Abstract
Selenium (Se) is an essential trace element in human. Recent studies of Se supplementation on the effect of Hashimoto’s thyroiditis (HT) have been reported, but the exact benefit is unclear as well as the underlying immunologic mechanism. We aimed to evaluate the clinical effect of Se supplement in patients with HT, and explore the potential mechanism against thyroid autoimmunity. A prospective, randomized‑controlled study was performed in patients with HT assigned to two groups. Se‐treated group (n = 43) received selenious yeast tablet (SYT) for 6 months, whereas no treatment in control group (n = 47). The primary outcome is the change of thyroid peroxidase antibody (TPOAb) or thyroglobulin antibody (TGAb). Second, thyroid function, urinary iodine, Se, Glutathione peroxidase3 (GPx3), and Selenoprotein P1 (SePP1) levels were measured during the SYT treatment. Meanwhile, regulatory T cells (Tregs) and their subsets activated Tregs (aTregs), resting Tregs, and secreting Tregs, as well as Helios and PD‐1 expression on these cells were also detected. The results showed that SYT treatment significantly decreased TPOAb, TGAb, and thyroid stimulating hormone (TSH) levels, accompanied with the increased Se, GPx3, and SePP1, compared with the control group. Subgroup analysis revealed that subclinical HT may benefit more from this treatment in the decrease of TSH levels by interaction test. Moreover, the percentage of aTregs, Helios/Tregs, and Helios/aTregs were significantly higher in the Se‐treated group than control. In conclusion, Se supplementation may have a beneficial effect on thyroid autoantibodies and thyroid function by increasing the antioxidant activity and upregulating the activated Treg cells.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Selenium (Se) is an essential trace mineral for human health that has a wide range of biological effects, ranging from antioxidant and anti‐inflammatory capacity to thyroid hormone metabolism. The clinical effect of Se supplementation in patients with Hashimoto’s thyroiditis (HT) is inconsistent, and the immunological mechanism is weakly understood.
WHAT QUESTION DID THIS STUDY ADDRESS?
The aim was to perform a prospective, randomized‑controlled study to evaluate the clinical effect of Se in patients with HT without levothyroxine treatment, and explore the potential impact on regulatory T cells (Tregs).
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study showed that Se supplementation was associated with reductions in thyroid autoantibodies and thyroid stimulating hormone levels, which may be explained by the enhanced antioxidant ability and upregulated activated Treg cells in vivo of patients with HT.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Se supplementation may benefit patients with HT with low Se status, and have potential to prevent thyroid dysfunction in the clinical course of the disease.
INTRODUCTION
Hashimoto’s thyroiditis (HT), also named chronic lymphocytic thyroiditis, is the most common organ‐specific autoimmune thyroid disease (AITD) and is associated with lymphocytic infiltration and high levels of thyroid autoantibodies, such as thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TGAb). The etiology of HT is multifactorial, including genetic susceptibility, environmental factors, and trace elements, which could lead to autoimmune disorder and abnormal thyroid function. 1 There is no specific therapeutic approach to suppress immune destruction, thus thyroxine replacement has been the generally accepted therapy for patients with HT with hypothyroidism. Recently, some clinical studies 2 , 3 , 4 , 5 have demonstrated that selenium (Se) supplementation decreased TPOAb and/or TGAb titers, improving glandular echogenicity and thyroid function in patients with HT, suggesting Se might be helpful to thyroid autoimmunity. However, a 2016 web survey conducted by Italian endocrinologists, as well as subsequent critical publications throw doubt on the efficacy of Se treatments, 6 , 7 further studies on therapeutic effects and concrete mechanism are therefore warranted.
Se, an essential trace element, has multiple biological functions for human health. The thyroid gland contains the highest concentration of Se, which is incorporated into selenoproteins, such as glutathione peroxidase (GPx), selenoprotein P (SePP), thioredoxin reductase, and iodothyronine deiodinases. These selenoenzymes play important roles in thyroid hormone metabolism by acting as antioxidants and immunomodulators. 8 Given that the extracellular GPx3 and Se‐transporter SePP1 show plateaued expression in plasma, we measured these two plasma selenoproteins to evaluate the nutritional status of Se. Moreover, iodine as the primary factor to thyroid hormone synthesis is closely related to pathogenesis of autoimmune thyroiditis. The status of iodine deficiency or excessive iodine intake results in an increase in the prevalence of HT. 9 Therefore, adjusting the iodine intake to the recommended level will help us better understand the impact of Se in the treatment of HT.
It is notable that the reduction or functional regulatory T cells (Tregs) probably contribute to the pathogenesis of HT. 10 , 11 , 12 , 13 Treg cell is a crowd of CD4+T cells, which exert the ability of immunosuppression to excessive immune responses. CD4+CD25+Foxp3+Treg is at present considered the typical phenotype of Treg cell, and then based on the expression of CD45RO and Foxp3, it is divided into three subpopulations, activated Foxp3highCD45RO+Treg (aTreg), resting Foxp3lowCD45RO− Treg (rTreg), and cytokine‐secreting Foxp3lowCD45RO+ Treg (sTreg). 14 The former two groups, especially the aTreg cells, are known to have major suppressive effects on activated effector T cells. Besides, two novel markers Helios and PD‐1 have been found to enhance Treg cell immune tolerance ability, and thus regarded as important functional indicators for Treg. In our previous study, 15 we demonstrated reduced Treg frequency and aberrant Helios and PD‐1 expressions were related to thyroiditis development. These suggest that the progression of HT may be prevented by improving Treg cells in vivo.
Although some studies on the effects of Se supplementation on HT have been reported, the results are controversial and immunological mechanism has not been elucidated. Our aim was to conduct a prospective, randomized‑controlled trial to evaluate whether the selenious yeast tablet (SYT) treatment is a benefit to the thyroid antibodies and thyroid function. Furthermore, we first explored the impact of Se on CD4+CD25+Foxp3+Treg numbers and function in patients with HT.
METHODS
Study design and outcomes
A 6‐month prospectively, single‐center, open‐label, randomized, parallel controlled trial was conducted in the Endocrinology Department of the First Affiliated Hospital of Nanjing Medical University, between October 2017 and February 2020.
The primary outcome was thyroid antibody titer (TPOAb or TGAb). The secondary outcomes were thyroid hormones, Treg numbers and function, serum Se and selenoprotein (GPx3 and SePP1), and urine iodine (UI) levels.
Subjects
A total of 155 individuals were prospectively recruited from the Endocrinology Department of the First Affiliated Hospital of Nanjing Medical University, and finally 126 subjects (90 patients with HT and 36 healthy people) were eligible and completed this study (Flow diagram: Figure S1), inclusion and exclusion criteria were described in Supplementary Experimental Procedures. The patients with HT were assigned into two groups by a random‐number generator program: Se‐treated group (n = 43) received 200 μg/day SYT for at least 6 months, and no treatment was given in the control group (n = 47). Dietary iodine intervention is recommended in both two groups, and the specific method is to instruct patients to adjust iodine intake to an appropriate level (100–199 μg/L) by Out‐Patient Questionnaire, urinary iodine detection, dietary education, and follow‐up survey. No changes in medication protocols were allowed throughout the study. The patients would be followed‐up at 3 months and 6 months to detect TPOAb and TGAb titers, thyroid hormones, serum Se and UI levels, GPx3 and SePP1 concentrations, Treg numbers, and function. Treatment compliance and physical condition of participants were visited at each month.
Ethics statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2017‐SR‐141), and the protocol has been registered in the Chinese clinical trial registry with the No. ChiCTR2000032905.
