A 24-year-old Fontan procedure patient underwent surveillance liver cardiac magnetic resonance imaging. Findings were suggestive of hepatocellular…
Key Words: awareness, congenital heart defect, cyanotic heart disease, imaging, treatment
Abbreviations and Acronyms: AASLD, American Association for Study of Liver Disease; AFP, alpha-fetoprotein; ALT, lanine aminotransferase; AST, aspartate aminotransferase; AV, atrioventricular; CT, computed tomography; FALD, Fontan associated liver disease; FNH, focal nodular hyperplasia; GS, glutamine synthetase; HCC, hepatocellular carcinoma; INR, international normalized ratio; MRI, magnetic resonance imaging; US, ultrasound
Abstract
A 24-year-old Fontan procedure patient underwent surveillance liver cardiac magnetic resonance imaging. Findings were suggestive of hepatocellular carcinoma (HCC). Currently, HCC is diagnosed based on imaging alone. Given her otherwise reassuring clinical profile, she underwent liver biopsy. Pathology demonstrated focal nodular hyperplasia. This raises concern for overdiagnosis of HCC in Fontan patients without tissue confirmation. (Level of Difficulty: Advanced.)
Graphical abstract
History of Presentation
A 24-year-old female presented with a history of right-sided heterotaxy including asplenia, an unbalanced atrioventricular canal with hypoplastic left ventricle and double-outlet right ventricle, and pulmonary artery hypoplasia. She had undergone a Blalock-Taussig shunt procedure in infancy until adequate growth of the pulmonary arteries was achieved. A bidirectional Glenn operation was performed within her first year of life. The third stage of single-ventricle palliation was completed with a lateral tunnel Fontan procedure at age 12. She was then converted to an extracardiac Fontan procedure at 13 years of age.
Learning Objectives
-
•
To recognize that Fontan-associated liver disease is a different disease entity from more prevalent types of cirrhosis.
-
•
To highlight the need for more Fontan-specific imaging guidelines.
-
•
To consider biopsy in the evaluation of lesions concerning for hepatocellular carcinoma in the Fontan patient.
In 2016, she presented for follow-up. She was assessed New York Heart Association functional class I, and her review of symptoms was negative, including any abdominal symptoms. An echocardiogram demonstrated a moderately dilated systemic right ventricle with mildly reduced systolic function. Her liver function test results were within normal limits, with a total bilirubin concentration of 0.7 mg/dl, an aspartate aminotransferase concentration of 19 IU/l, an alanine aminotransferase level of 42 IU/l, and an alkaline phosphatase level of 71 IU/l. Her alpha-fetoprotein level (AFP) was normal at 3 ng/ml. Review of her liver function test results dating back to 2009 showed normal values. Her hepatitis C antibody was negative, and her hepatitis B test results were consistent with immunization. Platelets were 286 × 103/μl, international standardize ratio was 1.0, and albumin concentration was 4.1 g/dl.
Medical History
The patient was a nonsmoker and did not drink alcohol. Her medications included losartan, aspirin, and combined oral estrogen/progesterone contraceptive pills. She had no history of prior hepatotoxic medications or exposures.
