Abstract
Periodontal disease is driven by dysbiosis in the oral microbiome, resulting in over-representation of species that induce the release of pro-inflammatory cytokines, chemokines, and tissue-remodeling matrix metalloproteinases (MMPs) in the periodontium. These chronic tissue-destructive inflammatory responses result in gradual loss of tooth-supporting alveolar bone. The oral spirochete Treponema denticola, is consistently found at significantly elevated levels in periodontal lesions. Host-expressed Toll-Like Receptor 2 (TLR2) senses a variety of bacterial ligands, including acylated lipopolysaccharides and lipoproteins. T. denticola dentilisin, a surface-expressed protease complex comprised of three lipoproteins has been implicated as a virulence factor in periodontal disease, primarily due to its proteolytic activity. While the role of acylated bacterial components in induction of inflammation is well-studied, little attention has been given to the potential role of the acylated nature of dentilisin. The purpose of this study was to test the hypothesis that T. denticola dentilisin activates a TLR2-dependent mechanism, leading to upregulation of tissue-destructive genes in periodontal tissue. RNA-sequencing of periodontal ligament cells challenged with T. denticola bacteria revealed significant upregulation of genes associated with extracellular matrix organization and degradation including potentially tissue-specific inducible MMPs that may play novel roles in modulating host immune responses that have yet to be characterized within the context of oral disease. The Gram-negative oral commensal, Veillonella parvula, failed to upregulate these same MMPs. Dentilisin-induced upregulation of MMPs was mediated via TLR2 and MyD88 activation, since knockdown of expression of either abrogated these effects. Challenge with purified dentilisin upregulated the same MMPs while a dentilisin-deficient T. denticola mutant had no effect. Finally, T. denticola-mediated activation of TLR2/MyD88 lead to the nuclear translocation of the transcription factor Sp1, which was shown to be a critical regulator of all T. denticola-dependent MMP expression. Taken together, these data suggest that T. denticola dentilisin stimulates tissue-destructive cellular processes in a TLR2/MyD88/Sp1-dependent fashion.
Author summary
Periodontal disease is driven by dysbiosis of the oral microbiome, which interacts with host tissues and thereby induces the release of pro-inflammatory cytokines, chemokines, and tissue-remodeling matrix metalloproteinases (MMPs), leading to destruction of the periodontal tissues. Even after clinical intervention, patients with severe periodontal disease are left with a persistent pro-inflammatory transcriptional profile throughout the periodontium. The oral spirochete, Treponema denticola is consistently found at elevated levels in periodontal lesions and is associated with several pathophysiological effects driving periodontal disease progression. The T. denticola surface-expressed protease complex (dentilisin) has cytopathic effects consistent with periodontal disease pathogenesis. To date, few direct links have been reported between dentilisin and the cellular and tissue processes that drive periodontal tissue destruction at the transcriptional and/or epigenetic levels. Here, we utilize wild type and dentilisin-deficient T. denticola as well as purified dentilisin to characterize dentilisin-dependent activation of intracellular pathways controlling MMP expression and activity. Our results define a role for dentilisin in initiating this signal cascade. Also, our study identified tissue-specific inducible MMPs that may play novel roles in modulating as-yet uncharacterized host responses in periodontal disease. Lastly, T. denticola dentilisin stimulates tissue-destructive cellular processes in a TLR2/MyD88/Sp1-dependent fashion. Taken together, our study provides new insights into the molecular mechanisms underpinning periodontal disease progression which could lead to the development of more efficacious therapeutic treatments.
Introduction
Periodontitis or Periodontal Disease is characterized as a chronic oral inflammatory disease that compromises the integrity of the tooth-supporting tissues, which include the gingiva, periodontal ligament and alveolar bone, and are collectively known as the periodontium. In its severe form, which afflicts 8.5% of adults in the United States [1], periodontitis may cause tooth loss, and also affect systemic health by increasing the patients’ risk for atherosclerosis, adverse pregnancy outcomes, rheumatoid arthritis, aspiration pneumonia and cancer [2–5]. Additionally, patients with chronic forms of periodontal disease are left with a non-resolving pro-inflammatory transcriptional profile throughout the periodontium, even after clinical intervention, leading to tissue-destruction and tooth loss [6–8]. This suggests that previously uncharacterized cellular and molecular mechanisms underlying periodontal disease pathophysiology may explain why many patients do not respond to the conventional treatment schema.