Laboratory testing
Peripheral venous blood samples were obtained from each participant on the morning after overnight fast at baseline, 3 months and 6 months, then centrifuged at 1000 g for 10 min to separate the serum. Serum FT4, FT3, thyroid stimulating hormone (TSH), TPOAb, and TGAb were measured by electrochemiluminescence immunoassay using Roche cobas e601 reagent kits. The manufacturer’s reference values were FT3: 3.10 to 6.8 pmol/L, FT4: 12.00 to 22.00 pmol/L, TSH: 0.27–4.20 mIU/L, TPOAb less than 34 IU/ml, and TGAb less than 115.0 IU/ml.
Subjects were instructed to collect the specimen of midstream urine in a sterile screw‐top container. The urinary iodine concentration was detected by arsenic‐cerium catalytic spectrophotometry (WS/T 107‐2016). According to the World Health Organization (WHO) and International Council for Control of Iodine Deficiency Disorders (IC‐CIDD) guidelines, 16 median urinary iodine (MUI) value was disaggregated into: iodine deficiency (< 100 μg/L), sufficient iodine level (100–199 μg/L), above iodine requirements (200–299 μg/L), and excessive iodine intake (> 300 μg/L).
Ultrasound examination
Thyroid ultrasound examination is described in Supplementary Experimental Procedures.
Serum selenium and selenoprotein determination
Serum Se concentration test was run by inductively coupled plasma mass spectrometry. Blood serum was diluted 10‐fold with acidic solution consisting of nitric acid (0.05%) and Triton X‐100 (0.01%), then an internal standard of 89Y was added to correct for matrix effect and ion signal instability. In subanalysis of our data, subjects were divided into two groups (S1 < 80 μg/L and S2 > 80 μg/L) by baseline serum Se.
Serum GPx3 and SePP1 levels were assayed by enzyme‐linked immunosorbent assay kit according to the manufacturer’s instructions (GENE MEI BIOTECH Co., Ltd.). More detailed steps are described in Supplementary Experimental Procedures.
Flow cytometric analysis of Treg cells
Peripheral blood mononuclear cells were isolated from heparinized venous blood by Ficoll‐Hypaque density centrifugation, and stained with the surface antibodies (CD3, CD4, CD25, CD45RO, and PD‐1) and intracellular antigens (Foxp3 and Helios), details are described in Supplementary Experimental Procedures. Flow cytometric analysis was performed to sort and quantify the stained cells on a seven‐color FACScan flow cytometer (FCM, FACS Verse, BD Biosciences). Treg cells were gated as the population of CD3+CD4+CD25+Foxp3+ cells, which can further be divided into three subsets of Foxp3hiCD45RO+ aTregs, CD45RO+Foxp3lo sTregs, and CD45RO−Foxp3lo rTregs based on CD45RO and Foxp3 expression. Finally, Treg function was analyzed by the positive percentage of Helios or PD‐1 in the presence of Treg cells and subsets.
Statistical analysis
The SPSS 25.0 software (IBM) was used to analyze the data. The sample size takes into account the variation in thyroid antibody with a type I error α = 0.05 and a type II error β = 0.2 in a two‐sided test (80% power). Normality of the continuous variables was detected by the Shapiro‐Wilk normality test. Normal distributions and measurement data were expressed as means ± SDs, whereas skewed distributions were expressed as median (25th–75th percentiles). The categorical variables were represented as percentages. The differences between two or more groups in quantitative variables were analyzed by Student’s t‐test or one‐way analysis of variance when data were normal or log transformed to normalize values, and by Mann–Whitney U tests or Kruskal–Wallis test for abnormal distribution data. The χ 2 test was used to compare the enumeration data. Subgroup analyses were conducted by interaction and stratified test. Pairwise correlations between parameters were determined by Pearson’s or Spearman’s test. This multiplicity is handled by Bonferroni statistical tests or Dunn’s post hoc tests. Differences were regarded as statistically significant at p < 0.05.
.
RESULTS
Characteristics of subjects
The baseline demographic data of the study population were shown in Table 1. There were no significant differences in age, gender, and body mass index (BMI) between patients with HT (n = 90) and healthy people (n = 36). The MUI concentration of patients with HT was significantly higher than that of healthy people (246.6 μg/L vs. 159.5 μg/L, p = 0.004), and the percent distributions (Table S1) between the two groups were significantly different (p = 0.033), whereas no statistical significance between the patients with subclinical HT (SHT) and euthyroid HT (EHT) (p = 0.695). Basal Se status of subjects has markedly individual variation (30–200 μg/L), but most persons showed moderate‐severe SE deficiency according to the optimal level (≈125 μg/L). 17 Although serum Se, GPx3, and SePP1 were lower in patients with HT compared with those in healthy people, the only significant difference was GPx3 (p = 0.003).
Table 1.
Baseline demographic and clinical characteristics of the subjects
| Healthy people | Patients with HT | p value | Se group | Control group | p value | |
|---|---|---|---|---|---|---|
| No. of subjects | 36 | 90 | 43 | 47 | ||
| Thyroid function (n) | ||||||
| Euthyroidism | 36 | 61 | 27 | 34 | ||
| Subclinical hypothyroidism | 0 | 29 | 16 | 13 | ||
| Age, years | 36.2 ± 8.4 | 38.6 ± 11.6 | 0.205 | 39.4 ± 12.0 | 37.8 ± 11.2 | 0.513 |
| Sex, F/M | 31/5 | 80/10 | 0.664 | 39/4 | 41/6 | 0.601 |
| BMI, kg/m2 | 21.7 ± 1.8 | 21.0 ± 2.1 | 0.122 | 21.0 ± 2.5 | 21.1 ± 1.7 | 0.877 |
| MUI, μg/L | 159.5 (120, 230) | 246.6 (132.2, 327.6) | 0.004 | 267.0 (129.2, 380) | 232.0 (132.3, 288.8) | 0.255 |
| Se, μg/L | 76.9 (42.3, 104.2) | 73.3 (35.6, 118.5) | 0.493 | 73.6 (57.8, 95.7) | 65.0 (42.1, 131.0) | 0.218 |
| GPx3, ng/ml | 22.1 (19.5, 29.5) | 18.7 (15.0, 27.6) | 0.003 | 18.8 (15.6, 34.8) | 18.5 (13.8, 24.9) | 0.162 |
| SePP1, ng/ml | 16.7 (14.3, 20.3) | 14.8 (10.5, 20.8) | 0.085 | 16.0 (11.9, 26.6) | 12.9 (9.6, 18.9) | 0.094 |
| TPOAb, IU/ml | 13.2 (10.0, 20.9) | 208.1 (81.6 427.9) | <0.001 | 237.3 (148.2, 474.3) | 177.0 (59.2, 419.7) | 0.107 |
| TGAb, IU/ml | 20.1 (11.1, 37.7) | 370.8 (157.7, 370.9) | <0.001 | 435.0 (207.4, 615.7) | 370.7 (146.8, 509.7) | 0.253 |
| TSH, mIU/L | 2.01 (1.46, 3.04) | 2.92 (1.9, 4.66) | <0.001 | 3.16 (2.09, 5.5) | 2.81 (1.89, 4.28) | 0.503 |
| FT3, pmol/L | 4.47 ± 0.69 | 4.54 ± 0.67 | 0.587 | 4.43 ± 0.65 | 4.63 ± 0.68 | 0.168 |
| FT4, pmol/L | 16.37 ± 2.06 | 15.56 ± 2.49 | 0.088 | 15.42 ± 2.58 | 15.69 ± 2.42 | 0.612 |
Normally distributed data are described as mean ± SD. Non‐normal data are described as median (25th–75th percentile).
