016/j.jacc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Hokanson J.E., Austin M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A metaanalysis of population-based prospective studies. Eur. J. Cardiovasc. Prev. Rehabil. 1996;3:213–219. doi: 10.1177/174182679600300214. [DOI] [PubMed] [Google Scholar]
- 5.Wittrup H.H., Tybjærg-Hansen A., Nordestgaard B.G. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease: A meta-analysis. Circulation. 1999;99:2901–2907. doi: 10.1161/01.CIR.99.22.2901. [DOI] [PubMed] [Google Scholar]
- 6.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S.M., Khaw K.T., Gudnason V. Triglycerides and the risk of coronary heart disease: 10 158 Incident cases among 262 525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 7.Ando Y., Shimizugawa T., Takeshita S., Ono M., Shimamura M., Koishi R., Furukawa H. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipid Res. 2003;44:1216–1223. doi: 10.1194/jlr.M300031-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Stitziel N.O., Khera A.V., Wang X., Bierhals A.J., Vourakis A.C., Sperry A.E., Natarajan P., Klarin D., Emdin C.A., Zekavat S.M., et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J. Am. Coll. Cardiol. 2017;69:2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey F.E., Gusarova V., O’Dushlaine C., Gottesman O., Trejos J., Hunt C., Van Hout C.V., Habegger L., Buckler D., Lai K.-M.V., et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N. Engl. J. Med. 2016;374:1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smart-Halajko M.C., Kelley-Hedgepeth A., Montefusco M.C., Cooper J.A., Kopin A., McCaffrey J.M., Balasubramanyam A., Pownall H.J., Nathan D.M., Peter I., et al. ANGPTL4 variants E40K and T266M are associated with lower fasting triglyceride levels in Non-Hispanic White Americans from the Look AHEAD Clinical Trial. BMC Med. Genet. 2011;12:89. doi: 10.1186/1471-2350-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewey F.E., Gusarova V., Dunbar R.L., O’Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C.V., Bruse S., Dansky H.M., et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson M., Levin M., Skålén K., Perman J., Fridén V., Jirholt P., Olofsson S.O., Fazio S., Linton M.F., Semenkovich C.F., et al. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: Evidence for a role of lipoprotein lipase. Circ. Res. 2007;101:777–783. doi: 10.1161/CIRCRESAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 13.Korn E.D. Clearing factor, a Heparin-activated lipoprotein lipase: II. Substrate Specificity and activation of coconut oil. J. Biol. Chem. 1955;215:15–26. doi: 10.1016/S0021-9258(18)66012-0. [DOI] [PubMed] [Google Scholar]
- 14.Kirchgessner T.G., LeBouef R.C., Langner C.A., Zollman S., Chang C.H., Taylor B.A., Schotz M.C., Gordon J.I., Lusis A.J. Genetic and developmental regulation of the lipoprotein lipase gene: Loci both distal and proximal to the lipoprotein lipase structural gene control enzyme expression. J. Biol. Chem. 1989;264:1473–1482. doi: 10.1016/S0021-9258(18)94212-2. [DOI] [PubMed] [Google Scholar]
- 15.Scow R.O., Olivecrona T. Effect of albumin on products formed from chylomicron triacylglycerol by lipoprotein lipase in vitro. Biochim. Biophys. Acta Lipids Lipid Metab. 1977;487:472–486. doi: 10.1016/0005-2760(77)90217-X. [DOI] [PubMed] [Google Scholar]
- 16.Brunzell J.D., Hazzard W.R., Porte D., Bierman E.L. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J. Clin. Investig. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leth-Espensen K.Z., Kristensen K.K., Kumari A., Winther A.-M.L., Young S.G., Jørgensen T.J.D., Ploug M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc. Natl. Acad. Sci. USA. 2021;118:e2026650118. doi: 10.1073/pnas.2026650118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birrane G., Beigneux A.P., Dwyer B., Strack-Logue B., Kristensen K.K., Francone O.L., Fong L.G., Mertens H.D.T., Pan C.Q., Ploug M., et al. Structure of the lipoprotein lipase–GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc. Natl. Acad. Sci. USA. 2019;116:1723–1732. doi: 10.1073/pnas.1817984116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen K.K., Midtgaard S.R., Mysling S., Kovrov O., Hansen L.B., Skar-Gislinge N., Beigneux A.P., Kragelund B.B., Olivecrona G., Young S.G., et al. A disordered acidic domain in GPIHBP1 harboring a sulfated tyrosine regulates lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 2018;115:E6020–E6029. doi: 10.1073/pnas.1806774115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mysling S., Kristensen K.K., Larsson M., Beigneux A.P., Gårdsvoll H., Fong L.G., Bensadouen A., Jørgensen T.J., Young S.G., Ploug M. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. Elife. 2016;5:e12095. doi: 10.7554/eLife.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmerich J., Beg O.U., Peterson J., Previato L., Brunzell J.D., Brewer H.B., Santamarina-Fojo S. Human lipoprotein lipase. Analysis of the catalytic triad by site-directed mutagenesis of Ser-132, Asp-156, and His-241. J. Biol. Chem. 1992;267:4161–4165. doi: 10.1016/S0021-9258(19)50642-1. [DOI] [PubMed] [Google Scholar]
- 22.Dugi K.A., Dichek H.L., Talley G.D., Brewer H.B., Santamarina-Fojo S. Human lipoprotein lipase: The loop covering the catalytic site is essential for interaction with lipid substrates. J. Biol. Chem. 1992;267:25086–25091. doi: 10.1016/S0021-9258(19)74009-5. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y., Henderson H.E., Liu M.S., Zhang H., Forsythe I.J., Clarke-Lewis I., Hayden M.R., Brunzell J.D. Mutagenesis in four candidate heparin binding regions (residues 279–282, 291–304, 390–393, and 439–448) and identification of residues affecting heparin binding of human lipoprotein lipase. J. Lipid Res. 1994;35:2049–2059. doi: 10.1016/S0022-2275(20)39951-X. [DOI] [PubMed] [Google Scholar]
- 24.Lookene A., Nielsen M.S., Gliemann J., Olivecrona G. Contribution of the carboxy-terminal domain of lipoprotein lipase to interaction with heparin and lipoproteins. Biochem. Biophys. Res. Commun. 2000;271:15–21. doi: 10.1006/bbrc.2000.2530. [DOI] [PubMed] [Google Scholar]
- 25.Lutz E.P., Merkel M., Kako Y., Melford K., Radner H., Breslow J.L., Bensadoun A., Goldberg I.J. Heparin-binding defective lipoprotein lipase is unstable and causes abnormalities in lipid delivery to tissues. J. Clin. Investig. 2001;107:1183–1192. doi: 10.1172/JCI11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olafsen T., Young S.G., Davies B.S.J., Beigneux A.P., Kenanova V.E., Voss C., Young G., Wong K.P., Barnes R.H., Tu Y., et al. Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) in mouse tissues revealed by positron emission tomography scanning. J. Biol. Chem. 2010;285:39239–39248. doi: 10.1074/jbc.M110.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterniti J.R., Brown W.V., Ginsberg H.N., Artzt K. Combined lipase deficiency (cld): A lethal mutation on chromosome 17 of the mouse. Science. 1983;221:167–169. doi: 10.1126/science.6857276. [DOI] [PubMed] [Google Scholar]
- 28.Péterfy M., Ben-Zeev O., Mao H.Z., Weissglas-Volkov D., Aouizerat B.E., Pullinger C.R., Frost P.H., Kane J.P., Malloy M.J., Reue K., et al. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat. Genet. 2007;39:1483–1487. doi: 10.1038/ng.2007.24. [DOI] [PubMed] [Google Scholar]