The periodontal ligament (PDL) has two primary functions: 1) to absorb and respond to mechanical stresses, and 2) to provide vascular supply and nutrients to the cementum, alveolar bone and the PDL itself. Osteoblasts, osteoclasts, cementoblasts and endothelial cells make up the PDL and reside on the surface of the lamina dura and endosteal surfaces of the alveolar bone and cementum [9,10]. The most prominent cell type are human periodontal ligament fibroblasts (hPDL), which specialize in mechanosensing and tissue remodeling through robust expression of various hydrolytic enzymes such as matrix metalloproteinases (MMPs)[10,11]. They also function as immune-like cells demarcated by the production of inflammatory cytokines, chemokines and expression of pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), which are responsible for monitoring the local environment for signs of danger in the form of highly conserved microbial molecules present during periodontal disease [12–15]. When TLR signaling is left unchecked, downstream genes, such as MMPs can become significantly upregulated and constitutively activated, thereby contributing to the overall destruction of the periodontium by degrading extracellular matrix (ECM) proteins, such as collagen and fibronectin [11,16–20]. Although the role of MMPs has been primarily ascribed to turnover of the ECM, the great number of new substrates discovered for MMPs in the last few years suggest they are capable of regulating many signaling pathways, cell behaviors and diseases through novel mechanisms [21]. Therefore, PDL cells likely play a significant role in initiating and exacerbating tissue degradation and suggest that pharmacological anti-inflammatory treatment of periodontal disease should target dysregulated MMP activity and levels in the PDL tissues.
The pathogenic processes of periodontal disease are primarily due to the host response, which propagates the destructive responses initiated by microbes [22–24]. While a large focus has been placed on species such as Porphyromonas gingivalis, Fusbacterium nucleatum, and Aggrigatibacter actinomycetemcomitans, recent metagenomic and metatranscriptomic studies have implicated the Gram negative-oral spirochete, T. denticola with advanced or aggressive forms of periodontal disease and recurrence of disease [25–40]. Interactions between bacteria, viruses and host cells have been associated with shifts from health to disease in oral tissues as well [41–44].
Greatly increased numbers of T. denticola levels in periodontal disease vs health has been well-documented in the literature for >40 years [45]. Our lab has readily demonstrated that T. denticola was ~15-fold higher in the subgingival biofilm of periodontal lesions [46]. Additionally, elevated T. denticola biofilm levels combined with elevated MMP levels in host tissues display robust combinatorial characteristics in predicting advanced periodontal disease severity [46,47]. Further, Lee et al. and colleagues demonstrated that T. denticola infection was sufficient to induce alveolar bone resorption in a mouse model of periodontal disease [48]. Thus, clinical data regarding the increased presence of T. denticola in periodontal lesions, together with basic and in vivo studies involving the role of T. denticola products suggest that it plays a pivotal role in driving periodontal disease progression.
Among the various T. denticola effector molecules that have been described, its acylated chymotrypsin-like protease complex (CTLP), more recently called dentilisin, is a major virulence factor which facilitates numerous cytopathic effects that align with periodontal disease pathophysiology [30,49,50]. A few examples including adhesion, degradation of endogenous ECM-substrates [35], tissue penetration [51], complement evasion [52], ectopic activation of host MMPs [39] and degradation of host chemokines and cytokines, such as IL-1β and IL-6, primarily due to its potent proteolytic activity [38]. However, as in other spirochetes, conserved lipid moieties of the protease complex recognized by host TLR2 receptor complexes may contribute to activation of innate immune responses [53]. Because predominant host responses to lipoproteins are believed to be to their lipid moieties, most studies have focused on diacylated lipopeptide, Pam2CSK4, and triacylated lipopeptide, Pam3CSK4, which mimic bacterial lipoproteins for their potent immunostimulatory and osteoclastogenic activities by preferentially activating TLR2-dependent pathways [53–55]. Recent studies have demonstrated that synthetic di- and tri-acylated lipopeptides which preferentially activate TLR2/6 and TLR2/1-dependent pathways respectively, are sufficient to induce alveolar bone loss in mice [54,56], broadening the avenues of investigation into the role of lipoproteins underpinning the pathogenesis of periodontal disease. However, studies which utilize endogenously expressed bacterial lipopeptides are greatly lacking.
While a clear role for dentilisin in the context of periodontal disease has been delineated at the protein level, its ability to influence host ECM metabolism in a gene-centric context has yet to be characterized. Thus, the aim of this study was to determine the extent to which T. denticola and its highly expressed acylated dentilisin protease complex influence the transcriptional regulation of MMPs through TLR2-dependent pathways in hPDL cells.
