“Thus far an aberrant course of the right subclavian artery has not been reported in a living patient. In these circumstances it seems appropriate to publish an observation that I have made while performing a radiologic examination of the stomach.”
—Burckhard F. Kommerell, 1936 (1)
“Prevention is preferable to cure.”
—Hippocrates (2)
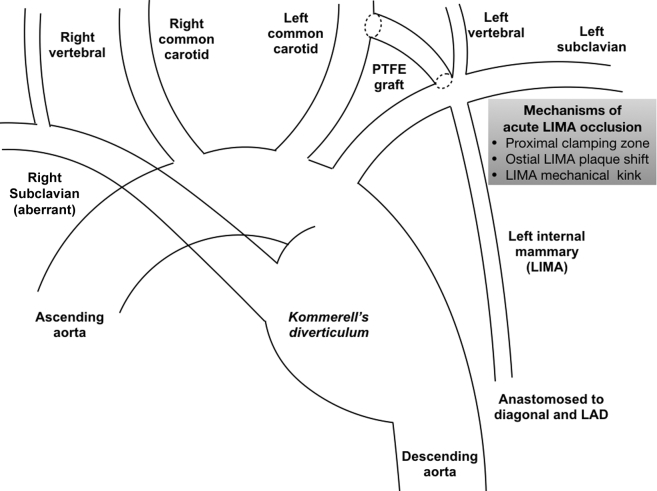
The reported case (3) in this issue of JACC: Case Reports represents an unusual complication in the management of a patient with aberrant right subclavian artery and associated Kommerell’s diverticulum (4). On the basis of the angiograms provided, the patient had an aberrant right subclavian artery (most common congenital anomaly of the aortic arch, product of abnormal embryonic development of the arch vessels), which arose distal to the takeoff of the left subclavian artery (Figure 1). In addition to the aberrant right subclavian artery, the patient also had aneurysmal changes of both the proximal portion of the aberrant right subclavian artery, as well as of the associated segment of the descending thoracic aorta. These aneurysmal changes are described as Kommerell’s diverticulum after the German radiologist Burckhard F. Kommerell (1901 to 1990), who first described this pathology in 1936 (5). The most common approach to treating patients with Kommerell’s diverticulum uses a hybrid plan, involving extra-anatomic bypass of the aortic arch vessels involved in the aneurysmal changes followed by endograft repair of the aneurysmal distal arch and proximal descending thoracic aorta and exclusion of the Kommerell’s aneurysmal changes 6, 7.
Figure 1.
The Anatomic Complexity of Kommerell’s Diverticulum
Illustration of the anatomic complexity of Kommerell’s diverticulum in the reported patient. Mechanisms of acute left internal mammary artery (LIMA) occlusion after left carotid to left subclavian artery bypass.
The reported patient, with a history of coronary bypass surgery, underwent a left common carotid to left subclavian bypass most likely to allow for creation of enough length of nonaneurysmal distal arch that could serve as the proximal attachment site for the placement of a descending thoracic aortic endograft (Figure 1). It appears that in the process of the performance of this bypass, the ostium of the left internal mammary artery (LIMA) became totally occluded. The patient went into cardiogenic shock, and emergent Impella-supported (Impella; Abiomed, Danvers, Massachusetts) percutaneous intervention of the ostial/proximal LIMA was performed from the left radial artery, after a failed attempt from the femoral access and initially unclear coronary bypass graft anatomy. Following successful intervention, the LIMA was found to be a skip graft, supplying a large first diagonal branch and left anterior descending artery. In follow-up, the left ventricular systolic function fully recovered.
In this case, the acute LIMA occlusion was most likely the result of 1 or more of the following issues:
-
1.
Incomplete pre-operative understanding of the anatomy of the LIMA and its role as the bypass conduit for both the large proximal diagonal and the left anterior descending coronary artery. This is likely the result of the unavailability of the medical records indicating the prior coronary bypass anatomy. This problem could have been circumvented with more detailed evaluation of the pre-operative computed tomography angiography and other potential pre-operative imaging, as well as access to the appropriate medical records.
-
2.
Use of the segment of the left subclavian artery out of which the LIMA originated as the site that received the distal anastomosis of the left common carotid to left subclavian bypass. Clamping of this segment of the left subclavian artery either stopped the flow of the LIMA leading to thrombosis, or possibly ruptured/dislocated an existing plaque of the LIMA ostium, resulting in total occlusion of the LIMA. This problem could have been avoided if a more distal segment of the left subclavian artery was selected as the recipient arterial segment for the bypass, allowing for placement of all clamps needed for the performance of the bypass at sites distal to the ostium of the LIMA.
-
3.
A post-operative change in the geometry of the LIMA producing a kink. It is possible that the placement of the short bypass graft that connected the left common carotid to the left subclavian artery produced a cephalad pull on the subclavian, which altered the geometry of the proximal LIMA against its takeoff from the left subclavian and introduced a local kink that compromised the patency of the LIMA. One can probably deal with such a technical issue by avoiding any tension on the prosthetic graft and by making sure the subclavian and carotid arteries easily return to their normal positions after the PTFE graft is sewn.