- 29.Sha H., Sun S., Francisco A.B., Ehrhardt N., Xue Z., Liu L., Lawrence P., Mattijssen F., Guber R.D., Panhwar M.S., et al. The ER-associated degradation adaptor protein sel1l regulates LPL secretion and lipid metabolism. Cell Metab. 2014;20:458–470. doi: 10.1016/j.cmet.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundberg E.L., Deng Y., Burd C.G. Syndecan-1 Mediates Sorting of Soluble Lipoprotein Lipase with Sphingomyelin-Rich Membrane in the Golgi Apparatus. Dev. Cell. 2019;51:387.e4–398.e4. doi: 10.1016/j.devcel.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cefalù A.B., Noto D., Arpi M.L., Yin F., Spina R., Hilden H., Barbagallo C.M., Carroccio A., Tarugi P., Squatrito S., et al. Novel LMF1 nonsense mutation in a patient with severe hypertriglyceridemia. J. Clin. Endocrinol. Metab. 2009;94:4584–4590. doi: 10.1210/jc.2009-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allan C.M., Larsson M., Jung R.S., Ploug M., Bensadoun A., Beigneux A.P., Fong L.G., Young A.S.G. Mobility of “HSPG-bound” LPL explains how LPL is able to reach GPIHBP1 on capillaries. J. Lipid Res. 2017;58:216–225. doi: 10.1194/jlr.M072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Rodrigues B. Intrinsic and extrinsic regulation of cardiac lipoprotein lipase following diabetes. Biochim. Biophys. Acta. 2014;1851:163–171. doi: 10.1016/j.bbalip.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Bishop J.R., Passos-Bueno M.R., Fong L., Stanford K.I., Gonzales J.C., Yeh E., Young S.G., Bensadoun A., Witztum J.L., Esko J.D., et al. Deletion of the basement membrane heparan sulfate proteoglycan type XVIII collagen causes hypertriglyceridemia in mice and humans. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies B.S.J., Beigneux A.P., Barnes R.H., Tu Y., Gin P., Weinstein M.M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beigneux A.P., Davies B.S.J., Gin P., Weinstein M.M., Farber E., Qiao X., Peale F., Bunting S., Walzem R.L., Wong J.S., et al. Glycosylphosphatidylinositol-Anchored High-Density Lipoprotein-Binding Protein 1 Plays a Critical Role in the Lipolytic Processing of Chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng X., Zeng W., Young S.G., Fong L.G. GPIHBP1, a partner protein for lipoprotein lipase, is expressed only in capillary endothelial cells. J. Lipid Res. 2020;61:591. doi: 10.1194/jlr.ILR120000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ioka R.X., Kang M.J., Kamiyama S., Kim D.H., Magoori K., Kamataki A., Ito Y., Takei Y.A., Sasaki M., Suzuki T., et al. Expression cloning and characterization of a novel glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein, GPI-HBP1. J. Biol. Chem. 2003;278:7344–7349. doi: 10.1074/jbc.M211932200. [DOI] [PubMed] [Google Scholar]
- 39.Olivecrona G., Ehrenborg E., Semb H., Makoveichuk E., Lindberg A., Hayden M.R., Gin P., Davies B.S.J., Weinstein M.M., Fong L.G., et al. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J. Lipid Res. 2010;51:1535–1545. doi: 10.1194/jlr.M002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charrière S., Peretti N., Bernard S., Di Filippo M., Sassolas A., Merlin M., Delay M., Debard C., Lefai E., Lachaux A., et al. GPIHBP1 C89F neomutation and hydrophobic C-terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia. J. Clin. Endocrinol. Metab. 2011;96:1675–1679. doi: 10.1210/jc.2011-1444. [DOI] [PubMed] [Google Scholar]
- 41.Rios J.J., Shastry S., Jasso J., Hauser N., Garg A., Bensadoun A., Cohen J.C., Hobbs H.H. Deletion of GPIHBP1 causing severe chylomicronemia. J. Inherit. Metab. Dis. 2012;2:531–540. doi: 10.1007/s10545-011-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plengpanich W., Young S.G., Khovidhunkit W., Bensadoun A., Karnman H., Ploug M., Gardsvoll H., Leung C.S., Adeyo O., Larsson M., et al. Multimerization of GPIHBP1 and Familial Chylomicronemia from a Serine-to-Cysteine Substitution in GPIHBP1’s Ly6 Domain. J. Biol. Chem. 2014;289:19491–19499. doi: 10.1074/jbc.M114.558528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franssen R., Young S.G., Peelman F., Hertecant J., Sierts J.A., Schimmel A.W.M., Bensadoun A., Kastelein J.J.P., Fong L.G., Dallinga-Thie G.M., et al. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ. Cardiovasc. Genet. 2010;3:169–178. doi: 10.1161/CIRCGENETICS.109.908905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beigneux A.P., Miyashita K., Ploug M., Blom D.J., Ai M., Linton M.F., Khovidhunkit W., Dufour R., Garg A., McMahon M.A., et al. Autoantibodies against GPIHBP1 as a Cause of Hypertriglyceridemia. N. Engl. J. Med. 2017;376:1647–1658. doi: 10.1056/NEJMoa1611930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutz J., Dunaj-Kazmierowska M., Arcan S., Kassner U., Miyashita K., Murakami M., Ploug M., Fong L.G., Young S.G., Nakajima K., et al. Chylomicronemia From GPIHBP1 Autoantibodies Successfully Treated With Rituximab: A Case Report. Ann. Intern. Med. 2020;173:764–765. doi: 10.7326/L20-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goulbourne C.N., Gin P., Tatar A., Nobumori C., Hoenger A., Jiang H., Grovenor C.R.M., Adeyo O., Esko J.D., Goldberg I.J., et al. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 2014;19:849–860. doi: 10.1016/j.cmet.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gin P., Beigneux A.P., Voss C., Davies B., Beckstead J.A., Ryan R.O., Bensadoun A., Fong L.G., Young S.G. Binding preferences for GPIHBP1, a glycosylphosphatidylinositol-anchored protein of capillary endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2011;31:176–182. doi: 10.1161/ATVBAHA.110.214718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dugi K.A., Dichek H.L., Santamarina-Fojo S. Human hepatic and lipoprotein lipase. The loop covering the catalytic site mediates lipase substrate specificity. J. Biol. Chem. 1995;270:25396–25401. doi: 10.1074/jbc.270.43.25396. [DOI] [PubMed] [Google Scholar]
- 49.Keiper T., Schneider J.G., Dugi K.A. Novel site in lipoprotein lipase (LPL415-438) essential for substrate interaction and dimer stability. J. Lipid Res. 2001;42:1180–1186. doi: 10.1016/S0022-2275(20)31567-4. [DOI] [PubMed] [Google Scholar]
- 50.Van Tilbeurgh H., Roussel A., Lalouel J.M., Cambillau C. Lipoprotein lipase. Molecular model based on the pancreatic lipase X-ray structure: Consequences for heparin binding and catalysis. J. Biol. Chem. 1994;269:4626–4633. doi: 10.1016/S0021-9258(17)41822-9. [DOI] [PubMed] [Google Scholar]
- 51.Williams S.E., Inoue I., Tran H., Fry G.L., Pladet M.W., Iverius P.H., Lalouel J.M., Chappell D.A., Strickland D.K. The carboxyl-terminal domain of lipoprotein lipase binds to the low density lipoprotein receptor-related protein/α2-macroglobulin receptor (LRP) and mediates binding of normal very low density lipoproteins to LRP. J. Biol. Chem. 1994;269:8653–8658. doi: 10.1016/S0021-9258(17)37017-5. [DOI] [PubMed] [Google Scholar]
- 52.Lookene A., Groot N.B., Kastelein J.J.P., Olivecrona G., Bruin T. Mutation of tryptophan residues in lipoprotein lipase: Effects on stability, immunoreactivity, and catalytic properties. J. Biol. Chem. 1997;272:766–772. doi: 10.1074/jbc.272.2.766. [DOI] [PubMed] [Google Scholar]
- 53.Kristensen K.K., Leth-Espensen K.Z., Mertens H.D.T., Birrane G., Meiyappan M., Olivecrona G., Jørgensen T.J.D., Young S.G., Ploug M. Unfolding of monomeric lipoprotein lipase by ANGPTL4: Insight into the regulation of plasma triglyceride metabolism. Proc. Natl. Acad. Sci. USA. 2020;117:4337–4346. doi: 10.1073/pnas.1920202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He C., Weston T.A., Jung R.S., Fong L.G., Young S.G., Jiang H., He C., Weston T.A., Jung R.S., Heizer P., et al. NanoSIMS Analysis of Intravascular Lipolysis and Lipid Movement across Capillaries and into Article NanoSIMS Analysis of Intravascular Lipolysis and Lipid Movement across Capillaries and into Cardiomyocytes. Cell Metab. 2018;27:1055.e3–1066.e3. doi: 10.1016/j.cmet.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaffer J.E. Death by lipids: The role of small nucleolar RNAs in metabolic stress. J. Biol. Chem. 2020;295:8628–8635. doi: 10.1074/jbc.AW120.011105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Tilbeurgh H., Sarda L., Verger R., Cambillau C. Structure of the pancreatic lipase-procolipase complex. Nature. 1992;359:159–162. doi: 10.1038/359159a0. [DOI] [PubMed] [Google Scholar]