Results
T. denticola upregulates genes associated with ECM and degradation in hPDL cells
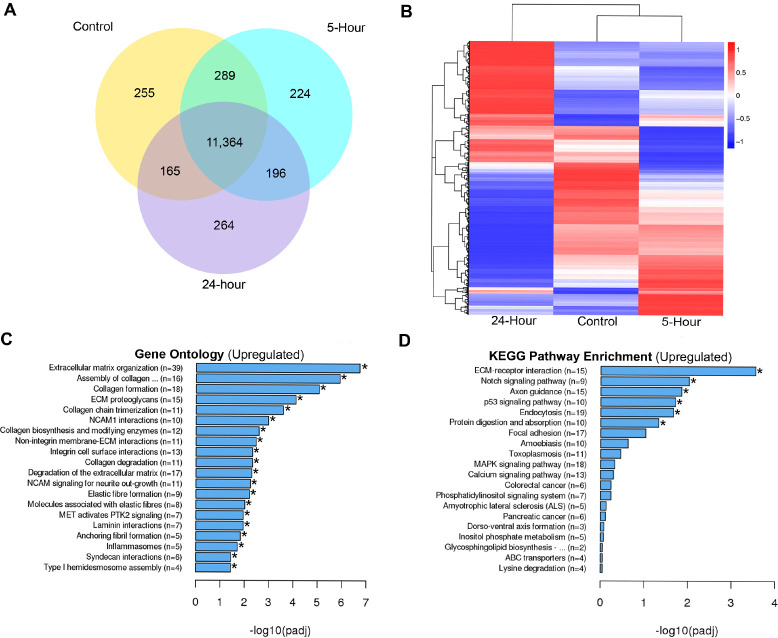
To better understand the extent to which T. denticola influences host transcriptomes in an unbiased and gene-centric context, we challenged three healthy patient replicates of hPDL cells with wild-type Td-WT for 2 hours. The cells were then incubated for an additional 3 or 22 hours (5 and 24-hours, respectively) before extracting total RNA for sequencing. Unchallenged hPDL cells were used as a negative control. The results are shown in Fig 1. In order to include moderately up- or downregulated genes in the analysis, genes below our cutoff criterion with a significant difference (p < 0.05) were examined. Differential expression analysis revealed a large overlap in gene expression between the control and 5-hour incubation groups (289 genes), whereas the control and 24-hour incubation groups showed significantly less overlap (165 genes) (Fig 1A). Hierarchical clustering analysis was carried out with the log10 (FPKM+1) of union differential expression genes of all comparison groups under different experimental conditions. Essentially, experimental groups which have more unique clusters diverge further away from the control group. The 24-hour incubation group clustered away from the 5-hour incubation and control groups (Fig 1B). For the gene ontology analysis, we utilized the mean FPKM values for each gene across experimental groups. Gene clusters at or above a 0.05 adjusted p-value were delineated as statically significant increases. ECM (~6-fold change), collagen degradation (~2-fold change) and degradation of the ECM (~2-fold change) amongst the top 20 significantly enriched biological processes (Fig 1C). Downregulated GO and KEGG Terms can be found in S1 Fig. KEGG pathway analysis revealed ECM-Receptor (~4-fold change), Notch (~2-fold change), endocytosis (~2-fold change) and protein digestion (~2-fold change) signaling amongst the top 20 enriched signaling pathways upon T. denticola challenge (Fig 1D). Interestingly, Membrane-type matrix metalloproteinase 4 (MMP-17), Stromelysin-3 (MMP-11) and Epilysin (MMP-28) were consistently clustered with various GO terms associated with ECM remodeling and catabolism and have not been associated with periodontal disease to date. Although RNA-seq analysis revealed increases in MMP-2 and MMP-14 read counts, they were not found to be statistically significant. Both genes, however, have been found to be chronically upregulated in hPDL fibroblasts upon T. denticola challenge for up to 12 days in vitro [46]. Additionally, while the primary functions of these enzymes have been associated with tissue turnover, both MMP-2 and MMP-14 participate in the active degradation of various cytokines [57], chemokines [57], ECM proteins [58] and various intracellular substrates [21,59,60] expressed by hPDL cells expressed during periodontal disease infections, delineating their potentially important role in driving periodontal induced tissue destruction. Next, we sought to determine if this upregulation of tissue destructive genes is common among pathogens and commensal bacteria.
Fig 1. Differential expression analysis of T. denticola challenged hPDL cells.
Total RNA was extracted from healthy patient-derived hPDL cells challenged with Td-WT bacteria at a MOI of 50 for 2-hours in media free of supplements followed by 3 and 22-hours in media supplemented with 10% FBS, 1% Pen Strep and 1% Amphotericin B. Mean FPKM values were used for downstream analysis (n = 3 patient replicates). A) Overlapping and differentially expressed genes between control, 5-hour and 24-hour incubation groups visualized using a Venn diagram. B) Hierarchical clustering analysis was used to determine similarity of transcriptome profiles based on differential expression as a heatmap. Red (Upregulation) to blue (Downregulation) color gradient of heatmap represents normalized gene expression as row Z-scores. C) Top 20 enriched Gene Ontology terms of hPDL cells challenged for 2-hours followed by a 22-hour incubation using the Reactome nomenclature. Statistical significance was assessed using a Kolmogorov-Smirnov test followed by Benjamini-Hochberg correction (p<0.05). D) Top 20 enriched signaling pathways of hPDL cells challenged for 2-hours followed by a 22-hour incubation using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Statistical significance was assessed using a Kolmogorov-Smirnov test followed Benjamini-Hochberg correction (p<0.05).