Abbreviations: BMI, body mass index; F, female, FT3, free triiodothyronine; FT4, free thyroxine; GPx3, glutathione peroxidase3; HT, Hashimoto thyroiditis; M, male; MUI, median urinary iodine; Se, selenium; SePP1, selenoprotein P1; TgAb, antithyroglobulin antibodies; TPOAb, antithyroid peroxidase antibodies; TSH, thyroid stimulating hormone.
Of the patients with HT, the age, gender, BMI, serum Se, GPx3, SePP1, thyroid function, and thyroid autoantibodies were similar at baseline between the Se group and the control group. The patients completed the follow‐up visits, and nobody suffered any adverse effects with 6 months of Se treatment, such as loss of hair and nails, nausea, vomiting, diarrhea, and dizziness.
Selenium supplementation in HT
The effect of SYT on clinical parameters in patients with HT are presented in Table 2. The MUI concentration of patients with HT presented a trend of gradual decrease after the limit of iodine intake, and there are similar number of subjects who had decrease in iodine intake as an intervention between the Se‐treated group (n = 27) and the control group (n = 29). Iodine nutritional status was at an appropriate level at 6 months in both the Se‐treated and control groups, but with no significant difference between two groups (150.5 μg/L vs. 145.4 μg/L, p = 0.793). Serum Se levels in the Se‐treated group were increased remarkably, from a basal median of 73.6 μg/L to 145.6 μg/L at 3 months, and to 187.2 μg/L at 6 months, with significant changes compared with the control group. In parallel to an increase in levels of Se, the GPx3 increase was more pronounced in the Se‐treated group compared with control after 6 months of treatment (p = 0.028). Another significant increase of SePP1 was observed in the Se‐treated group after 6 months, but significant decreases in the control group after 3 and 6 months were compared with baseline values. These resulted in higher levels of SePP1 in the Se‐treated group compared with the control group at 3 months (16.2 [9.3, 22.7] vs. 10.8 [8.2, 14.4], p = 0.001) and 6 months (17.2 [9.8, 22.1] vs. 10.7 [8.9, 14.6], p = 0.007).
Table 2.
Serum Se, selenoproteins, thyroid antibody titers, and thyroid function in groups during Se supplement
| Patients with HT | p value | |||
|---|---|---|---|---|
| Se group | Control group | |||
| N | 43 | 47 | ||
| MUI, μg/L | Baseline | 267.0 (129.2, 380) | 232.0 (132.3, 288.8) | 0.255 |
| 3 months | 201.5 (145.1, 271.5) | 178.1 (126.3, 254.6) | 0.262 | |
| 6 months | 150.5* (108.2, 217.6) | 145.4* (111.5, 224.0) | 0.793 | |
| Se, μg/L | Baseline | 73.6 (57.8, 95.7) | 65.0 (42.1, 131.0) | 0.218 |
| 3 months | 145.6** (80.1, 193.5) | 87.0* (42.6, 145.5) | 0.001 | |
| 6 months | 187.2** (102.4, 234.5) | 72.0 (46.0, 132.5) | <0.001 | |
| GPx3, ng/ml | Baseline | 18.8 (15.6, 34.8) | 18.5 (13.8, 24.9) | 0.162 |
| 3 months | 25.9** (19.1, 44.2) | 22.3* (15.7, 44.7) | 0.08 | |
| 6 months | 45.2** (23.7, 68.4) | 24.2* (15.3, 50.4) | 0.028 | |
| SePP1, ng/ml | baseline | 16.0 (11.9, 26.6) | 12.9 (9.6, 18.9) | 0.094 |
| 3 months | 16.2 (9.3, 22.7) | 10.8** (8.2, 14.4) | 0.001 | |
| 6 months | 17.2* (9.8, 22.1) | 10.7** (8.9, 14.6) | 0.007 | |
| TPOAb, IU/ml | Baseline | 237.3 (148.2, 474.3) | 177.0 (59.2, 419.7) | 0.107 |
| 3 months | 191.9** (100, 381) | 188.5 (59, 328.8) | 0.484 | |
| 6 months | 178.3** (78.8, 339.4) | 211.3 (62.0, 354.7) | 0.942 | |
| ΔTPOAb, IU/ml | 3 months‐baseline | −8.7 (−81.1, 3.3) | −1.1 (−28.4, 14.3) | 0.074 |
| 6 months‐baseline | −28.4 (−103.9, 0) | 0 (−18.1, 20.5) | 0.001 | |
| TGAb, IU/ml | Baseline | 435.0 (207.4, 615.7) | 370.7 (146.8, 509.7) | 0.253 |
| 3 months | 434.4 (187.5, 627.3) | 331.9 (164.0, 534.4) | 0.203 | |
| 6 months | 388.0 (175.4, 529.7) | 364.6 (186.0, 556.5) | 0.891 | |
| ΔTGAb, IU/mLl3 months‐baseline | −19.2 (−98.4, 43.3) | 0.7 (−48.0, 60.4) | 0.267 | |
| 6 months‐baseline | −48.8 (−139.7, −2.0) | 18.3 (−23.5, 77.4) | 0.001 | |
| TSH, mIU/L | Baseline | 3.16 (2.09, 5.5) | 2.81 (1.89, 4.28) | 0.503 |
| 3 months | 2.83 (2.01, 4.39) | 2.76 (2.11, 3.95) | 0.977 | |
| 6 months | 2.4 (1.92, 3.54) | 3.24** (2.32, 4.65) | 0.021 | |
| ΔTSH, mIU/L | 3 months‐baseline | −0.2 (−1.2, 0.49) | 0.18 (−0.39, 0.69) | 0.084 |
| 6 months‐baseline | −0.16 (−2.1, 0.28) | 0.48 (−0.15, 1.47) | 0.001 | |
| FT3, pmol/L | Baseline | 4.43 ± 0.65 | 4.63 ± 0.68 | 0.168 |
| 3 months | 4.72 ± 0.74 | 4.64 ± 0.74 | 0.611 | |
| 6 months | 4.69 ± 0.67 | 4.63 ± 0.69 | 0.691 | |
| FT4, pmol/L | Baseline | 15.42 ± 2.58 | 15.69 ± 2.42 | 0.612 |
| 3 months | 15.69 ± 2.54 | 16.45 ± 2.42 | 0.152 | |
| 6 months | 16.41 ± 2.61 | 15.77 ± 1.94 | 0.191 |
Δ = value after treatment − baseline value.
*p < 0.05, **p < 0.01 vs. baseline.
Abbreviations: FT3, free triiodothyronine; GPx3, glutathione peroxidase3; HT, Hashimoto thyroiditis; MUI, median urinary iodine; Se, selenium; SePP1, selenoprotein P1; TgAb, antithyroglobulin antibodies; TPOAb, antithyroid peroxidase antibodies; TSH, thyroid stimulating hormone.
In the Se‐treated group, TPOAb levels were significantly lower than that in study entry after 3 and 6 months, and the reductions were statistically different compared with the control group at 6 months (ΔTPOAb [IU/ml] = −28.4 [−103.9, 0] vs. 0 [−18.1, 20.5], p = 0.001). Meanwhile, a significant difference of TGAb titers between the Se‐treated group and the control group was appeared at 6 months (ΔTGAb [IU/ml] = −48.8 [−139.7, −2.0] vs. 18.3 [−23.5, 77.4], p = 0.001; Figure S2). Compared with baseline, TSH presented slightly lower levels in the Se‐treated group, whereas there was a statistical increase in the control group after 6 months, thus this difference approached significance between the 2 groups at 6 months (ΔTSH [mIU/L] = −0.16 [−2.1, 0.28] vs. 0.48 [−0.15, 1.47], p = 0.001). However, neither FT3 nor FT4 levels had a significantly difference between the 2 groups at 3 or 6 months.