- 57.Sonnenburg W.K., Yu D., Lee E.C., Xiong W., Gololobov G., Key B., Gay J., Wilganowski N., Hu Y., Zhao S., et al. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J. Lipid Res. 2009;50:2421–2429. doi: 10.1194/jlr.M900145-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arora R., Nimonkar A.V., Baird D., Wang C., Chiu C.-H., Horton P.A., Hanrahan S., Cubbon R., Weldon S., Tschantz W.R., et al. Structure of lipoprotein lipase in complex with GPIHBP1. Proc. Natl. Acad. Sci. USA. 2019;116:10360–10365. doi: 10.1073/pnas.1820171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukonina V., Lookene A., Olivecrona T., Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lookene A., Zhang L., Hultin M., Olivecrona G. Rapid subunit exchange in dimeric lipoprotein lipase and properties of the inactive monomer. J. Biol. Chem. 2004;279:49964–49972. doi: 10.1074/jbc.M407419200. [DOI] [PubMed] [Google Scholar]
- 61.Beigneux A.P., Allan C.M., Sandoval N.P., Cho G.W., Heizer P.J., Jung R.S., Stanhope K.L., Havel P.J., Birrane G., Meiyappan M., et al. Lipoprotein lipase is active as a monomer. Proc. Natl. Acad. Sci. USA. 2019;116:6319–6328. doi: 10.1073/pnas.1900983116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kristensen K.K., Leth-Espensen K.Z., Young S.G., Ploug M. ANGPTL4 inactivates lipoprotein lipase by catalyzing the irreversible unfolding of LPL’s hydrolase domain. J. Lipid Res. 2020;61:1253. doi: 10.1194/jlr.ILR120000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osborne J.C., Lee N.S., Bengtsson-Olivecrona G., Olivecrona T. Studies on Inactivation of Lipoprotein Lipase: Role of the Dimer to Monomer Dissociation. Biochemistry. 1985;24:5606–5611. doi: 10.1021/bi00341a048. [DOI] [PubMed] [Google Scholar]
- 64.Nilsson S.K., Anderson F., Ericsson M., Larsson M., Makoveichuk E., Lookene A., Heeren J., Olivecrona G. Triacylglycerol-rich lipoproteins protect lipoprotein lipase from inactivation by ANGPTL3 and ANGPTL4. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2012;1821:1370–1378. doi: 10.1016/j.bbalip.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Lund Winther A.-M., Kumari A., Young S.G., Ploug M. ANGPTL4 sensitizes lipoprotein lipase to PCSK3 cleavage by catalyzing its unfolding. J. Lipid Res. 2021 doi: 10.1016/j.jlr.2021.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin N., Matter W.F., Michael L.F., Qian Y., Gheyi T., Cano L., Perez C., Lafuente C., Broughton H.B., Espada A. The Angiopoietin-Like Protein 3 and 8 Complex Interacts with Lipoprotein Lipase and Induces LPL Cleavage. ACS Chem. Biol. 2021;16:457–462. doi: 10.1021/acschembio.0c00954. [DOI] [PubMed] [Google Scholar]
- 67.Vilella E., Joven J., Fernandez M., Vilaro S., Brunzell J.D., Olivecrona T., Bengtsson-Olivecrona G. Lipoprotein lipase in human plasma is mainly inactive and associated with cholesterol-rich lipoproteins. J. Lipid Res. 1993;34:1555–1564. doi: 10.1016/S0022-2275(20)36948-0. [DOI] [PubMed] [Google Scholar]
- 68.Peterson J., Fujimoto W.Y., Brunzell J.D. Human lipoprotein lipase: Relationship of activity, heparin affinity, and conformation as studied with monoclonal antibodies. J. Lipid Res. 1992;33:1165–1170. doi: 10.1016/S0022-2275(20)40768-0. [DOI] [PubMed] [Google Scholar]
- 69.Nielsen M.S., Brejning J., García R., Zhang H., Hayden M.R., Vilaró S., Gliemann J. Segments in the C-terminal folding domain of lipoprotein lipase important for binding to the low density lipoprotein receptor-related protein and to heparan sulfate proteoglycans. J. Biol. Chem. 1997;272:5821–5827. doi: 10.1074/jbc.272.9.5821. [DOI] [PubMed] [Google Scholar]
- 70.Merkel M., Kako Y., Radner H., Cho I.S., Ramasamy R., Brunzell J.D., Goldberg I.J., Breslow J.L. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: Direct evidence that lipoprotein lipase bridging occurs in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:13841–13846. doi: 10.1073/pnas.95.23.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loeffler B., Heeren J., Blaeser M., Radner H., Kayser D., Aydin B., Merkel M. Lipoprotein lipase-facilitated uptake of LDL is mediated by the LDL receptor. J. Lipid Res. 2007;48:288–298. doi: 10.1194/jlr.M600292-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Pentikäinen M.O., Öörni K., Kovanen P.T. Lipoprotein lipase (LPL) strongly links native and oxidized low density lipoprotein particles to decorin-coated collagen. Roles for both dimeric and monomeric forms of LPL. J. Biol. Chem. 2000;275:5694–5701. doi: 10.1074/jbc.275.8.5694. [DOI] [PubMed] [Google Scholar]
- 73.Brown W.V., Levy R.I., Fredrickson D.S. Further characterization of apolipoproteins from the human plasma very low density lipoproteins. J. Biol. Chem. 1970;245:6588–6594. doi: 10.1016/S0021-9258(18)62574-8. [DOI] [PubMed] [Google Scholar]
- 74.LaRosa J.C., Levy R.I., Herbert P., Lux S.E., Fredrickson D.S. A specific apoprotein activator for lipoprotein lipase. Biochem. Biophys. Res. Commun. 1970;41:57–62. doi: 10.1016/0006-291X(70)90468-7. [DOI] [PubMed] [Google Scholar]
- 75.Bier D.M., Havel R.J. Activation of lipoprotein lipase by lipoprotein fractions of human serum. J. Lipid Res. 1970;11:565–570. doi: 10.1016/S0022-2275(20)42941-4. [DOI] [PubMed] [Google Scholar]
- 76.Havel R.J., Fielding C.J., Olivecrona T., Shore V.G., Fielding P.E., Egelrud T. Cofactor Activity of Protein Components of Human Very Low Density Lipoproteins in the Hydrolysis of Triglycerides by Lipoprotein Lipase from Different Sources. Biochemistry. 1973;12:1828–1833. doi: 10.1021/bi00733a026. [DOI] [PubMed] [Google Scholar]
- 77.Segrest J.P., Jones M.K., De Loof H., Brouillette C.G., Venkatachalapathi Y.V., Anantharamaiah G.M. The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. J. Lipid Res. 1992;33:141–166. doi: 10.1016/S0022-2275(20)41536-6. [DOI] [PubMed] [Google Scholar]
- 78.Zdunek J., Martinez G.V., Schleucher J., Lycksell P.O., Yin Y., Nilsson S., Shen Y., Olivecrona G., Wijmenga S.S. Global structure and dynamics of human apolipoprotein CII in complex with micelles: Evidence for increased mobility of the helix involved in the activation of lipoprotein lipase. Biochemistry. 2003;42:1872–1889. doi: 10.1021/bi0267184. [DOI] [PubMed] [Google Scholar]
- 79.Musliner T.A., Herbert P.N., Church E.C. Activation of lipoprotein lipase by native and acylated peptides of apolipoprotein C-II. Biochim. Biophys. Acta Lipids Lipid Metab. 1979;573:501–509. doi: 10.1016/0005-2760(79)90224-8. [DOI] [PubMed] [Google Scholar]
- 80.Shen Y., Lookene A., Nilsson S., Olivecrona G. Functional analyses of human apolipoprotein CII by site-directed mutagenesis: Identification of residues important for activation of lipoprotein lipase. J. Biol. Chem. 2002;277:4334–4342. doi: 10.1074/jbc.M105421200. [DOI] [PubMed] [Google Scholar]
- 81.Hill J.S., Yang D., Nikazy J., Curtiss L.K., Sparrow J.T., Wong H. Subdomain chimeras of hepatic lipase and lipoprotein lipase. Localization of heparin and cofactor binding. J. Biol. Chem. 1998;273:30979–30984. doi: 10.1074/jbc.273.47.30979. [DOI] [PubMed] [Google Scholar]
- 82.McIlhargey T.L., Yang Y., Wong H., Hill J.S. Identification of a Lipoprotein Lipase Cofactor-binding Site by Chemical Cross-linking and Transfer of Apolipoprotein C-II-responsive Lipolysis from Lipoprotein Lipase to Hepatic Lipase. J. Biol. Chem. 2003;278:23027–23035. doi: 10.1074/jbc.M300315200. [DOI] [PubMed] [Google Scholar]
- 83.Meyers N.L., Larsson M., Olivecrona G., Small D.M. A pressure-dependent model for the regulation of lipoprotein lipase by apolipoprotein C-II. J. Biol. Chem. 2015;290:18029–18044. doi: 10.1074/jbc.M114.629865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shachter N.S., Hayek T., Leff T., Smith J.D., Rosenberg D.W., Walsh A., Ramakrishnan R., Goldberg I.J., Ginsberg H.N., Breslow J.L. Overexpression of apolipoprotein CII causes hypertriglyceridemia in transgenic mice. J. Clin. Investig. 1994;93:1683–1690. doi: 10.1172/JCI117151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatters D.M., MacPhee C.E., Lawrence L.J., Sawyer W.H., Howlett G.J. Human Apolipoprotein C-II Forms Twisted Amyloid Ribbons and Closed Loops. Biochemistry. 2000;39:8276–8283. doi: 10.1021/bi000002w. [DOI] [PubMed] [Google Scholar]