MMPs are not upregulated by commensal oral Gram-negative Veillonella parvula
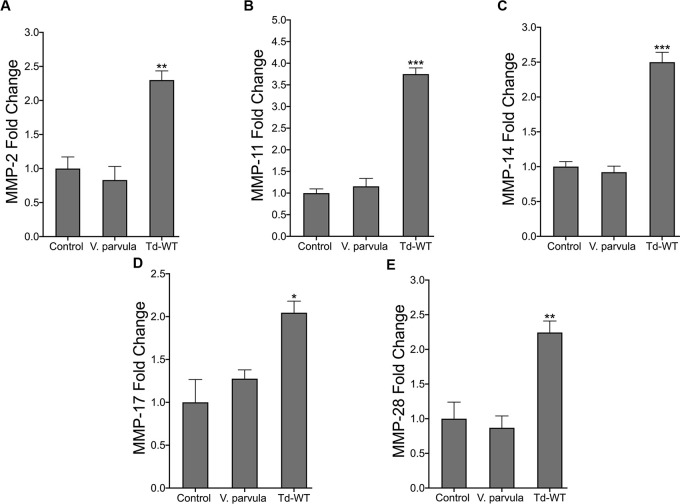
To determine if the induction of these MMPs was specific to T. denticola, we utilized Veillonella parvula, a Gram-negative anaerobic oral commensal that is prevalent in the gut and oral cavity and is not associated with oral diseases [61]. hPDL cells were challenged with wild-type T. denticola or V. parvula bacteria for 2 hours at 50 MOI followed by a 22-hour incubation period in α-MEM media free of antibiotics and supplemented with 10% FBS. V. parvula challenge did not induce the upregulation of MMPs 2, 11, 14, 17 or 28 (Fig 2). By contrast, T. denticola elicited a ~2-fold upregulation or more of all target MMP genes (Fig 2).
Fig 2. T. denticola upregulates MMPs in hPDL cells while V. parvula does not.
(A-E) RT-qPCR for MMP-2, MMP-11, MMP-14, MMP-17 and MMP-28 mRNA expression of healthy hPDL cells challenged with V. parvula (ATCC 10790) and Td-WT bacteria at a MOI of 50 for 2-hours, followed by a 22-hour incubation in media supplemented with 10% FBS and 1% Pen/Strep and 1% Amphotericin B. Expression of each gene was normalized to that of GAPDH. Statistical significance was determined using a One-Way ANOVA followed by Post-Hoc Tukey’s multiple comparisons. Bars represent mean ± SEM (n = 5). *p < .05 versus control. **p < .01 versus control. ***p < .001 versus control.
T. denticola dentilisin mediates the upregulation of various MMPs in hPDL cells
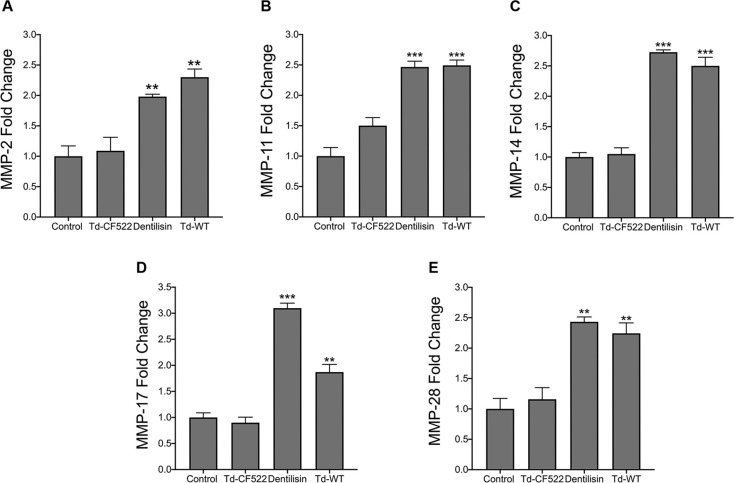
Among the various TLR2 stimulatory bacterial ligands, lipoproteins are considered a major virulence factor because of their strong immunostimulating capacity [55,62,63]. Interestingly, while differential innate immune responses have been reported depending on the acylation status and tissue type [63–65], both di- and triacylated synthetic lipopeptides have demonstrated the ability to sufficiently drive alveolar and long bone resorption in mice through TLR2/MyD88 dependent mechanisms [54,56]. We investigated whether purified dentilisin can drive MMP gene regulation in hPDL cells and the results can be seen in Fig 3. hPDL cells were challenged with dentilisin purified from T. denticola ATCC 35405 at a final concentration of 1 μg/mL for 2-hours in MEM-media supplemented 1% P/S. Enzymatic activity of dentilisin protein was verified by collecting conditioned media from dentilisin stimulated hPDL cells as described above and analyzing samples using gelatin zymography (S2 Fig). While, dentilisin enzymatic activity (98 kDa) increased in a dose dependent manner, MMP-2 enzymatic activity (72 kDa) concomitantly increased in a statistically significant manner (~2.0-fold) across all 3 experimental conditions.