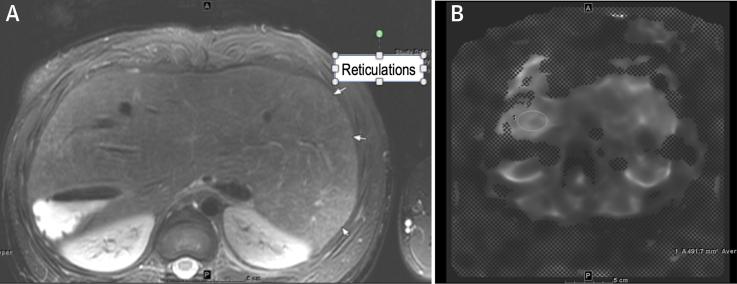
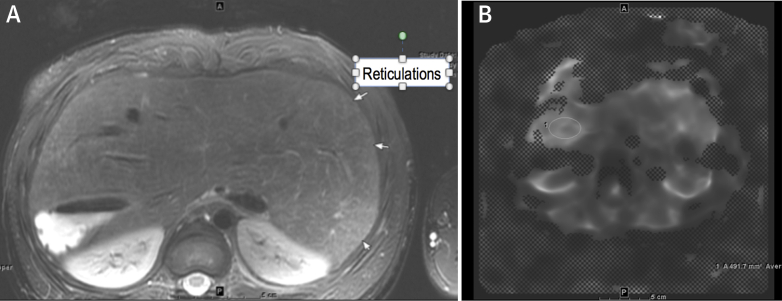
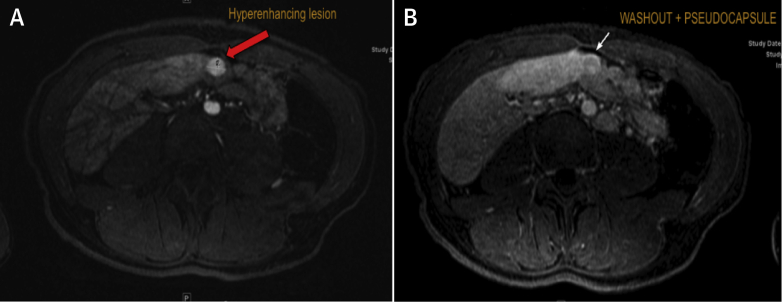
A routine liver cardiac magnetic resonance (CMR) performed to screen for cirrhosis showed evidence of fibrosis on the basis of hyperintense reticulations (Figure 1A) and elastography (Figure 1B). Two lesions (Figure 2) were visualized that exhibited arterial enhancement and delayed phase washout, findings believed to be consistent with hepatocellular carcinoma (HCC). One lesion measured 1.2 × 0.8 cm, and the second lesion measured 1.2 × 1.4 cm. Both lesions had a pseudocapsule.
Figure 1.
Hyperintense Reticulations Consistent With Fibrosis
(A) Axial T2, fine T2 hyperintense reticulations consistent with fibrosis. (B) MR elastogram, heterogeneous in distribution but highest area measured was 3.5 kPa. Elastography of 3.5 kPa has been demonstrated to correlate with stage F0 to F1 fibrosis.
Figure 2.
Arterial Phase Enhancement and Venous Phase Washout
(A) Arterial phase shows hyperenhancement. Oval circle in A indicates a nodule that shows hyperenhancement during the arterial phase of the scan. (B) Venous phase shows slightly hypointense/washout with a suggestion of pseudocapsule.
According to current guidelines, in the setting of cirrhosis, HCC is diagnosed by imaging alone, due in part to the 2.7% risk of tumor seeding with biopsy (1). Following 2010 American Association for Study of Liver Disease criteria, if a lesion is >1 cm, has early post-contrast arterial enhancement, and has delayed phase washout, HCC can be diagnosed with a high pretest probability. Based on these guidelines, elective chemoembolization is generally recommended.
The patient underwent cardiac catheterization after the findings of the liver CMR images, which revealed normal Fontan hemodynamics. Cardiac index was 4.9 l/min per m2 by the Fick method with a Fontan pressure of 8 mm Hg. Transpulmonary gradient was 4 mm Hg, and her right (systemic) ventricular pressure was 109/4 mm Hg. There were no significant gradients throughout the Fontan pathway or across her aorta. Her pulmonary vascular resistance was 0.8 WU. Her mixed venous saturation was 81% with a systemic saturation of 93%.
The patient’s data were reviewed by a multidisciplinary and multi-institutional group of adult congenital heart disease and hepatology experts. Given the patient’s unremarkable Fontan hemodynamics, her normal liver tests, and lack of cirrhosis history, the consensus recommendation was to take a biopsy of the liver lesions. The risks were discussed with the patient.