- 86.Medeiros L.A., Khan T., El Khoury J.B., Pham C.L.L., Hatters D.M., Howlett G.J., Lopez R., O’Brien K.D., Moore K.J. Fibrillar Amyloid Protein Present in Atheroma Activates CD36 Signal Transduction. J. Biol. Chem. 2004;279:10643–10648. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- 87.Mak P.A., Laffitte B.A., Desrumaux C., Joseph S.B., Curtiss L.K., Mangelsdorf D.J., Tontonoz P., Edwards P.A. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages: A critical role for nuclear liver X receptors α and β. J. Biol. Chem. 2002;277:31900–31908. doi: 10.1074/jbc.M202993200. [DOI] [PubMed] [Google Scholar]
- 88.Wolska A., Lo L., Sviridov D.O., Pourmousa M., Pryor M., Ghosh S.S., Kakkar R., Davidson M., Wilson S., Pastor R.W., et al. A dual apolipoprotein C-II mimetic-apolipoprotein C-III antagonist peptide lowers plasma triglycerides. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aaw7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van der Vliet H.N., Sammels M.G., Leegwater A.C.J., Levels J.H.M., Reitsma P.H., Boers W., Chamuleau R.A.F.M. Apolipoprotein A-V: A novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 90.Pennacchio L.A., Olivier M., Hubacek J.A., Cohen J.C., Cox D.R., Fruchart J.C., Krauss R.M., Rubin E.M. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 91.Grosskopf I., Baroukh N., Lee S.-J., Kamari Y., Harats D., Rubin E.M., Pennacchio L.A., Cooper A.D. Apolipoprotein A-V Deficiency Results in Marked Hypertriglyceridemia Attributable to Decreased Lipolysis of Triglyceride-Rich Lipoproteins and Removal of Their Remnants. Arterioscler. Thromb. Vasc. Biol. 2005;25:2573–2579. doi: 10.1161/01.ATV.0000186189.26141.12. [DOI] [PubMed] [Google Scholar]
- 92.Van der Vliet H.N., Schaap F.G., Levels J.H.M., Ottenhoff R., Looije N., Wesseling J.G., Groen A.K., Chamuleau R.A.F.M. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem. Biophys. Res. Commun. 2002;295:1156–1159. doi: 10.1016/S0006-291X(02)00808-2. [DOI] [PubMed] [Google Scholar]
- 93.Johansen C.T., Kathiresan S., Hegele R.A. Genetic determinants of plasma triglycerides. J. Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Brien P.J., Alborn W.E., Sloan J.H., Ulmer M., Boodhoo A., Knierman M.D., Schultze A.E., Konrad R.J. The Novel Apolipoprotein A5 Is Present in Human Serum, Is Associated with VLDL, HDL, and Chylomicrons, and Circulates at Very Low Concentrations Compared with Other Apolipoproteins. Clin. Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 95.Weinberg R.B., Cook V.R., Beckstead J.A., Martin D.D.O., Gallagher J.W., Shelness G.S., Ryan R.O. Structure and Interfacial Properties of Human Apolipoprotein A-V. J. Biol. Chem. 2003;278:34438–34444. doi: 10.1074/jbc.M303784200. [DOI] [PubMed] [Google Scholar]
- 96.Nilsson S.K., Lookene A., Beckstead J.A., Gliemann J., Ryan R.O., Olivecrona G. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry. 2007;46:3896–3904. doi: 10.1021/bi7000533. [DOI] [PubMed] [Google Scholar]
- 97.Lookene A., Beckstead J.A., Nilsson S., Olivecrona G., Ryan R.O. Apolipoprotein A-V-heparin interactions: Implications for plasma lipoprotein metabolism. J. Biol. Chem. 2005;280:25383–25387. doi: 10.1074/jbc.M501589200. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y.Q., Pottanat T.G., Zhen E.Y., Siegel R.W., Ehsani M., Qian Y.-W., Konrad R.J. ApoA5 lowers triglyceride levels via suppression of ANGPTL3/8-mediated LPL inhibition. J. Lipid Res. 2021;62:100068. doi: 10.1016/j.jlr.2021.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mansouri R.M., Baugé E., Gervois P., Fruchart-Najib J., Fiévet C., Staels B., Fruchart J.C. Atheroprotective effect of human apolipoprotein a5 in a mouse model of mixed dyslipidemia. Circ. Res. 2008;103:450–453. doi: 10.1161/CIRCRESAHA.108.179861. [DOI] [PubMed] [Google Scholar]
- 100.Grosskopf I., Shaish A., Afek A., Shemesh S., Harats D., Kamari Y. Apolipoprotein A–V modulates multiple atherogenic mechanisms in a mouse model of disturbed clearance of triglyceride-rich lipoproteins. Atherosclerosis. 2012;224:75–83. doi: 10.1016/j.atherosclerosis.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Gonzales J.C., Gordts P.L.S.M., Foley E.M., Esko J.D. Apolipoproteins e and AV mediate lipoprotein clearance by hepatic proteoglycans. J. Clin. Investig. 2013;123:2742–2751. doi: 10.1172/JCI67398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams K.J., Chen K. Recent insights into factors affecting remnant lipoprotein uptake. Curr. Opin. Lipidol. 2010;21:218–228. doi: 10.1097/MOL.0b013e328338cabc. [DOI] [PubMed] [Google Scholar]
- 103.Foley E.M., Esko J.D. Progress in Molecular Biology and Translational Science. Volume 93. Elsevier Inc.; New York, NY, USA: 2010. Hepatic heparan sulfate proteoglycans and endocytic clearance of triglyceride-rich lipoproteins; pp. 213–233. [DOI] [PubMed] [Google Scholar]
- 104.Schaap F.G., Rensen P.C.N., Voshol P.J., Vrins C., Van Der Vliet H.N., Chamuleau R.A.F.M., Havekes L.M., Groen A.K., Van Dijk K.W. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglycerides (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 105.Sharma V., Beckstead J.A., Simonsen J.B., Nelbach L., Watson G., Forte T.M., Ryan R.O. Gene transfer of apolipoprotein A-V improves the hypertriglyceridemic phenotype of apoa5 (-/-) mice. Arterioscler. Thromb. Vasc. Biol. 2013;33:474–480. doi: 10.1161/ATVBAHA.112.301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shu X., Nelbach L., Weinstein M.M., Burgess B.L., Beckstead J.A., Young S.G., Ryan R.O., Forte T.M. Intravenous Injection of Apolipoprotein A-V Reconstituted High-Density Lipoprotein Decreases Hypertriglyceridemia in apoav−/− Mice and Requires Glycosylphosphatidylinositol-Anchored High-Density Lipoprotein–Binding Protein 1. Arterioscler. Thromb. Vasc. Biol. 2010;30:2504–2509. doi: 10.1161/ATVBAHA.110.210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fuior E.V., Gafencu A.V. Apolipoprotein c1: Its pleiotropic effects in lipid metabolism and beyond. Int. J. Mol. Sci. 2019;20:5939. doi: 10.3390/ijms20235939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jackson R.L., Sparrow J.T., Baker H.N. The primary structure of apolipoprotein serine. J. Biol. Chem. 1974;249:5308–5313. doi: 10.1016/S0021-9258(19)42365-X. [DOI] [PubMed] [Google Scholar]
- 109.Shulman R.S., Herbert P.N., Wehrly K., Fredrickson D.S. The complete amino acid sequence of C I (ApoLp Ser), an apolipoprotein from human very low density lipoproteins. J. Biol. Chem. 1975;250:182–190. doi: 10.1016/S0021-9258(19)41998-4. [DOI] [PubMed] [Google Scholar]
- 110.Björkegren J., Hamsten A., Milne R.W., Karpe F. Alterations of VLDL composition during alimentary lipemia. J. Lipid Res. 1997;38:301–314. doi: 10.1016/S0022-2275(20)37443-5. [DOI] [PubMed] [Google Scholar]
- 111.Björkegren J., Karpe F., Milne R.W., Hamsten A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low density lipoproteins isolated from fasting and postprandial plasma. J. Lipid Res. 1998;39:1412–1420. doi: 10.1016/S0022-2275(20)32522-0. [DOI] [PubMed] [Google Scholar]
- 112.Rozek A., Buchko G.W., Cushley R.J. Conformation of Two Peptides Corresponding to Human Apolipoprotein C-I Residues 7-24 and 35-53 in the Presence of Sodium Dodecyl Sulfate by CD and NMR Spectroscopy. Biochemistry. 1995;34:7401–7408. doi: 10.1021/bi00022a013. [DOI] [PubMed] [Google Scholar]
- 113.Berbée J.F.P., Van Der Hoogt C.C., Sundararaman D., Havekes L.M., Rensen P.C.N. Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J. Lipid Res. 2005;46:297–306. doi: 10.1194/jlr.M400301-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Masaki S., Norihiro S., Roger L.B., Kohji S., Richard L.J. Effect of apolipoproteins on the hepatic lipase-catalyzed hydrolysis of human plasma high density lipoprotein2-triacylglycerols. Biochim. Biophys. Acta Lipids Lipid Metab. 1982;713:292–299. doi: 10.1016/0005-2760(82)90247-8. [DOI] [PubMed] [Google Scholar]