Fig 3. T. denticola surface-expressed dentilisin mediates the upregulation of MMP 2, 11, 14, 17 and 28 mRNA levels in hPDL cells.
(A-E) RT-qPCR for MMP-2, MMP-11, MMP-14, MMP-17 and MMP-28 mRNA expression of healthy hPDL cells challenged with isogenic Td-CF522 bacteria, Td-WT bacteria and purified dentilisin. Cells were stimulated at an MOI of 50 and a final concentration of of 1 μg/mL. Cells were challenged for 2-hours in alpha-MEM media supplemented with 10% FBS followed by a 22-hour incubation in alpha-MEM media with 10% FBS, 1% PenStrep and 1% Amphotericin B. The expression of each gene was normalized to that of GAPDH. Statistical significance was determined using a One-Way ANOVA followed by Tukey’s Post-Hoc multiple comparisons. Bars represent mean ± SEM (n = 4). **p < .01 versus control. ***p < .001 versus control.
Total RNA from hPDL cells (unchallenged and challenged with T. denticola strains or purified dentilisin) was extracted, processed for cDNA synthesis and rendered to RT-qPCR. Cells stimulated with purified dentilisin resulted in statistically significant increases in all MMP targets with MMP-17 being the most responsive (Fig 3). Similarly, hPDL cells challenged with T. denticola 35405 (Td-WT) resulted in statistically significant increases across all MMP targets (~2–2.5-fold) (Fig 3). By contrast, hPDL cells challenged with dentilisin-deficient T. denticola (Td-CF522) resulted in no statistically significant upregulation across all MMP targets compared to the control group (Fig 3).
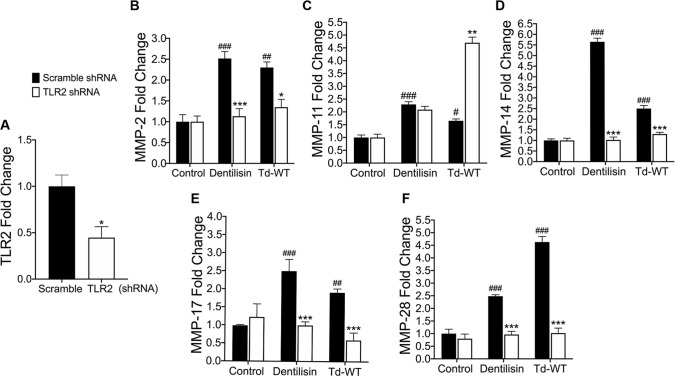
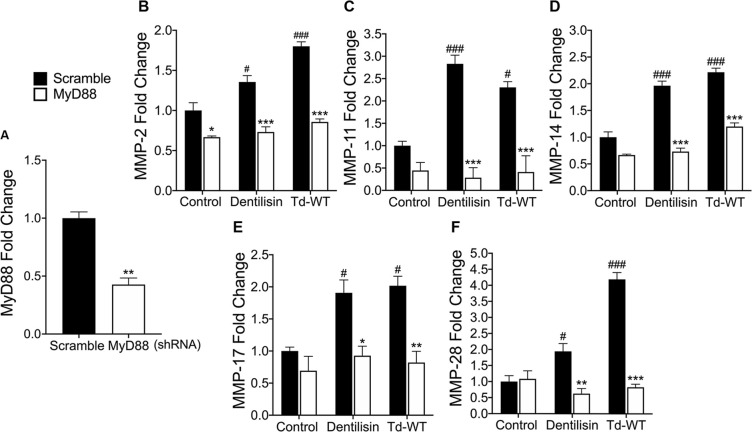
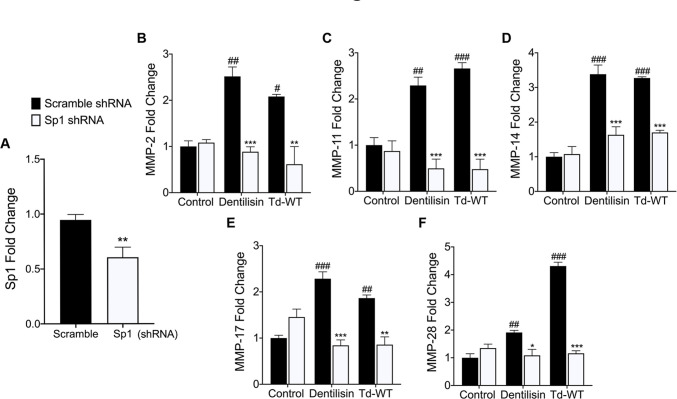
shRNA knockdown of TLR2 inhibits T. denticola-induced upregulation of MMPs 2, 14 17 and 28 in hPDL cells while synergistically increasing MMP 11 expression
Thus, after determining dentilisin plays an important role as a regulator of MMP expression in host hPDL cells, we sought to establish a direct link between TLR2 activation and MMP gene regulation. TLRs, particularly TLR2, and to a much lesser extent TLR4, regulate important immune responses to periodontal bacteria in vivo and in vitro [34,66–72]. TLR2 has been identified as a receptor for an array of ligands comprising T. denticola bacteria, including lipoteichoic acid, beta barrel proteins, and acylated lipopeptides, such as dentilisin [73,74]. Thus, to examine the role of TLR2 and MMP transcriptional regulation in hPDL cells, we generated a primary hPDL TLR2 knockdown cell line using shRNA targeted against the TLR2 gene and transduced using lentiviral particles. Basal TLR2 expression was reduced ~60% across 3 clonal replicates as validated using RT-qPCR; reduction was found to be statistically significant using an unpaired t-test (Fig 4A). Next, TLR2 knockdown cells were challenged with either purified dentilisin or wild-type T. denticola bacteria. MMP 2, 11, 14, 17 and 28 expression increased upon stimulation with both purified dentilisin and Td-WT in the shRNA control group (Fig 