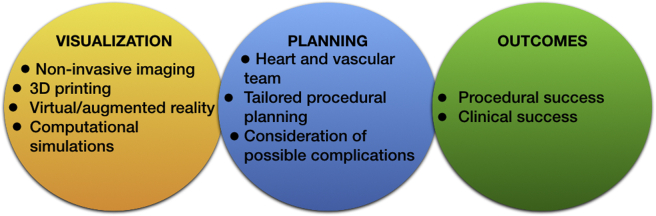
This case highlights the importance of thorough pre-procedural planning, especially when it comes to rare and anatomically complex percutaneous or surgical cardiovascular interventions (e.g., coronary, vascular, congenital) (Figure 2). Advanced noninvasive imaging tools, including computer tomography or magnetic resonance imaging, both equipped with post-processing 3-dimensional (3D) rendering capabilities can be indispensable in our hands to better understand the anatomical complexities in 3D space and plan accordingly. Modern approaches, including 3D printing 8, 9, virtual and augmented reality (10), and computational simulations 11, 12 can provide unique aid in forming a patient-specific surgical plan, prevent catastrophic complications, and know how to react and what to do when complications come. The heart and vascular team should be always formed and discuss those complex cases upfront, considering potential complications and alternative plans, ultimately achieving the best possible outcomes.
Figure 2.
Approach of Anatomically Complex Cardiovascular Interventions
3D = 3-dimensional.
When Dr. Kommerell saw for the first time an aberrant right subclavian artery compressing the esophagus in one of his patients, he called it lusus naturae, which in English means “freak of nature” (1). It was the lack of knowledge and understanding of the anatomic complexity of this rare entity that made him use the word freak. In fact, this is another anatomic variation of embryologic origin, which sometimes might require surgical correction. Modern medicine has all the right tools to help us visualize these rare anatomic entities, plan thoroughly, and prevent possible complications rather than cure them.
Footnotes
Dr. Chatzizisis has received consultation fees, speaker honoraria, and research support from Boston Scientific; and has received research support from Medtronic.
References
- 1.Kommerell B. Verlagerung des Ösophagus durch eine abnorm verlaufende Arteria subclavia dextra (Arteria lusoria) Fortschr Geb Roentgenstrahlen. 1936;54:590–595. [Google Scholar]
- 2.The Hippocratic Oath: Modern Version. https://www.pbs.org/wgbh/nova/ doctors/oath_modern.html Available at:
- 3.Rizzi S., Schneibel E., Park K. Myocardial infarction secondary to left internal mammary artery graft occlusion after left subclavian bypass. JACC Case Rep. 2019;1:120–123. doi: 10.1016/j.jaccas.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baig M.S., Timaran C.H. Upper extremity aneurysms. In: Sidawy A.P., Perler B.A., editors. Rutherford's Vascular Surgery and Endovascular Therapy. 9th edition. Elsevier; Philadelphia, PA: 2019. pp. 1095–1108. [Google Scholar]
- 5.van Son J.A.M., Konstantinov I.E. Burckhard F. Kommerell and Kommerell’s diverticulum. Tex Heart Inst J. 2002;29:109–112. [PMC free article] [PubMed] [Google Scholar]
- 6.Verzini F., Isernia G., Simonte G., De Rango P., Cao P., Italian AARSA Collaborative Group Results of aberrant right subclavian artery aneurysm repair. J Vasc Surg. 2015;62:343–350. doi: 10.1016/j.jvs.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Kim K.M., Cambria R.P., Isselbacher E.M. Contemporary surgical approaches and outcomes in adults with Kommerell diverticulum. Ann Thorac Surg. 2014;98:1347–1354. doi: 10.1016/j.athoracsur.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Giannopoulos A.A., Mitsouras D., Yoo S.J., Liu P.P., Chatzizisis Y.S., Rybicki F.J. Applications of 3D printing in cardiovascular diseases. Nat Rev Cardiol. 2016;13:701–718. doi: 10.1038/nrcardio.2016.170. [DOI] [PubMed] [Google Scholar]
- 9.Dugas C.M., Schussler J.M. Advanced technology in interventional cardiology: a roadmap for the future of precision coronary interventions. Trends Cardiovasc Med. 2016;26:466–473. doi: 10.1016/j.tcm.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Fischer G., Stadie A., Schwandt E. Minimally invasive superficial temporal artery to middle cerebral artery bypass through a minicraniotomy: benefit of three-dimensional virtual reality planning using magnetic resonance angiography. Neurosurg Focus. 2009;26:E20. doi: 10.3171/2009.2.FOCUS0917. [DOI] [PubMed] [Google Scholar]
- 11.Willaert W.I., Aggarwal R., Van Herzeele I., European Virtual Reality Endovascular Research Team EVEResT Patient-specific endovascular simulation influences interventionalists performing carotid artery stenting procedures. Eur J Vasc Endovasc Surg. 2011;41:492–500. doi: 10.1016/j.ejvs.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Antoniadis A.P., Mortier P., Kassab G. Biomechanical modeling to improve coronary artery bifurcation stenting: expert review document on techniques and clinical implementation. J Am Coll Cardiol Intv. 2015;8:1281–1296. doi: 10.1016/j.jcin.2015.06.015. [DOI] [PubMed] [Google Scholar]