- 115.Poensgen J. Apolipoprotein C-1 inhibits the hydrolysis by phospholipase A2 of phospholipids in liposomes and cell membranes. Biochim. Biophys. Acta Lipids Lipid Metab. 1990;1042:188–192. doi: 10.1016/0005-2760(90)90006-J. [DOI] [PubMed] [Google Scholar]
- 116.Gautier T., Masson D., Pais De Barros J.P., Athias A., Gambert P., Aunis D., Metz-Boutigue M.H., Lagrost L. Human apolipoprotein C-I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J. Biol. Chem. 2000;275:37504–37509. doi: 10.1074/jbc.M007210200. [DOI] [PubMed] [Google Scholar]
- 117.Westerterp M., Van Eck M., de Haan W., Offerman E.H., Van Berkel T.J.C., Havekes L.M., Rensen P.C.N. Apolipoprotein CI aggravates atherosclerosis development in ApoE-knockout mice despite mediating cholesterol efflux from macrophages. Atherosclerosis. 2007;195:e9–e16. doi: 10.1016/j.atherosclerosis.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 118.Verine A., Benkirane M., Meignen J.M., Boyer J. Lipoprotein lipase in rat heart-II. Influence of apolipoproteins and nutritional factors on tri- di- and monoacylglycerol lipase activities in post-heparin effluents. Comp. Biochem. Physiol. Part B Biochem. 1989;94:19–25. doi: 10.1016/0305-0491(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 119.Weisgraber K.H., Mahley R.W., Kowal R.C., Herz J., Goldstein J.L., Brown M.S. Apolipoprotein C-I modulates the interaction of apolipoprotein E with β-migrating very low density lipoproteins (β-VLDL) and inhibits binding of β-VLDL to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990;265:22453–22459. doi: 10.1016/S0021-9258(18)45726-2. [DOI] [PubMed] [Google Scholar]
- 120.Sehayek E., Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 1991;266:18259–18267. doi: 10.1016/S0021-9258(18)55263-7. [DOI] [PubMed] [Google Scholar]
- 121.De Haan W., Out R., Berbée J.F.P., van der Hoogt C.C., van Dijk K.W., van Berkel T.J.C., Romijn J.A., Wouter Jukema J., Havekes L.M., Rensen P.C.N. Apolipoprotein CI inhibits scavenger receptor BI and increases plasma HDL levels in vivo. Biochem. Biophys. Res. Commun. 2008;377:1294–1298. doi: 10.1016/j.bbrc.2008.10.147. [DOI] [PubMed] [Google Scholar]
- 122.Soutar A.K., Sigler G.F., Smith L.C., Gotto A.M., Sparrow J.T. Lecithin:cholesterol aeyltransferase activation and lipid binding by synthetic fragments of apolipoprotein c-i. Scand. J. Clin. Lab. Investig. 1978;38:53–58. doi: 10.1080/00365517809104900. [DOI] [PubMed] [Google Scholar]
- 123.Lauer S.J., Walker D., Elshourbagy N.A., Reardon C.A., Levy-Wilson B., Taylor J.M. Two copies of the human apolipoprotein C-I gene are linked closely to the apolipoprotein E gene. J. Biol. Chem. 1988;263:7277–7286. doi: 10.1016/S0021-9258(18)68638-7. [DOI] [PubMed] [Google Scholar]
- 124.Björkegren J., Silveira A., Boquist S., Tang R., Karpe F., Bond M.G., De Faire U., Hamsten A. Postprandial enrichment of remnant lipoproteins with ApoC-I in healthy normolipidemic men with early asymptomatic atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002;22:1470–1474. doi: 10.1161/01.ATV.0000029972.42487.42. [DOI] [PubMed] [Google Scholar]
- 125.Hamsten A., Silveira A., Boquist S., Tang R., Bond M.G., De Faire U., Björkegren J. The apolipoprotein CI content of triglyceride-rich lipoproteins independently predicts early atherosclerosis in healthy middle-aged men. J. Am. Coll. Cardiol. 2005;45:1013–1017. doi: 10.1016/j.jacc.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 126.Domschke G., Linden F., Pawig L., Hafner A., Akhavanpoor M., Reymann J., Doesch A.O., Erbel C., Weber C., Katus H.A., et al. Systematic RNA-interference in primary human monocyte-derived macrophages: A high-throughput platform to study foam cell formation. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-28790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan L.R., Wang D.X., Liu H., Zhang X.X., Zhao H., Hua L., Xu P., Li Y.S. A pro-atherogenic HDL profile in coronary heart disease patients: An iTRAQ labelling-based proteomic approach. PLoS ONE. 2014;9:e98368. doi: 10.1371/journal.pone.0098368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gautier T., Deckert V., Aires V., Le Guern N., Proukhnitzky L., Patoli D., Lemaire S., Maquart G., Bataille A., Xolin M., et al. Human apolipoprotein C1 transgenesis reduces atherogenesis in hypercholesterolemic rabbits. Atherosclerosis. 2021;320:10–18. doi: 10.1016/j.atherosclerosis.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Jørgensen A.B., Frikke-Schmidt R., Nordestgaard B.G., Tybjærg-Hansen A. Loss-of-Function Mutations in APOC3 and Risk of Ischemic Vascular Disease. N. Engl. J. Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 130.Pollin T.I., Damcott C.M., Shen H., Ott S.H., Shelton J., Horenstein R.B., Post W., McLenithan J.C., Bielak L.F., Peyser P.A., et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van Capelleveen J.C., Moens S.J.B., Yang X., Kastelein J.J.P., Wareham N.J., Zwinderman A.H., Stroes E.S.G., Witztum J.L., Kees Hovingh G., Khaw K.T., et al. Apolipoprotein C-III Levels and Incident Coronary Artery Disease Risk: The EPIC-Norfolk Prospective Population Study. Arterioscler. Thromb. Vasc. Biol. 2017;37:1206–1212. doi: 10.1161/ATVBAHA.117.309007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taskinen M.R., Packard C.J., Borén J. Emerging Evidence that ApoC-III Inhibitors Provide Novel Options to Reduce the Residual CVD. Curr. Atheroscler. Rep. 2019;21 doi: 10.1007/s11883-019-0791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brown W.V., Baginsky M.L. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem. Biophys. Res. Commun. 1972;46:375–382. doi: 10.1016/S0006-291X(72)80149-9. [DOI] [PubMed] [Google Scholar]
- 134.Kinnunen P.K.J., Ehnholm C. Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett. 1976;65:354–357. doi: 10.1016/0014-5793(76)80145-7. [DOI] [PubMed] [Google Scholar]
- 135.McConathy W.J., Gesquiere J.C., Bass H., Tartar A., Fruchart J.C., Wang C.S. Inhibition of lipoprotein lipase activity by synthetic peptides of apolipoprotein C-III. J. Lipid Res. 1992;33:995–1003. doi: 10.1016/S0022-2275(20)41415-4. [DOI] [PubMed] [Google Scholar]
- 136.Breyer E.D., Le N.A., Li X., Martinson D., Brown W.V. Apolipoprotein C-III displacement of apolipoprotein E from VLDL: Effect of particle size. J. Lipid Res. 1999;40:1875–1882. doi: 10.1016/S0022-2275(20)34904-X. [DOI] [PubMed] [Google Scholar]
- 137.Ito Y., Azrolan N., O’Connell A., Walsh A., Breslow J. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249:790–793. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- 138.Yao Z., Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr. Opin. Lipidol. 2012;23:206–212. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]
- 139.Meyers N.L., Larsson M., Vorrsjö E., Olivecrona G., Small D.M. Aromatic residues in the C terminus of apolipoprotein C-III mediate lipid binding and LPL inhibition. J. Lipid Res. 2017;58:840–852. doi: 10.1194/jlr.M071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Witztum J.L., Gaudet D., Freedman S.D., Alexander V.J., Digenio A., Williams K.R., Yang Q., Hughes S.G., Geary R.S., Arca M., et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019;381:531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 141.Paik J., Duggan S. Volanesorsen: First Global Approval. Drugs. 2019;79:1349–1354. doi: 10.1007/s40265-019-01168-z. [DOI] [PubMed] [Google Scholar]