4B–4F). Compared with hPDL cells transfected with the scrambled shRNA control, induction of expression of MMPs 2, 14, 17 and 28 was suppressed in TLR2-deficient hPDL cells challenged with T. denticola or dentilisin, while MMP 11 was significantly upregulated in T. denticola-challenged TLR2-deficient hPDL cells. After determining TLR2 activation plays an integral role in the regulation of MMP expression, we next sought out to determine which downstream mediator is required to propagate this signal.
Fig 4. Suppression of TLR2 inhibits T. denticola-stimulated upregulation of MMPs 2, 14, 17 and 28 while exacerbating MMP 11 expression in hPDL cells.
A) RT-qPCR validation of stable gene suppression using shRNA vectors targeted against TLR2 in healthy hPDL cells. Cells transduced with scrambled shRNA vectors were used as a control. Statistical significance was determined using an unpaired t-test. Bars represent ± SEM of mean value (n = 3 clones). *p < .05 versus control. B-F) RT-qPCR for MMP-2, MMP-11, MMP-14, MMP-17 and MMP-28 mRNA expression of scrambled shRNA control and TLR2 shRNA hPDL cells challenged or stimulated with Td-CF522, purified dentilisin or Td-WT at an MOI of 50 and concentration of 1 μg/mL for 2-hours in alpha-MEM media with no supplementation followed by a 22-hour incubation in alpha-MEM media supplemented with 10% FBS, 1% Pen/Strep and 1% Amphotericin B. The expression of each gene was normalized to that of GAPDH. Statistical significance was determined using a Two-Way ANOVA followed by post-hoc Tukey’s multiple comparisons. Bars represent mean ± SEM (n = 3). #p < .05 versus scramble control group. ##p < .01 versus scramble control group. ###p < .001 versus scramble control group. *p < .05 versus TLR2 shRNA equivalent group. ***p < .001 versus TLR2 equivalent shRNA group.
shRNA knockdown of MyD88 inhibits T. denticola-stimulated upregulation of MMPs 2, 11, 14, 17 and 28 in hPDL cells
All TLRs signal through MyD88 with the exception of TLR3 and TLR4 which utilize TIRAP [75]. However, numerous recent reports suggest that MyD88-independent TLR2 pathways may also contribute to periodontal disease progression [76]. Thus, to determine if T. denticola-induced TLR2 activation requires MyD88 to regulate targeted MMPs, we generated an hPDL MyD88 knockdown cell line, achieving approximately ~60% reduction in MyD88 basal expression across 3 validated clonal replicates (Fig 5A). MyD88-deficient hPDL cells were stimulated with either Td-WT or purified dentilisin as described above. In the negative shRNA control group, all MMP targets showed increased mRNA levels following challenge with both Td-WT and dentilisin (Fig 5B–5F). By contrast, MyD88-deficient cells challenged with Td-WT bacteria and purified dentilisin revealed unanimous depression of all MMP targets as compared to the respective scrambled shRNA control samples (Fig 5B–5F).
Fig 5. Suppression of MyD88 inhibits T. denticola-stimulated upregulation of MMP 2, 11, 14, 17 and 28 in hPDL cells.