Intervention
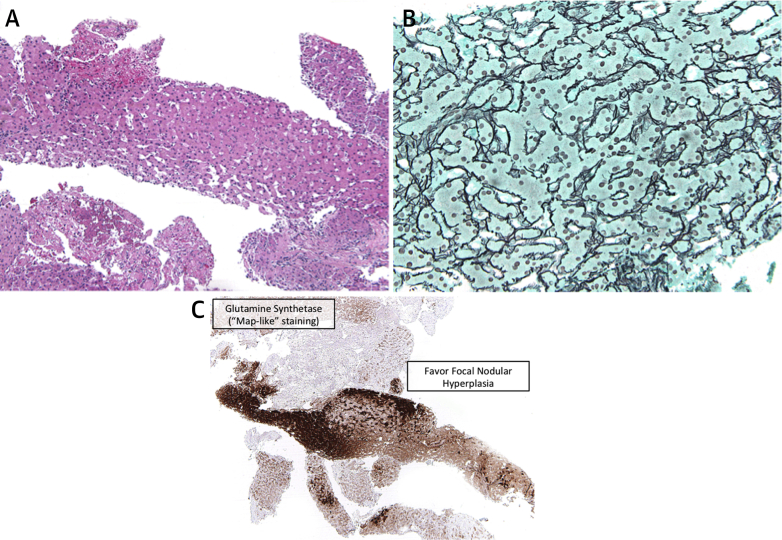
A CT-guided liver biopsy was performed. Fifteen core samples were taken from the right-sided hepatic lesion concerning in appearance for HCC. Histopathology was consistent with focal nodular hyperplasia (FNH). Tissue samples showed the characteristic fibrosis with ductules at the interface between hepatocytes and the fibrous region, and prominent arteries, all intrinsic to FNH (Figure 3A). Copper-binding protein, also expressed in FNH, was seen (Figure 3B). Finally, staining for glutamine synthetase, which is overproduced in FNH in a “map-like” pattern, was also positive (Figure 3C). The other lesions were not biopsied.
Figure 3.
Ductular Reaction With Varied Intensity at the Junction of Fibrous Septa With a Hepatocellular Component
(A) Presence of ductular reaction with varied intensity at the junction of the fibrous septa with a hepatocellular component. Thick abnormal arteries are also present. (B) Evidence of copper-binding protein (demonstrated by Victoria blue stain) suggests FNH. (C) “Map-like” staining of hepatocytes. Glutamine synthetase (GS) staining of hepatocytic cytoplasm creates a “map-like” pattern, often surrounding hepatic veins, whereas GS staining is not expressed in hepatocytes close to fibrotic bands containing arteries and ductules. FNH = focal nodular hyperplasia.
Discussion
Fontan circulations have obligatory venous hypertension and decreased cardiac output, given the reliance on a single ventricle for complete circulatory support. Venous hypertension has been associated with greater transmission of mechanical tension to the liver matrix, which is believed to then cause fibrosis. Depressed cardiac output and cyanosis are also likely to contribute to signaling for fibrosis through hypoxia-inducible factors. The net result is the development of Fontan-associated liver disease (FALD).
The process is insidious and may lead to overt cirrhosis and liver failure. Cirrhosis in this setting is associated with survival of 35% at 5 years after diagnosis (2). Additionally, HCC has been demonstrated in Fontan patients and presents significant concern for long-term prognosis.
Routine screening for liver disease in Fontan patients remains a challenge. Noninvasive measurements of hepatic function remain intact until advanced stages of hepatic fibrosis. Hypervascular nodules are often present in Fontan patients 3, 4. Determining which lesions are potentially benign, reactive lesions versus HCC is one of the difficulties in FALD evaluation. Case reports have been published documenting the presence of HCC in patients approximately 14 to 22 years after Fontan procedure (3). Most of these patients had documented cirrhosis and elevated AFP.