- 142.Santulli G. Angiopoietin-like proteins: A comprehensive look. Front. Endocrinol. 2014;5:5–10. doi: 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ge H., Cha J.Y., Gopal H., Harp C., Yu X., Repa J.J., Li C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J. Lipid Res. 2005;46:1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 144.Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P., Gonzalez F.J., Desvergne B., Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 145.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H.F.J., Hesselink M.K., Schrauwen P., Müller M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2009;29:969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 146.Ruppert P.M.M., Michielsen C.C.J.R., Hazebroek E.J., Pirayesh A., Olivecrona G., Afman L.A., Kersten S. Fasting induces ANGPTL4 and reduces LPL activity in human adipose tissue. Mol. Metab. 2020;40:101033. doi: 10.1016/j.molmet.2020.101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cushing E.M., Chi X., Sylvers K.L., Shetty S.K., Potthoff M.J., Davies B.S.J. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 2017;6:809–818. doi: 10.1016/j.molmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Köster A., Chao Y.B., Mosior M., Ford A., Gonzalez-DeWhitt P.A., Hale J.E., Li D., Qiu Y., Fraser C.C., Yang D.D., et al. Transgenic angiopoietin-like (Angptl)4 overexpression and targeted disruption of Angptl4 and Angptl3: Regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 149.Robciuc M.R., Naukkarinen J., Ortega-Alonso A., Tyynismaa H., Raivio T., Rissanen A., Kaprio J., Ehnholm C., Jauhiainen M., Pietiläinen K.H. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J. Lipid Res. 2011;52:1575–1582. doi: 10.1194/jlr.P015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robciuc M.R., Tahvanainen E., Jauhiainen M., Ehnholm C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J. Lipid Res. 2010;51:824–831. doi: 10.1194/jlr.M002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dijk W., Ruppert P.M.M., Oost L.J., Kersten S. Angiopoietin-like 4 promotes the intracellular cleavage of lipoprotein lipase by PCSK3/furin in adipocytes. J. Biol. Chem. 2018;293:14134–14145. doi: 10.1074/jbc.RA118.002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dijk W., Beigneux A.P., Larsson M., Bensadoun A., Young S.G., Kersten S. Angiopoietin-like 4 (ANGPTL4) promotes intracellular degradation of lipoprotein lipase in adipocytes. J. Lipid Res. 2016;7:956–963. doi: 10.1194/jlr.M067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Makoveichuk E., Sukonina V., Kroupa O., Thulin P., Ehrenborg E., Olivecrona T., Olivecrona G. Inactivation of lipoprotein lipase occurs on the surface of THP-1 macrophages where oligomers of angiopoietin-like protein 4 are formed. Biochem. Biophys. Res. Commun. 2012;425:138–143. doi: 10.1016/j.bbrc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 154.Mysling S., Kristensen K.K., Larsson M., Kovrov O., Bensadouen A., Jørgensen T.J.D., Olivecrona G., Young S.G., Ploug M. The angiopoietin-like protein angptl4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein gpihbp1 counteracts this unfolding. Elife. 2016;5:e20958. doi: 10.7554/eLife.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chi X., Shetty S.K., Shows H.W., Hjelmaas A.J., Malcolm E.K., Davies B.S.J. Angiopoietin-like 4 modifies the interactions between lipoprotein lipase and its endothelial cell transporter GPIHBP1. J. Biol. Chem. 2015;290:11865–11877. doi: 10.1074/jbc.M114.623769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Helgadottir A., Gretarsdottir S., Thorleifsson G., Hjartarson E., Sigurdsson A., Magnusdottir A., Jonasdottir A., Kristjansson H., Sulem P., Oddsson A., et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat. Genet. 2016;48:634–639. doi: 10.1038/ng.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yin W., Romeo S., Chang S., Grishin N.V., Hobbs H.H., Cohen J.C. Genetic variation in ANGPTL4 provides insights into protein processing and function. J. Biol. Chem. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lichtenstein L., Mattijssen F., de Wit N.J., Georgiadi A., Hooiveld G.J., van der Meer R., He Y., Qi L., Köster A., Tamsma J.T., et al. Angptl4 Protects against Severe Proinflammatory Effects of Saturated Fat by Inhibiting Fatty Acid Uptake into Mesenteric Lymph Node Macrophages. Cell Metab. 2010;12:580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oteng A.B., Ruppert P.M.M., Boutens L., Dijk W., Van Dierendonck X.A.M.H., Olivecrona G., Stienstra R., Kersten S. Characterization of ANGPTL4 function in macrophages and adipocytes using Angptl4-knockout and Angptl4-hypomorphic mice. J. Lipid Res. 2019;60:1741–1754. doi: 10.1194/jlr.M094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Georgiadi A., Wang Y., Stienstra R., Tjeerdema N., Janssen A., Stalenhoef A., Van Der Vliet J.A., De Roos A., Tamsma J.T., Smit J.W.A., et al. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2013;33:1529–1537. doi: 10.1161/ATVBAHA.113.301698. [DOI] [PubMed] [Google Scholar]
- 161.Koishi R., Ando Y., Ono M., Shimamura M., Yasumo H., Fujiwara T., Horikoshi H., Furukawa H. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 162.Romeo S., Yin W., Kozlitina J., Pennacchio L.A., Boerwinkle E., Hobbs H.H., Cohen J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R., Ueda K., Inaba T., Minekura H., Kohama T., et al. ANGPTL3 decreases very low density lipoprotein triglyceride cleara ance by inhibition of lipoprotein lipase. J. Biol. Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 164.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 165.Hirata K.I., Dichek H.L., Cioffi J.A., Choi S.Y., Leeper N.J., Quintana L., Kronmal G.S., Cooper A.D., Quertermous T. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 1999;274:14170–14175. doi: 10.1074/jbc.274.20.14170. [DOI] [PubMed] [Google Scholar]
- 166.Ma K., Cilingiroglu M., Otvos J.D., Ballantyne C.M., Marian A.J., Chan L. Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure and metabolism. Proc. Natl. Acad. Sci. USA. 2003;100:2748–2753. doi: 10.1073/pnas.0438039100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Musunuru K., Pirruccello J.P., Do R., Peloso G.M., Guiducci C., Sougnez C., Garimella K.V., Fisher S., Abreu J., Barry A.J., et al. Exome Sequencing, ANGPTL3 Mutations, and Familial Combined Hypolipidemia. N. Engl. J. Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Minicocci I., Santini S., Cantisani V., Stitziel N., Kathiresan S., Arroyo J.A., Martí G., Pisciotta L., Noto D., Cefalù A.B., et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: A pooled analysis. J. Lipid Res. 2013;54:3481–3490. doi: 10.1194/jlr.P039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Adam R.C., Mintah I.J., Alexa-Braun C.A., Shihanian L.M., Lee J.S., Banerjee P., Hamon S.C., Kim H.I., Cohen J.C., Hobbs H.H., et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipid Res. 2020;61:1271–1286. doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y., Koishi R., Furukawa H. Protein Region Important for Regulation of Lipid Metabolism in Angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 2003;278:41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 171.Chi X., Britt E.C., Shows H.W., Hjelmaas A.J., Shetty S.K., Cushing E.M., Li W., Dou A., Zhang R., Davies B.S.J. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol. Metab. 2017;6:1137–1149. doi: 10.1016/j.molmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu J., Afroza H., Rader D.J., Jin W. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J. Biol. Chem. 2010;285:27561–27570. doi: 10.1074/jbc.M110.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haller J.F., Mintah I.J., Shihanian L.M., Stevis P., Buckler D., Alexa-Braun C.A., Kleiner S., Banfi S., Cohen J.C., Hobbs H.H., et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J. Lipid Res. 2017;58:1166–1173. doi: 10.1194/jlr.M075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sueishi K., Yonemitsu Y., Nakagawa K., Kaneda Y., Kumamoto M., Nakashima Y. Atherosclerosis and Angiogenesis: Its Pathophysiological Significance in Humans as Well as in an Animal Model Induced by the Gene Transfer of Vascular Endothelial Growth Factor. Ann. N. Y. Acad. Sci. 1997;811:311–324. doi: 10.1111/j.1749-6632.1997.tb52011.x. [DOI] [PubMed] [Google Scholar]