Subgroup analysis by basal Se levels or thyroid function status
We have demonstrated significant differences in SYT treatment‐induced changes of TPOAb, TGAb, and TSH titers between the Se‐treated group and the control group at 6 months. The subgroup analysis was performed to identify certain patients who would be more likely to benefit from Se supplementation (Table 3), based on the positive outcomes above. We divided patients into subgroups defined by thyroid function (EHT and SHT) and Se‐level, because these may be key influencers in clinical outcomes during SYT treatment. Considering that Se deficiency requires a serum Se concentration of ~80 μg/L, also the median Se concentration of subjects in this study approaches 80 μg/L, so we selected a cutoff level of 80 μg/L (S1 < 80 μg/L and S2 > 80 μg/L). In the S1 group, there was a significant difference between the Se‐treated group and the control group in TPOAb, TGAb, or TSH amounts decreased (p = 0.001, p = 0.002, and p = 0.001, respectively), although the trend seems to exist in the S2 subgroup, no statistical difference was found. However, the treatment effect did not differ between treatment groups and Se‐level category by interaction test (p‐interaction = 0.113 for ΔTPOAb, 0.687 for ΔTGAb, 0.116 for ΔTSH). In thyroid function subgroups, a significant reduction of TPOAb between the treatment and control groups occurred in the SHT group, whereas TGAb occurred both in the EHT and SHT groups, but with a similar amount across subgroups (p‐interaction = 0.054 for ΔTPOAb, 0.853 for ΔTGAb). It is noteworthy that the effect of SYT treatment on TSH changes are not consistent across different thyroid functions, a significant decrease was observed in SHT but not in EHT, and with statistical evidence of an interaction (p‐interaction = 0.003).
Table 3.
Subgroup analysis of treatment‐induced changes in thyroid antibody and TSH titers by basal Se levels or thyroid function status
| Se‐treated group | Control group | p value | Interaction p value | ||
|---|---|---|---|---|---|
| N | Total | 43 | 47 | ||
| Outcomes | Subgroup | ||||
| ΔTPOAb, IU/ml | Se level | 0.113 | |||
|
S1 < 80 μg/L (n = 49) |
−37.1 (n = 25) (−121.7, 0.0) |
2.9 (n = 24) (−19.6, 33.7) |
0.001 | ||
|
S2 ≥ 80 μg/L (n = 41) |
−6.45 (n = 18) (−50.9, 0.0) |
−1.2 (n = 23) (−18.1, 2.5) |
0.180 | ||
| Thyroid function | 0.054 | ||||
| EHT (n = 61) |
−6.8 (n = 27) (−47.1, 0.0) |
−0.5 (n = 34) (−18.8, 32.6) |
0.077 | ||
| SHT (n = 29) |
−86.5 (n = 16) (−195.7, −28.3) |
0.0 (n = 13) (−12.7, 12.8) |
0.003 | ||
| ΔTGAb, IU/ml | Se level | 0.687 | |||
|
S1 <80 μg/L (n = 49) |
−43.3 (n = 25) (−114.4, −3.4) |
30.5 (n = 24) (−18.8, 73.4) |
0.002 | ||
|
S2 ≥ 80 μg/L (n = 41) |
−56.5 (n = 18) (−375.1, 22.7) |
12.7 (n = 23) (−36.5, 95.6) |
0.098 | ||
| Thyroid function | 0.853 | ||||
| EHT (n = 61) |
−62.3 (n = 27) (−181.8, −4.2) |
18.1 (n = 34) (−32.9, 77.3) |
0.004 | ||
| SHT (n = 29) |
−21.3 (n = 16) (−126.1, 27.7) |
26.6 (n = 13) (−5.3, 112.8) |
0.039 | ||
| ΔTSH, mIU/L | Se level | 0.116 | |||
|
S1 < 80 μg/L (n = 49) |
−0.63 (n = 25) (−2.83, 0.27) |
0.49 (n = 24) (1.11, 0.12) |
0.001 | ||
|
S2 ≥ 80 μg/L (n = 41) |
−0.01 (n = 18) (−0.67, 0.52) |
0.29 (n = 23) (−0.91, 1.74) |
0.293 | ||
| Thyroid function | 0.003 | ||||
| EHT (n = 61) |
0.01 (n = 27) (−0.61, 0.68) |
0.38 (n = 34) (−0.05, 1.06) |
0.079 | ||
| SHT (n = 29) |
−2.5 (n = 16) (−4.02, −0.28) |
1.91 (n = 13) (−1.36, 2.76) |
0.002 |
Δ = value after treatment − baseline value.
Abbreviations: EHT, euthyroid Hashimoto thyroiditis; S1: Se <80 μg/L; S2: >80 μg/L; Se, selenium; SHT, subclinical Hashimoto thyroiditis; TgAb, antithyroglobulin antibodies; TPOAb, antithyroid peroxidase antibodies; TSH, thyroid stimulating hormone.
Effects of selenium on Treg cells
The percentage of Treg cells in peripheral blood were evaluated (an example is shown in Figure 1), all data are summarized in Table 4. There were no significant differences in baseline covariates of Treg cells between study groups. After application of SYT, the percentages of Treg cells were increased after 6 months, but there was no significant difference compared with the control group (5.3 ± 1.74 vs. 4.89 ± 1.36, p = 0.214). Notably, the proportion of aTreg cells in the Se‐treated group was significantly higher than the control group at 6 months (13.19 ± 3.5 vs. 11.49 ± 2.79, p = 0.012), whereas there were no statistical differences between groups of rTreg or sTreg cells. In addition, the Se‐treated group showed Helios expression was upregulated significantly in Treg cells compared with the control group at 6 months (78.07 ± 8.07 vs. 72.55 ± 8.78, p = 0.003), as well as the trends were observed in aTreg cells at 3 months (82.98 ± 4.71 vs. 79.84 ± 4.83, p = 0.002) and 6 months (83.32 ± 5.18 vs. 79.97 ± 4.85, p = 0.002). However, no significant changes of PD‐1 expression on Treg cells or their subsets occurred in the study groups.
Figure 1.