A) RT-qPCR validation of stable gene suppression using shRNA vectors targeted against MyD88 in healthy hPDL cells. Cells transduced with scrambled shRNA vectors were used as a control. Statistical significance was determined using an unpaired t-test. Bars represent ± SEM of mean values (n = 3 clones). *p < .05 versus control. B-F) RT-qPCR for MMP-2, MMP-11, MMP-14, MMP-17 and MMP-28 mRNA expression of scrambled shRNA control and MyD88 shRNA hPDL cells challenged or stimulated with Td-CF522, purified dentilisin or Td-WT at an MOI of 50 and concentration of 1 μg/mL for 2-hours in alpha-MEM media with no supplementation followed by a 22-hour incubation in alpha-MEM media supplemented with 10% FBS, 1% Pen/Strep and 1% Amphotericin B. The expression of each gene was normalized to that of GAPDH. Statistical significance was determined using a Two-Way ANOVA followed by post-hoc Tukey’s multiple comparisons. Bars represent mean ± SEM (n = 3). #p < .05 versus scramble control group. ###p < .001 versus scramble control group *p < .05 versus MyD88 shRNA equivalent group. **p < .01 versus MyD88 shRNA equivalent group. ***p < .001 versus MyD88 shRNA equivalent group.
shRNA knockdown of TLR2/MyD88-dependent signaling inhibits nuclear translocation of transcription factor Sp1 in hPDL cells stimulated with T. denticola or dentilisin
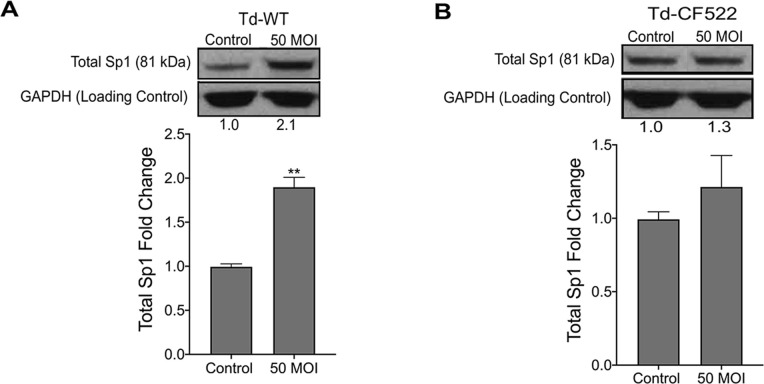
One of the many downstream targets of TLR/MyD88 signaling is the transcription factor Specificity Protein 1 (Sp1). Sp1 regulates a variety of genes associated with cell cycle, pro-inflammation and tissue-destruction, including MMPs 2, 11, 14 17 and 28. Interestingly, Larsson and colleagues have readily demonstrated that mRNA levels of Sp1 are consistently upregulated at periodontally involved sites in humans [77]. Thus, to determine if T. denticola influences Sp1 expression in the PDL, hPDL cells were challenged with Td-WT and Td-CF522 bacteria at a multiplicity of infection of 50 for 2-hours followed by a 22-hour incubation period in media. Cells were collected and used for western blot analysis utilizing a monoclonal antibody against total Sp1 protein. Total Sp1 protein expression levels increased in the groups challenged with Td-WT at 50 MOI compared to controls and were statistically significant (Fig 6). By contrast, challenge with Td-CF522 failed to recapitulate this effect (Fig 6).
Fig 6.
Healthy hPDL cells were challenged with A) Td-WT and B) isogenic Td-CF522 bacteria at an MOI of 50 as previously described. Whole cell lysates were generated and used for Western Blot analysis utilizing total anti-Sp1 antibodies. Total Sp1 protein expression was normalized against GAPDH protein expression as a loading control. Statistical significance was determined using a paired t-test. Bars represent mean ± SD (n = 3). **p < .01 versus control.
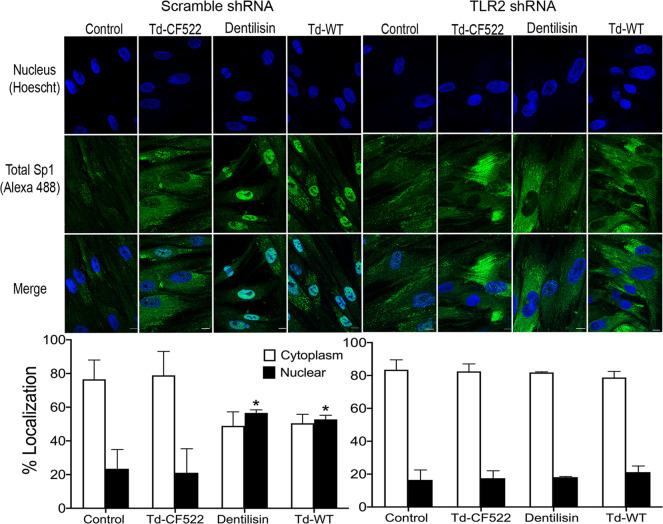
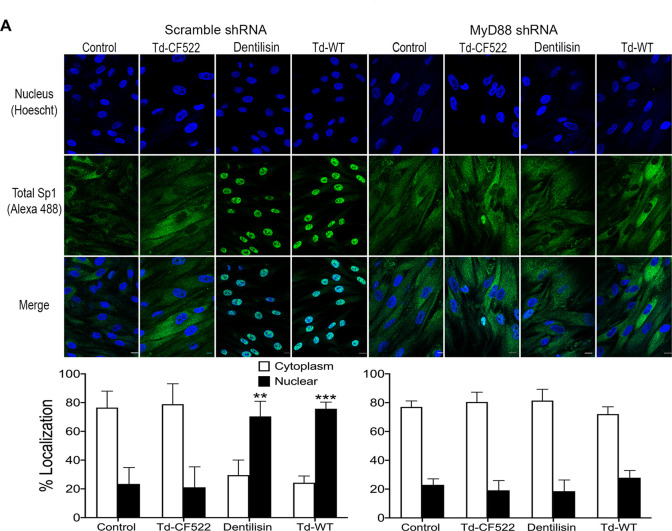
Transcription factors are activated post-translationally by TLR signaling, often at the step of nuclear translocation leading to the induction of the primary response genes [78]. After finding that T. denticola increases total Sp1 protein expression, we investigated whether T. denticola or dentilisin are able to induce nuclear translocation of Total Sp1 via TLR2 and MyD88 activation in hPDL cells and the results can be seen on Figs 7 and 8. TLR2, MyD88-deficient and shRNA control cells were stimulated with purified dentilisin, Td-WT or Td-CF522 as previously described. Protein localization was assessed using primary antibodies against Sp1 and a secondary