- 175.Hatsuda S., Shoji T., Shinohara K., Kimoto E., Mori K., Fukumoto S., Koyama H., Emoto M., Nishizawa Y. Association between plasma angiopoietin-like protein 3 and arterial wall thickness in healthy subjects. J. Vasc. Res. 2007;44:61–66. doi: 10.1159/000098153. [DOI] [PubMed] [Google Scholar]
- 176.Camenisch G., Pisabarro M.T., Sherman D., Kowalski J., Nagel M., Hass P., Xie M.H., Gurney A., Bodary S., Liang X.H., et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin αvβ3 and induces blood vessel formation in vivo. J. Biol. Chem. 2002;277:17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- 177.Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012;424:786–792. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 178.Kovrov O., Kristensen K.K., Larsson E., Ploug M., Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. J. Lipid Res. 2019;60:783–793. doi: 10.1194/jlr.M088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E., Valenzuela D.M., Murphy A.J., Cohen J.C., Hobbs H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen Y.Q., Pottanat T.G., Siegel R.W., Ehsani M., Qian Y.-W., Zhen E.Y., Regmi A., Roell W.C., Guo H., Luo M.J., et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J. Lipid Res. 2020;61:1203–1220. doi: 10.1194/jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oldoni F., Cheng H., Banfi S., Gusarova V., Cohen J.C., Hobbs H.H. ANGPTL8 has both endocrine and autocrine effects on substrate utilization. JCI Insight. 2020;5:1–18. doi: 10.1172/jci.insight.138777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol. 2016;6:150272. doi: 10.1098/rsob.150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brahm A., Hegele R.A. Hypertriglyceridemia. Nutrients. 2013;5:981. doi: 10.3390/nu5030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simha V. Management of hypertriglyceridemia. BMJ. 2020;371:m3109. doi: 10.1136/bmj.m3109. [DOI] [PubMed] [Google Scholar]
- 185.Surendran R.P., Visser M.E., Heemelaar S., Wang J., Peter J., Defesche J.C., Kuivenhoven J.A., Hosseini M., Péterfy M., Kastelein J.J.P., et al. Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J. Intern. Med. 2012;272:185–196. doi: 10.1111/j.1365-2796.2012.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yuan G., Al-Shali K.Z., Hegele R.A. Hypertriglyceridemia: Its etiology, effects and treatment. CMAJ. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Johansen C.T., Hegele R.A. Allelic and phenotypic spectrum of plasma triglycerides. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2012;1821:833–842. doi: 10.1016/j.bbalip.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 188.Rodrigues R., Artieda M., Tejedor D., Martínez A., Konstantinova P., Petry H., Meyer C., Corzo D., Sundgreen C., Klor H.U., et al. Pathogenic classification of LPL gene variants reported to be associated with LPL deficiency. J. Clin. Lipidol. 2016;10:394–409. doi: 10.1016/j.jacl.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 189.Gagné S.E., Larson M.G., Pimstone S.N., Schaefer E.J., Kastelein J.J.P., Wilson P.W.F., Ordovas J.M., Hayden M.R. A common truncation variant of lipoprotein lipase (Ser447X) confers protection against coronary heart disease: The Framingham Offspring Study. Clin. Genet. 1999;55:450–454. doi: 10.1034/j.1399-0004.1999.550609.x. [DOI] [PubMed] [Google Scholar]
- 190.Gaudet D., Méthot J., Kastelein J. Gene therapy for lipoprotein lipase deficiency. Curr. Opin. Lipidol. 2012;23:310–320. doi: 10.1097/MOL.0b013e3283555a7e. [DOI] [PubMed] [Google Scholar]
- 191.Felts J.M., Itakura H., Thomas Crane R. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants—A new theory. Biochem. Biophys. Res. Commun. 1975;66:1467–1475. doi: 10.1016/0006-291X(75)90524-0. [DOI] [PubMed] [Google Scholar]
- 192.Merkel M., Heeren J., Dudeck W., Rinninger F., Radner H., Breslow J.L., Goldberg I.J., Zechner R., Greten H. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J. Biol. Chem. 2002;277:7405–7411. doi: 10.1074/jbc.M107914200. [DOI] [PubMed] [Google Scholar]
- 193.Beisiegel U., Weber W., Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 1991;88:8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Takahashi S., Kawarabayasi Y., Nakai T., Sakai J., Yamamoto T. Rabbit very low density lipoprotein receptor: A low density lipoprotein receptor-like protein with distinct ligand specificity. Proc. Natl. Acad. Sci. USA. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mulder M., Lombardi P., Jansen H., van Berkel T.J.C., Frants R.R., Havekes L.M. Heparan sulphate proteoglycans are involved in the lipoprotein lipase-mediated enhancement of the cellular binding of very low density and low density lipoproteins. Biochem. Biophys. Res. Commun. 1992;185:582–587. doi: 10.1016/0006-291X(92)91664-C. [DOI] [PubMed] [Google Scholar]
- 196.Mulder M., Lombardi P., Jansen H., Van Berkel T.J.C., Frants R.R., Havekes L.M. Low density lipoprotein receptor internalizes low density and very low density lipoproteins that are bound to heparan sulfate proteoglycans via lipoprotein lipase. J. Biol. Chem. 1993;268:9369–9375. doi: 10.1016/S0021-9258(18)98359-6. [DOI] [PubMed] [Google Scholar]
- 197.Chappell D.A., Fry G.L., Waknitz M.A., Muhonen L.E., Pladet M.W., Iverius P.H., Strickland D.K. Lipoprotein lipase induces catabolism of normal triglyceride-rich lipoproteins via the low density lipoprotein receptor-related protein/α2- macroglobulin receptor in vitro. A process facilitated by cell-surface proteoglycans. J. Biol. Chem. 1993;268:14168–14175. doi: 10.1016/S0021-9258(19)85223-7. [DOI] [PubMed] [Google Scholar]
- 198.Nykjaer A., Bengtsson-Olivecrona G., Lookene A., Moestrup S.K., Petersen C.M., Weber W., Beisiegel U., Gliemann J. The α2-macroglobulin receptor/low density lipoprotein receptor-related protein binds lipoprotein lipase and β-migrating very low density lipoprotein associated with the lipase. J. Biol. Chem. 1993;268:15048–15055. doi: 10.1016/S0021-9258(18)82436-X. [DOI] [PubMed] [Google Scholar]
- 199.Niemeier A., Gåfvels M., Heeren J., Meyer N., Angelin B., Beisiegel U. VLDL receptor mediates the uptake of human chylomicron remnants in vitro. J. Lipid Res. 1996;37:1733–1742. doi: 10.1016/S0022-2275(20)39116-1. [DOI] [PubMed] [Google Scholar]
- 200.Saxena U., Klein M.G., Vanni T.M., Goldberg I.J. Lipoprotein lipase increases low density lipoprotein retention by subendothelial cell matrix. J. Clin. Investig. 1992;89:373–380. doi: 10.1172/JCI115595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.O’Brien K.D., Olin K.L., Alpers C.E., Chiu W., Ferguson M., Hudkins K., Wight T.N., Chait A. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: Colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–527. doi: 10.1161/01.CIR.98.6.519. [DOI] [PubMed] [Google Scholar]
- 202.Olin K.L., Potter-Perigo S., Barrett P.H.R., Wight T.N., Chait A. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J. Biol. Chem. 1999;274:34629–34636. doi: 10.1074/jbc.274.49.34629. [DOI] [PubMed] [Google Scholar]
- 203.Skålén K., Gustafsson M., Knutsen Rydberg E., Hultén L.M., Wiklund O., Innerarity T.L., Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 204.Joris I., Zand T., Nunnari J.J., Krolikowski F.J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am. J. Pathol. 1983;113:341–358. [PMC free article] [PubMed] [Google Scholar]
- 205.Huff M.W., Daugherty A., Lu H. Atherosclerosis. 6th ed. Elsevier; New York, NY, USA: 2016. [Google Scholar]
- 206.Chen C., Khismatullin D.B. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS ONE. 2015;10:e123088. doi: 10.1371/journal.pone.0123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stocker R., Keaney J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 208.Thomas A.C., Sala-Newby G.B., Ismail Y., Johnson J.L., Pasterkamp G., Newby A.C. Genomics of foam cells and nonfoamy macrophages from rabbits identifies arginase-I as a differential regulator of nitric oxide production. Arterioscler. Thromb. Vasc. Biol. 2007;27:571–577. doi: 10.1161/01.ATV.0000256470.23842.94. [DOI] [PubMed] [Google Scholar]