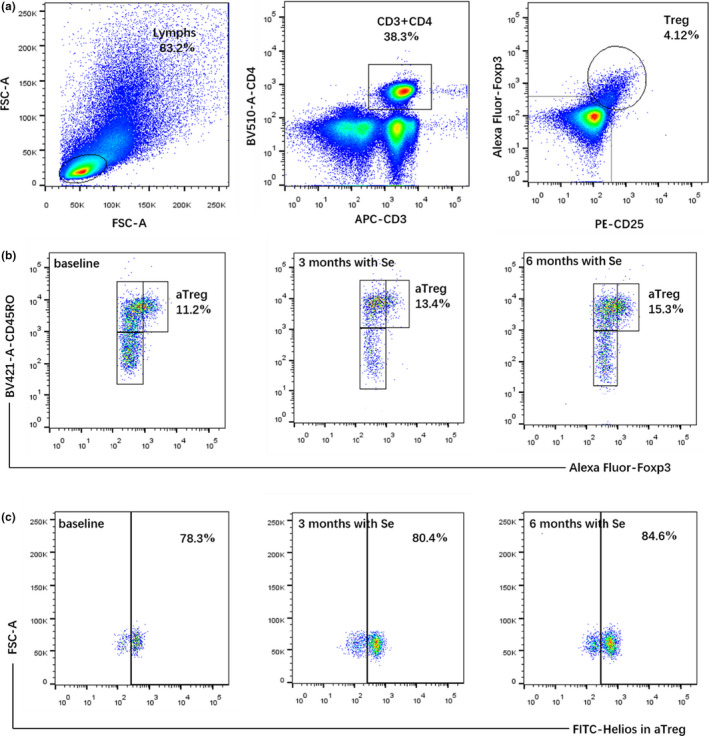
An example of a flow cytometry analysis in patients with HT. (a) The method for the assessment of Treg cells. (b) Se supplementation increased the percentage of aTreg cells. (c) Helios expressing in aTreg cells were increased after SYT treatment. aTreg, activated regulatory T cells; HT, Hashimoto thyroiditis; Treg, regulatory T cells; SYT, selenious yeast tablet
Table 4.
Se effect on Treg cells in patients with HT
| Patients with HT | p value | |||
|---|---|---|---|---|
| Se group | Control group | |||
| Treg, % | Baseline | 4.60 ± 1.38 | 4.82 ± 1.02 | 0.387 |
| 3 months | 4.98 ± 1.56 | 4.65 ± 0.87 | 0.225 | |
| 6 months | 5.30 ± 1.74** | 4.89 ± 1.36 | 0.214 | |
| aTreg, % | Baseline | 11.7 ± 3.30 | 11.63 ± 3.31 | 0.908 |
| 3 months | 12.20 ± 3.44 | 11.33 ± 2.13 | 0.154 | |
| 6 months | 13.19 ± 3.5** | 11.49 ± 2.79 | 0.012 | |
| rTreg, % | Baseline | 36.08 ± 9.86 | 36.44 ± 11.60 | 0.844 |
| 3 months | 35.69 ± 9.01 | 35.22 ± 11.28 | 0.829 | |
| 6 months | 33.90 ± 8.49 | 35.56 ± 11.52 | 0.447 | |
| sTreg, % | Baseline | 53.51 ± 8.69 | 53.21 ± 10.33 | 0.884 |
| 3 months | 51.74 ± 9.16 | 53.53 ± 11.13 | 0.405 | |
| 6 months | 52.02 ± 9.95 | 51.71 ± 10.21 | 0.884 | |
| Helios/Treg, % | Baseline | 72.97 ± 8.35 | 72.21 ± 8.4 | 0.668 |
| 3 months | 75.41 ± 7.99 | 73.82 ± 8.94 | 0.379 | |
| 6 months | 78.07 ± 8.07** | 72.55 ± 8.78 | 0.003 | |
| Helios/aTreg, % | Baseline | 79.78 ± 3.57 | 81.11 ± 4.29 | 0.119 |
| 3 months | 82.98 ± 4.71** | 79.84 ± 4.83 | 0.002 | |
| 6 months | 83.32 ± 5.18** | 79.97 ± 4.85 | 0.002 | |
| Helios/rTreg, % | Baseline | 67.79 ± 12.70 | 63.38 ± 18.18 | 0.182 |
| 3 months | 67.77 ± 13.21 | 64.65 ± 19.26 | 0.370 | |
| 6 months | 67.84 ± 13.24 | 64.88 ± 19.05 | 0.392 | |
| Helios/sTreg, % | baseline | 68.96 ± 11.46 | 71.32 ± 12.25 | 0.347 |
| 3 months | 71.51 ± 7.91 | 70.58 ± 8.78 | 0.601 | |
| 6 months | 70.14 ± 8.59 | 72.44 ± 8.64 | 0.210 | |
| PD‐1/Treg, % | Baseline | 20.81 ± 8.93 | 18.66 ± 7.32 | 0.214 |
| 3 months | 20.63 ± 8.98 | 19.75 ± 8.05 | 0.628 | |
| 6 months | 21.20 ± 8.61 | 20.03 ± 8.20 | 0.512 | |
| PD‐1/aTreg, % | Baseline | 36.13 ± 16.51 | 34.1 ± 14.6 | 0.539 |
| 3 months | 35.57 ± 14.81 | 33.63 ± 17.1 | 0.567 | |
| 6 months | 36.22 ± 17.76 | 32.42 ± 14.30 | 0.266 | |
| PD‐1/rTreg, % | Baseline | 3.8 ± 2.31 | 4.32 ± 2.49 | 0.306 |
| 3 months | 3.89 ± 2.27 | 4.20 ± 2.50 | 0.537 | |
| 6 months | 3.90 ± 2.36 | 4.46 ± 2.45 | 0.277 | |
| PD‐1/sTreg, % | Baseline | 25.81 ± 9.2 | 23.36 ± 7.44 | 0.166 |
| 3 months | 25.29 ± 8.16 | 25.03 ± 7.89 | 0.876 | |
| 6 months | 24.96 ± 9.17 | 23.41 ± 8.04 | 0.395 | |
**Statistically significant difference (<0.01) between post‐treatment and baseline in the same group.
Abbreviations: aTreg, activated regulatory T cells; HT, Hashimoto thyroiditis; rTreg, resting regulatory T cells; Se, selenium; sTreg, secreting regulatory T cells; Treg, regulatory T cells.
Given the interaction of the treatment group with thyroid function was significant for TSH reduction, we further performed a subgroup analysis on established changes in Treg cells based on thyroid function status (Table 5). In parallel to the increased percentage of aTreg, Helios/Treg, and Helios/aTreg in patients with SYT treatment, the differences were similar between the EHT group and the SHT group, and without nominally significant interaction, demonstrating no distinct difference between the two groups in improving immune ability of Treg cells.
Table 5.
Subgroup analysis on Se effect on Treg in different thyroid function status
| EHT | p value | SHT | p value | Interaction p | |||
|---|---|---|---|---|---|---|---|
| Se group | Control group | Se group | Control group | ||||
| N | 27 | 34 | 16 | 13 | |||
| aTreg, % | 11.39 ± 3.08 | 11.45 ± 3.46 | 0.939 | 12.25 ± 3.69 | 12.08 ± 2.96 | 0.897 | |
| aTreg, %–6 mo | 13.07 ± 3.25** | 11.39 ± 2.90 | 0.037 | 13.41 ± 3.99** | 11.77 ± 2.57 | 0.211 | |
| ΔaTreg, %–6 mo | 1.68 ± 1.11 | −0.07 ± 2.07 | <0.001 | 1.16 ± 1.23 | −0.31 ± 1.97 | 0.030 | 0.720 |
| Helios/Treg, % | 72.48 ± 7.56 | 73.27 ± 7.73 | 0.690 | 73.79 ± 9.75 | 69.43 ± 9.73 | 0.240 | |
| Helios/Treg, %–6 mo | 76.66 ± 7.84* | 73.32 ± 8.57 | 0.123 | 80.46 ± 8.15* | 70.53 ± 9.34 | 0.005 | |
| ΔHelios/Treg, %–6 mo | 4.18 ± 7.81 | 0.05 ± 7.58 | 0.042 | 6.66 ± 7.8 | 1.09 ± 4.83 | 0.027 | 0.668 |
| Helios/aTreg, % | 79.87 ± 4.19 | 81.10 ± 4.65 | 0.241 | 79.86 ± 2.28 | 81.11 ± 3.32 | 0.241 | |
| Helios/aTreg, %–6 mo | 81.79 ± 4.58 | 79.95 ± 4.71 | 0.131 | 85.89 ± 5.24** | 80.0 ± 5.40 | 0.006 | |
| ΔHelios/aTreg, %–6 mo | 2.05 ± 5.42 | −1.15 ± 5.84 | 0.032 | 6.03 ± 6.26 | −1.11 ± 5.41 | 0.003 | 0.134 |
Values in italics are initial values (baseline). Δ = value after treatment − baseline value.