This patient did not have risk factors detailed in other case reports of HCC in Fontan patients (3). She had excellent Fontan pressures, serologic testing indicated good hepatic synthetic function, her cardiac index was normal, and her AFP levels were normal.
Current guidelines recommend diagnosing HCC by imaging alone in patients with cirrhosis or an otherwise high pre-test probability (5). The imaging guidelines for HCC screening and treatment are based primarily on patients with a history of chronic viral hepatitis or alcoholic cirrhosis, which are inflammatory states. In contrast, Fontan liver physiology is primarily a congestive state. Although there may be a final common pathway for these different disease states, in the absence of more definitive data, Fontan liver physiology cannot be treated using the same protocols.
In cases with atypical radiological features, tissue diagnosis by either fine needle aspiration or biopsy is recommended (6). Given that the long-term effects of FALD have not been well delineated, biopsy can aid in diagnosis and prognostication. Newer biopsy methods, such as the coaxial technique, have not resulted in tumor seeding along the biopsy track as seen with other methods (7).
Currently, there are no guidelines regarding liver surveillance in Fontan patients. The American Association for the Study of Liver Disease recommends liver imaging every 6 months in patients with hepatitis B and C infection, alcoholic cirrhosis, and hemochromatosis (5). A recent FALD stakeholder meeting suggested liver imaging in Fontan patients every 6 months if cirrhosis is detected (8). There is no consensus as to when to start screening or which imaging modality (ultrasonography, CMR, or CT) should be used.
More study is needed to determine the utility of liver surveillance and biopsy in FALD. Overscreening may lead to unnecessary testing and invasive procedures. However, if recommendations are too conservative, early stage cancer or cirrhosis may be missed. The diagnosis of HCC has important implications, as the treatment of choice is liver transplantation. In Fontan patients, liver transplantation generally would only be considered as part of a combined heart-liver transplantation (9).
The present case suggests the importance of liver biopsy in the evaluation of liver lesions suspicious for HCC. The present authors believe a full hepatic evaluation of the Fontan patient must include a biopsy if suspicious findings are present on imaging.
Follow-Up
Our patient continues to be closely monitored. Serial repeat CMR studies have demonstrated stable liver nodules without significant interval growth or the appearance of new lesions.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Silva M.A., Hegab B., Hyde C. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 2.Pundi K., Pundi K.N., Kamath P.S. Liver Disease in Patients After the Fontan Operation. Am J Cardiol. 2016;117:456–460. doi: 10.1016/j.amjcard.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Asrani K., Warnes C.A., Kamath P.S. Hepatocellular Carcinoma after the Fontan Procedure. N Engl J Med. 2013;368:1756–1757. doi: 10.1056/NEJMc1214222. [DOI] [PubMed] [Google Scholar]
- 4.Bryant T., Ahmad Z., Millward-Sadler H. Arterialised hepatic nodules in the Fontan circulation: hepatico-cardiac interactions. Int J Cardiol. 2011;151:268–272. doi: 10.1016/j.ijcard.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Marrero J.A., Kulik L.M., Sirlin C.B. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the AASLD. Hepatology. 2018;68:723. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 6.Jain D. Tissue diagnosis of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4 Suppl 3:S67–S73. doi: 10.1016/j.jceh.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maturen K.E., Ngheim H.V., Marrero J.A. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187:1184–1187. doi: 10.2214/AJR.05.1347. [DOI] [PubMed] [Google Scholar]
- 8.Daniels C.J., Bradley E.A., Landzberg M.J. Fontan-associated liver disease. J Am Coll Cardiol. 2017;70:3173–3194. doi: 10.1016/j.jacc.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Hollander S.A., Reinhartz O., Maeda K. Intermediate-term outcomes after combined heart-liver transplantation in children with a univentricular heart. J Heart Lung Transplant. 2013;32:368–370. doi: 10.1016/j.healun.2012.11.023. [DOI] [PubMed] [Google Scholar]