- 209.Nagai M., Granger D.N. Inflammatory Mechanisms in Ischemic Cerebrovascular Disease. Elsevier Inc.; New York, NY, USA: 2018. [Google Scholar]
- 210.Renier G., Skamene E., De Sanctis J.B., Radzioch D. High macrophage lipoprotein lipase expression and secretion are associated in inbred murine strains with susceptibility to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1993;13:190–196. doi: 10.1161/01.ATV.13.2.190. [DOI] [PubMed] [Google Scholar]
- 211.Babaev V.R., Fazio S., Gleaves L.A., Carter K.J., Semenkovich C.F., Linton M.F. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J. Clin. Investig. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Babaev V.R., Patel M.B., Semenkovich C.F., Fazio S., Linton M.F. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J. Biol. Chem. 2000;275:26293–26299. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- 213.Takahashi M., Yagyu H., Tazoe F., Nagashima S., Ohshiro T., Okada K., Osuga J.I., Goldberg I.J., Ishibashi S. Macrophage lipoprotein lipase modulates the development of atherosclerosis but not adiposity. J. Lipid Res. 2013;54:1124–1134. doi: 10.1194/jlr.M035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Van Eck M., Zimmermann R., Groot P.H., Zechner R., Van Berkel T.J. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000;20 doi: 10.1161/01.ATV.20.9.e53. [DOI] [PubMed] [Google Scholar]
- 215.Borén J., Williams K.J. The central role of arterial retention of cholesterolrich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016;27:473–483. doi: 10.1097/MOL.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 216.Rapp J.H., Lespine A., Hamilton R.L., Colyvas N., Chaumeton A.H., Tweedie-Hardman J., Kotite L., Kunitake S.T., Havel R.J., Kane J.P. Triglyceride-rich lipoproteins isolated by selected-affinity anti- apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler. Thromb. 1994;14:1767–1774. doi: 10.1161/01.ATV.14.11.1767. [DOI] [PubMed] [Google Scholar]
- 217.Nordestgaard B.G., Wootton R., Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo: Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler. Thromb. Vasc. Biol. 1995;15:534–542. doi: 10.1161/01.ATV.15.4.534. [DOI] [PubMed] [Google Scholar]
- 218.Nakajima K., Nakano T., Tanaka A. The oxidative modification hypothesis of atherosclerosis: The comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin. Chim. Acta. 2006;367:36–47. doi: 10.1016/j.cca.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 219.Tomono S., Kawazu S., Kato N., Ono T., Ishii C., Ito Y., Shimizu M., Shimoyama M., Nakano T., Nakajima K. Uptake of remnant like particles (RLP) in diabetic patients from mouse peritoneal macrophages. J. Atheroscler. Thromb. 1994;1:98–102. doi: 10.5551/jat1994.1.98. [DOI] [PubMed] [Google Scholar]
- 220.Nakajima K., Tanaka A. Atherogenic postprandial remnant lipoproteins; VLDL remnants as a causal factor in atherosclerosis. Clin. Chim. Acta. 2018;478:200–215. doi: 10.1016/j.cca.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 221.Chen S.H., Yang C.Y., Chen P.F., Setzer D., Tanimura M., Li W.H., Gotto A.M., Chan L. The complete cDNA and amino acid sequence of human apolipoprotein B-100. J. Biol. Chem. 1986;261:12918–12921. doi: 10.1016/S0021-9258(18)69248-8. [DOI] [PubMed] [Google Scholar]
- 222.Glickman R.M., Rogers M., Glickman J.N. Apolipoprotein B synthesis by human liver and intestine in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:5296–5300. doi: 10.1073/pnas.83.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen S., Habib G., Yang C., Gu Z., Lee B., Weng S., Silberman S., Cai S., Deslypere J., Rosseneu M., et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238:363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 224.Robinson J.G. Management of Familial Hypercholesterolemia: A Review of the Recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Manag. Care Pharm. 2013;19:139–149. doi: 10.18553/jmcp.2013.19.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nordestgaard B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights from Epidemiology, Genetics, and Biology. Circ. Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 226.Bergheanu S.C., Bodde M.C., Jukema J.W. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Netherlands Heart J. 2017;25:231–242. doi: 10.1007/s12471-017-0959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pedersen T.R., Faergeman O., Kastelein J.J.P., Olsson A.G., Tikkanen M.J., Holme I., Larsen M.L., Bendiksen F.S., Lindahl C., Palmer G. Design and baseline characteristics of the Incremental Decrease in End Points through Aggressive Lipid Lowering study. Am. J. Cardiol. 2004;94:720–724. doi: 10.1016/j.amjcard.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 228.Reiner Z. Management of patients with familial hypercholesterolaemia. Nat. Rev. Cardiol. 2015;12:565–575. doi: 10.1038/nrcardio.2015.92. [DOI] [PubMed] [Google Scholar]
- 229.Sampson U.K., Fazio S., Linton M.F. Residual Cardiovascular Risk Despite Optimal LDL Cholesterol Reduction with Statins: The Evidence, Etiology, and Therapeutic Challenges. Curr. Atheroscler. Rep. 2012;14:1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Briel M., Ferreira-Gonzalez I., You J.J., Karanicolas P.J., Akl E.A., Wu P., Blechacz B., Bassler D., Wei X., Sharman A., et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: Systematic review and meta-regression analysis. BMJ. 2009;338:1–8. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rosenson R.S., Brewer H.B., Ansell B.J., Barter P., Chapman M.J., Heinecke J.W., Kontush A., Tall A.R., Webb N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fan W., Philip S., Granowitz C., Toth P.P., Wong N.D. Residual hypertriglyceridemia and estimated atherosclerotic cardiovascular disease risk by statin use in U.S. Adults with diabetes: National health and nutrition examination survey 2007–2014. Diabetes Care. 2019;42:2307–2314. doi: 10.2337/dc19-0501. [DOI] [PubMed] [Google Scholar]
- 233.Chadwick A.C., Evitt N.H., Lv W., Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation. 2018;137:975–977. doi: 10.1161/CIRCULATIONAHA.117.031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Graham M.J., Lee R.G., Brandt T.A., Tai L.-J., Fu W., Peralta R., Yu R., Hurh E., Paz E., McEvoy B.W., et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 235.Gusarova V., Alexa C.A., Wang Y., Rafique A., Kim J.H., Buckler D., Mintah I.J., Shihanian L.M., Cohen J.C., Hobbs H.H., et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 2015;56:1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ahmad Z., Banerjee P., Hamon S., Chan K.C., Bouzelmat A., Sasiela W.J., Pordy R., Mellis S., Dansky H., Gipe D.A., et al. Inhibition of Angiopoietin-Like Protein 3 with a Monoclonal Antibody Reduces Triglycerides in Hypertriglyceridemia. Circulation. 2019;140:470–486. doi: 10.1161/CIRCULATIONAHA.118.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fu Z., Abou-Samra A.B., Zhang R. A lipasin/Angptl8 monoclonal antibody lowers mouse serum triglycerides involving increased postprandial activity of the cardiac lipoprotein lipase. Sci. Rep. 2015;5:1–9. doi: 10.1038/srep18502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gusarova V., Banfi S., Alexa-Braun C.A., Shihanian L.M., Mintah I.J., Lee J.S., Xin Y., Su Q., Kamat V., Cohen J.C., et al. ANGPTL8 Blockade With a Monoclonal Antibody Promotes Triglyceride Clearance, Energy Expenditure, and Weight Loss in Mice. Endocrinology. 2017;158:1252–1259. doi: 10.1210/en.2016-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pouwer M.G., Pieterman E.J., Worms N., Keijzer N., Jukema J.W., Gromada J., Gusarova V., Princen H.M.G. Alirocumab, evinacumab, and atorvastatin triple therapy regresses plaque lesions and improves lesion composition in mice. J. Lipid Res. 2020;61:365–375. doi: 10.1194/jlr.RA119000419. [DOI] [PMC free article] [PubMed] [Google Scholar]