*Statistically significant difference (<0.05), **Statistically significant difference (<0.01) between post‐treatment and baseline in the same group.
Abbreviations: aTreg, activated regulatory T cells; EHT, euthyroid Hashimoto thyroiditis; Se, selenium; SHT, subclinical Hashimoto thyroiditis; Treg, regulatory T cells.
Correlation analysis
The correlation analyses between variables were calculated additionally (Table S2). At baseline, TPOAb and TGAb titers correlated inversely with the percentage of Treg, aTreg cells, as well as with the expression of Helios in aTreg, which were similar to our previous study. After 6‐month treatment, Se levels showed significantly positive correlations with GPx3 (r = 0.325, p = 0.002), SePP1 (r = 0.225, p = 0.033), and MUI (r = 0.257, p = 0.014), and relationship with GPx3 or MUI was obtained by linear regression. Moreover, the increment of Se levels correlated with treatment‐induced changes in TPOAb (r = −0.278, p = 0.008), TGAb (r = −0. 437, p = 0.003), TSH (r = −0.314, p < 0.001), aTreg (r = 0.275, p = 0.009), Helios/Treg (r = 0.286, p = 0.006), and Helios/aTreg (r = 0.277, p = 0.008).
DISCUSSION
This study suggested that Se has the potential to reduce thyroid antibodies and TSH levels in the development of HT, and it was the first to provide a basis for the therapy mechanism by improving Treg cells via Se supplementation in patients with HT.
Se has been acknowledged for its essential roles in human health, and relation exhibits an inextricable U‐shaped, with possible harm occurring below or above the optimal range (80–120 μg/L). 17 Se status varies widely in different countries, where populations from the United States showed a mean serum Se level of 137 μg/L, 18 whereas a lower level in European residents was 85.6 μg/L, 19 but along with a higher prevalence of autoimmune thyroid disease. In China, our country’s selenium status covers a quite a wide range 22 to 550 μg/L, 20 an epidemiological study (n = 6, 152) showed the prevalence of pathological thyroid conditions was significantly higher in the low‐Se area (57.4 [39.4, 82.1] μg/L) than adequate‐Se area (103.6 [79.7, 135.9] μg/L). 21 These suggest that low Se status is associated with increased risk of thyroid disease. In this study, we investigated the Se levels in healthy people and patients with HT without levothyroxine treatment, and both groups showed Se deficiency status (≤80 μg/L), but no significant difference, which might be explained by small sample data and similar diets in Nanjing area. Further study needs to investigate the mean Se level in Nanjing and relationship with the incidence of HT. In human beings, the biological functions of Se are achieved by selenoproteins with antioxidant and immunoregulatory capacity. Serum GPx3 and SePP1 levels were also measured to evaluate Se status in this study, in line with the result of baseline Se, SePP1 was slightly lower in patients with HT than healthy subjects but the trend was not significant, whereas GPx3 showed statistical difference between groups, implying the reduced antioxidation ability in patients with HT. Moreover, we also detected the urinary iodine concentration of subjects, as iodine status is the main driver of multifactorial etiology of thyroid disease. Related studies suggest that the effect of Se on thyroid volume is more prominent in patients with iodine adequacy than in those with iodine deficiency. 22 , 23 In our study, the MUI of patients with HT was more than adequate, significantly higher than that of healthy people. This suggests that excess iodine intake may induce autoimmune thyroiditis, which is similar to previous studies. 24 , 25 , 26 Given that iodine‐deficient status may affect the treatment effect of Se, we did not strictly limit the iodine intake of patients with HT, but instructed them to achieve appropriate iodine status, the MUI concentrations were also monitored during the course of treatment.
Se supplementation may also protect against autoimmune thyroiditis, but the results have been ambiguous. The first meta‐analysis by Toulis et al. 27 involving patients with HT under L‐T4 treatment and concluded that Se supplementation is a promising adjuvant therapy for HT. A subsequent meta‐analysis by Wichman et al. 28 concluded that Se supplementation decreased TPOAb levels after 3, 6, and 12 months in the LT4‐treated AITD population, and after 3 months in the LT4‐untreated population. However, a recent meta‐analysis 29 found that no effect of Se supplementation on clinical importance, such as disease remission, levothyroxine dose, or health‐related quality of life. Therefore, although about 20 clinical trials have investigated the effect of Se treatment in HT, additional research is warranted to help explore a robust evidence of appropriate use of Se. Our study was to assess the effect of 3‐month and 6‐month administration of SYT on thyroid autoantibodies and thyroid function in patients with HT, and monitored serum Se, GPx3, SePP1, and MUI concentrations throughout the study. All enrolled participants were not treated with levothyroxine during the course of study, which enabled us to excluded the possible impact of exogenous thyroid hormones on immuno‐modulatory and clinical outcomes. 30 , 31 , 32 Our results showed that significant increases in Se, GPx3, and SePP1 after application of SYT in contrast to controls, whereas a marked decrease in MUI after control of iodine intake with respect to the baseline. More importantly, we found that patients with HT supplemented with SYT presented a slight treatment‐induced reduction either in TPOAb or TGAb at 3 months, which became significant at 6 months compared with controls. This phenomenon reached conflicting results in two studies, 33 , 34 but most researches have also established that 6 months of Se treatment caused a significant decrease in titer of antithyroid antibodies (mainly the TPOAb). 5 , 35 , 36 , 37 , 38 It is also noteworthy that we observed TSH levels moderately decreased afterapplication of SYT, whereas there was a significant increase of TSH levels in untreated patients, and the induced change between groups was statistically different. Correlative analysis revealed that Se levels positively related with GPx3 and SePP1 at 6 months, as well as the significant relationship between treatment‐induced changes of Se and that of TPOAb, TGAb, or TSH titers. These data support the view that patients with HT are likely to benefit from Se after 6 months of continuous treatment, by enhancing the antioxidant capacity.
To further explore who might benefit more with SYT treatment, we performed a subgroup analysis stratified by the baseline Se status or thyroid function, which is considered as an important parameter affecting the outcome of SYT treatment. In China, a lower bound of Se in an area of adequate status is about 80 μg/L, and maximal selenoprotein activity requires above 80 μg/L of selenium concentration. 39 Moreover, the median serum Se concentration of subjects in our study approaches 80 μg/L, thus we select 80 μg/L as a cutoff, which is a common statistical classification method. The data suggested that patients with Se less than 80 μg/L presented significant reductions in TPOAb, TGAb, and TSH levels after 6 months’ treatment, whereas this phenomenon was disappeared in patients with Se greater than or equal to 80 μg/L. However, any resulting misclassification would be expected to generate false‐positive or false‐negative results. We, therefore, conducted an interaction and stratified analysis and found no differences in main outcomes between the two subgroups, who stand to benefit from SYT treatment, regardless of the baseline Se level (<80 μg/L or ≥80 μg/L). We speculate that patients with HT whose serum Se less than 120 μg/L may be administered by oral Se (200 μg/day), according to the interquartile range (IQR) of Se levels (IQR ≈35–120 μg/L) in this study and referring to a recent article. 39 In addition, the substudy by thyroid function found consistent effect on thyroid antibodies with SYT treatment, based on interaction analysis between the EHT and the SHT groups. Notably, SYT benefited the SHT rather than the EHT in terms of reducing TSH levels, which is supported by a previous study of restoration of euthyroidism in subclinical HT treated with Se. 3 However, because the subgroup analyses were post hoc and small numbers were included in each group, the results of exploratory analyses served as the basis of further investigations. It warrants further prespecified trials with more samples of whether certain patients could benefit more from Se supplementation.
A possible mechanism for the beneficial effect of Se in HT is associated with the role of selenoenzymes in immunoregulatory processes, involving T cells activity and cytokine production. In line with this explanation, sodium selenite dose‐dependently inhibited HLA‐DR molecule expression of thyrocytes induced by IFN‐γ, and prevented apoptosis of thyroid follicular cells by TNF antagonism. 40 , 41 In a Polish study, the addition of selenomethionine (SeMet) to levothyroxine treatment inhibited lymphocyte release of IL‐2, IFN‐γ, and TNF, which was accompanied by a reduction of CRP. 31 Another Italian trial showed that supplementation with SeMet downregulated the IFN‐γ‐inducible chemokines (CXCL9 and CXCL10). 42 Conversely, an Austrian study with small samples found no significant differences in the cytokine production patterns of CD4+ or CD8+ T cells after Se administration in patients with AITD (autoimmune thyroiditis). 43 In recent years, mounting evidence indicates that Treg cells are a key subset indispensable in controlling immune responses and maintaining self‐tolerance. 44 The attenuation of Treg number and function is reported to be associated with HT. 45 , 46 Although an experimental animal study found that Se upregulated Treg cells of splenocyte and reduced lymphocytic infiltration of thyroid in NOD.H‐2(h4) mice with AITD, 47 the evidence in patients with HT is insufficient. Our previous data shed light on the deficient classical CD4+CD25+Foxp3+ Treg cells and their subsets, as well as the aberrant Helios and PD‐1 expression in the progression of HT. 15 The PD‐1 pathway may assist in controlling the complicated dynamic interactions between Tregs and T‐effector cells. PD‐1 expression on Tregs have the potential to indirectly suppress T‐effector responses by directly modulating DCs function. Helios, a member of Ikaros family, play an essential role in maintaining the functional stability of peripheral Treg cells especially in pro‐inflammatory conditions. Coexpression of Helios and Foxp3 in Treg function had more suppressive capacity compared with Foxp3 alone. In the present study, we observed that SYT treament not only increased the frequency of Foxp3highCD45RO+ aTreg cells, but also enhanced the expression of Helios in Treg and aTreg cells in patients with HT. A strength of our study was the finding of a series of relationships between treatment‐induced levels of serum Se and aTreg, Helios/Treg, or Helios/aTreg. These results suggest that SYT treatment affected activated Treg cells in patients with HT, and also implied an important role of Helios in evaluating the effect of selenium in Treg cells. Moreover, we discovered the similar effect of SYT on activated Treg cells between patients with EHT and SHT, which is consistent with that on thyroid antibodies, speculating that SYT administered in patients with HT may decrease thyroid antibodies by upregulating the activated Treg cells.
This study also suffers from some limitations. First, it was an open‐label, single centered small sample study without a placebo control, which may contribute to statistical error, thus the results need to be interpreted with some caution. Second, the data of subgroup analysis by Se status was not powered to establish an accurate result, although the interaction test was implicated in this study. Third, the exact mechanism of SYT treatment on the effect of Treg cells is not clearly explained, more functional trials are required to reveal the relationships. Last, the impact of Se on the clinical course of HT remains unclear, as these findings are restricted to biochemical parameters over a short period of time, we need to focus on the effects on symptoms, disease course, or eventual need for levothyroxine in our future work.
In conclusion, Se supplementation is associated with reductions in TPOAb, TGAb, and TSH levels, as well as increases in Se, GPx3, and SePP1 concentrations in patients with HT without levothyroxine replacement. This treatment is probably more helpful for patients with subclinical hypothyroid with HT in aspect of restoring euthyroidism. Furthermore, we speculate that patients with HT patients whose selenium levels less than 120 μg/L may be susceptible to the beneficial effect of Se supplementation. These results could be explained by assuming that Se may have the potential to enhance antioxidant ability and upregulated activated Treg cells in vivo of patients with HT.
CONFLICT OF INTEREST
All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
Y.F.H. wrote the manuscript. D.C., H.H.C., and L.J. designed the research. W.W.F., H.S., and X.Y.L. performed the research. X.Q.Z., W.S.Z., and Y.Q.G. analyzed the data. Y.L. contributed analytical tools.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to all participants and reviewers for their great contributions to this study.
Funding information
The research was supported by Six Talent Peaks Project in Jiangsu Province (2016‐WSN‐023), Thyroid Research Program of Young and Middle‐aged Physicians from China Health Promotion Foundation, National Key Research & Development plan of Ministry of Science and Technology of China (2018YFC1314900, 2018YFC1314901), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Contributor Information
Yun Liu, Email: liuyun@njmu.edu.cn.
Dai Cui, Email: cui_dai@163.com.
REFERENCES
- 1. Li Q, Wang B, Mu K, Zhang JA. The pathogenesis of thyroid autoimmune diseases: new T lymphocytes ‐ cytokines circuits beyond the Th1‐Th2 paradigm. J Cell Physiol. 2019;234:2204‐2216. [DOI] [PubMed] [Google Scholar]
- 2. de Farias CR, Cardoso BR, de Oliveira GMB, et al. A randomized‐controlled, double‐blind study of the impact of selenium supplementation on thyroid autoimmunity and inflammation with focus on the GPx1 genotypes. J Endocrinol Invest. 2015;38:1065‐1074. [DOI] [PubMed] [Google Scholar]
- 3. Pirola I, Gandossi E, Agosti B, Delbarba A, Cappelli C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol Pol. 2016;67:567‐571. [DOI] [PubMed] [Google Scholar]
- 4. Yu L, Zhou L, Xu E, et al. Levothyroxine monotherapy versus levothyroxine and selenium combination therapy in chronic lymphocytic thyroiditis. J Endocrinol Invest. 2017;40:1243‐1250. [DOI] [PubMed] [Google Scholar]
- 5. Wang W, Mao J, Zhao J, et al. Decreased thyroid peroxidase antibody titer in response to selenium supplementation in autoimmune thyroiditis and the influence of a SEPP gene polymorphism: a prospective, multicenter study in China. Thyroid. 2018;28(12):1674‐1681. [DOI] [PubMed] [Google Scholar]
- 6. Negro R, Attanasio R, Grimaldi F, Marcocci C, Guglielmi R, Papini E. A 2016 Italian survey about the clinical use of selenium in thyroid disease. Eur Thyroid J. 2016;5:164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hegedüs L, Bonnema SJ, Winther KH. Selenium in the treatment of thyroid diseases: an element in search of the relevant indications. Eur Thyroid J. 2016;5:149‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos LR, Neves C, Melo M, Soares P. Selenium and selenoproteins in immune mediated thyroid disorders. Diagnostics (Basel). 2018;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duntas LH. The role of iodine and selenium in autoimmune thyroiditis. Horm Metab Res. 2015;47:721‐726. [DOI] [PubMed] [Google Scholar]
- 10. Shao S, Yu X, Shen L. Autoimmune thyroid diseases and Th17/Treg lymphocytes. Life Sci. 2018;192:160‐165. [DOI] [PubMed] [Google Scholar]
- 11. Wei WZ, Jacob JB, Zielinski JF, et al. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell‐depleted mice. Cancer Res. 2005;65:8471‐8478. [DOI] [PubMed] [Google Scholar]
- 12. Zha B, Huang X, Lin J, Liu J, Hou Y, Wu G. Distribution of lymphocyte subpopulations in thyroid glands of human autoimmune thyroid disease. J Clin Lab Anal. 2014;28:249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xue H, Yu X, Ma L, et al. The possible role of CD4⁺CD25(high)Foxp3⁺/CD4⁺IL‐17A⁺ cell imbalance in the autoimmunity of patients with Hashimoto thyroiditis. Endocrine. 2015;50:665‐673. [DOI] [PubMed] [Google Scholar]
- 14. Miyara M, Yumiko Y, Akihiko K, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899‐911. [DOI] [PubMed] [Google Scholar]
- 15. Hu Y, Zhang L, Chen H, et al. Analysis of regulatory T cell subsets and their expression of Helios and PD‐1 in patients with Hashimoto thyroiditis. Int J Endocrinol. 2019;2019:5368473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sebotsa ML, Dannhauser A, Jooste PL, Joubert G. Assessment of the sustainability of the iodine‐deficiency disorders control program in Lesotho. Food Nutr Bull. 2007;28:337‐347. [DOI] [PubMed] [Google Scholar]
- 17. Rayman MP. Selenium and human health. Lancet. 2012;379:1256‐1268. [DOI] [PubMed] [Google Scholar]
- 18. Laclaustra M, Navas‐Acien A, Stranges S, Ordovas JM, Guallar E . Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009;117:1409‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes DJ, Fedirko V, Jenab M, et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer. 2015;136:1149‐1161. [DOI] [PubMed] [Google Scholar]
- 20. Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low‐selenium area of China. Am J Clin Nutr. 2005;81:829‐834. [DOI] [PubMed] [Google Scholar]
- 21. Wu Q, Rayman MP, Lv H, et al. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab. 2015;100:4037‐4047. [DOI] [PubMed] [Google Scholar]
- 22. Derumeaux H, Valeix P, Castetbon K, et al. Association of selenium with thyroid volume and echostructure in 35‐ to 60‐year‐old French adults. Eur J Endocrinol. 2003;148:309‐315. [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen LB, Schomburg L, Köhrle J, et al. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur J Endocrinol. 2011;164:585‐590. [DOI] [PubMed] [Google Scholar]
- 24. Teng X, Shan Z, Chen Y, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross‐sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943‐950. [DOI] [PubMed] [Google Scholar]
- 25. Doğan M, Acikgoz E, Acikgoz M, Cesur Y, Ariyuca S, Bektas MS. The frequency of Hashimoto thyroiditis in children and the relationship between urinary iodine level and Hashimoto thyroiditis. J Pediatr Endocrinol Metab. 2011;24:75‐80. [DOI] [PubMed] [Google Scholar]
- 26. Zhao H, Tian Y, Liu Z, Li X, Feng M, Huang T. Correlation between iodine intake and thyroid disorders: a cross‐sectional study from the South of China. Biol Trace Elem Res. 2014;162:87‐94. [DOI] [PubMed] [Google Scholar]
- 27. Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta‐analysis. Thyroid. 2010;20:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 28. Wichman J, Winther KH, Bonnema SJ, Hegedüs L. Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta‐analysis. Thyroid. 2016;26:1681‐1692. [DOI] [PubMed] [Google Scholar]
- 29. Winther KH, Wichman JE, Bonnema SJ, Hegedüs L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta‐analysis. Endocrine. 2017;55:376‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schloot N, Eisenbarth GS. Isohormonal therapy of endocrine autoimmunity. Immunol Today. 1995;16:289‐294. [DOI] [PubMed] [Google Scholar]
- 31. Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2011;96:2206‐2215. [DOI] [PubMed] [Google Scholar]
- 32. Mittag J, Behrends T, Hoefig CS, Vennström B, Schomburg L. Thyroid hormones regulate selenoprotein expression and selenium status in mice. PLoS One. 2010;5:e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anastasilakis AD, Toulis KA, Nisianakis P, et al. Selenomethionine treatment in patients with autoimmune thyroiditis: a prospective, quasi‐randomised trial. Int J Clin Pract. 2012;66:378‐383. [DOI] [PubMed] [Google Scholar]
- 34. Eskes SA, Endert E, Fliers E, et al. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin Endocrinol (Oxf). 2014;80:444‐451. [DOI] [PubMed] [Google Scholar]
- 35. Gärtner R, Gasnier BC. Selenium in the treatment of autoimmune thyroiditis. BioFactors. 2003;19:165‐170. [DOI] [PubMed] [Google Scholar]
- 36. Duntas LH, Mantzou E, Koutras DA. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol. 2003;148:389‐393. [DOI] [PubMed] [Google Scholar]
- 37. Mazokopakis EE, Papadakis JA, Papadomanolaki MG, et al. Effects of 12 months treatment with L‐selenomethionine on serum anti‐TPO levels in patients with Hashimoto's thyroiditis. Thyroid. 2007;17:609‐612. [DOI] [PubMed] [Google Scholar]
- 38. Balázs C. The effect of selenium therapy on autoimmune thyroiditis. Orv Hetil. 2008;149:1227‐1232. [DOI] [PubMed] [Google Scholar]
- 39. Winther KH, Rayman MP, Bonnema SJ, Hegedüs L. Selenium in thyroid disorders ‐ essential knowledge for clinicians. Nat Rev Endocrinol. 2020;16:165‐176. [DOI] [PubMed] [Google Scholar]
- 40. Balázs C, Kaczur V. Effect of selenium on HLA‐DR expression of thyrocytes. Autoimmune Dis. 2012;2012:374635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nettore IC, De Nisco E, Desiderio S, et al. Selenium supplementation modulates apoptotic processes in thyroid follicular cells. BioFactors. 2017;43:415‐423. [DOI] [PubMed] [Google Scholar]
- 42. Pilli T, Cantara S, Schomburg L, et al. IFNγ‐inducible chemokines decrease upon selenomethionine supplementation in women with euthyroid autoimmune thyroiditis: comparison between two doses of selenomethionine (80 or 160 μg) versus placebo. Eur Thyroid J. 2015;4:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karanikas G, Schuetz M, Kontur S, et al. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008;18:7‐12. [DOI] [PubMed] [Google Scholar]
- 44. Esensten JH, Muller YD, Bluestone JA, Tang Q. Regulatory T‐cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J Allergy Clin Immunol. 2018;142:1710‐1718. [DOI] [PubMed] [Google Scholar]
- 45. Glick AB, Wodzinski A, Fu P, Levine AD, Wald DN. Impairment of regulatory T‐cell function in autoimmune thyroid disease. Thyroid. 2013;23:871‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bossowski A, Moniuszko M, Dąbrowska M, et al. Lower proportions of CD4+CD25(high) and CD4+FoxP3, but not CD4+CD25+CD127(low) FoxP3+ T cell levels in children with autoimmune thyroid diseases. Autoimmunity. 2013;46:222‐230. [DOI] [PubMed] [Google Scholar]
- 47. Xue H, Wang W, Li Y, et al. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine‐induced autoimmune thyroiditis model of NOD.H‐2(h4) mice. Endocr J. 2010;57:595‐601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