antibody conjugated to an Alexa 488 fluorophore and subjected to confocal microscopy. shRNA control hPDL cells challenged with Td-CF522 resulted in an increased Sp1 protein signal compared to the control group but remained localized to the cytoplasm (Fig 7A). In contrast, shRNA control hPDL cells stimulated with purified dentilisin and Td-WT bacteria resulted in an increased Sp1 signal and translocation to the nucleus (Fig 7A). hPDL cells deficient in TLR2 and MyD88 and challenged with the Td-CF522 resulted in similar results as the shRNA control group (Figs 7 and 8). However, when challenged or stimulated with purified dentilisin and Td-WT bacteria, both TLR2 and MyD88 deficient cells inhibited the translocation of Sp1 into the nucleus (Figs 7 and 8A). Thus, after determining both TLR2 and MyD88 play an integral role in the translocation of Sp1 in our system, we sought out to determine if Sp1 is required or sufficient to regulate target MMPs.
Fig 7. Knockdown of TLR2-dependent signaling inhibits translocation of transcription factor Sp1 in both T. denticola- and dentilisin-stimulated hPDL cells.
A-B) Healthy hPDL cells were challenged with Td-WT and isogenic Td-CF522 bacteria at an MOI of 50 as previously described. Whole cell lysates were generated and used for Western blot analysis utilizing total Sp1-specific antibodies. Total Sp1 protein expression was normalized using GAPDH as a loading control. Statistical significance was determined using a paired t-test. Bars represent mean ± SD (n = 3). **p < .01 versus control. C) Scrambled shRNA control and TLR2 shRNA hPDL cells were challenged or stimulated with Td-CF522, purified dentilisin or Td-WT at an MOI of 50 and concentration of 1 μg/mL for 2-hours in alpha-MEM media with no supplementation followed by a 22-hour incubation in alpha-MEM media supplemented with 10% FBS, 1% Pen/Strep and 1% Amphotericin B. Cells were stained with Hoescht 33342 (Blue) and total Sp1-specific antibodies (Green), and subjected to confocal microscopy. Scale bar represents 10 μm.
Fig 8. Knockdown of MyD88-dependent signaling inhibits translocation of transcription factor Sp1 in both T. denticola- and dentilisin-stimulated hPDL cells.
A) Scrambled shRNA control and MyD88 shRNA hPDL cells were challenged or stimulated with Td-CF522, purified dentilisin or Td-WT at an MOI of 50 and concentration of 1 μg/mL for 2-hours in alpha-MEM media with no supplementation followed by a 22-hour incubation in alpha-MEM media supplemented with 10% FBS, 1% Pen/Strep and 1% Amphotericin B. Cells were stained with Hoescht 33342 (Blue) and total Sp1-specific antibodies (Green) and subjected to confocal microscopy. Scale bar represents 10 μm. Scale bar represents 10 μm.
Knockdown of transcription factor Sp1 inhibits T. denticola/dentilisin-stimulated upregulation of MMPs 2, 11, 14, 17 and 28 in hPDL cells
Promoter characterization and luciferase promoter activity assays have identified Sp1 as a regulator of most MMPs and TLRs, through interactions with other transcription factors and cis-regulatory elements such as distal enhancers [79–84]. Thus, it is possible that Sp1 may function as a critical regulator in response to T. denticola challenge. To answer this question, shRNA vectors against the Sp1 gene were used to transduce healthy patient-derived hPDL cells using lentiviral particles as previously described. Cells transfected with scrambled shRNA were used as a negative control. Average basal Sp1 mRNA levels were decreased ~40% across three clones as assessed using RT-qPCR (Fig 9A). These shRNA control and Sp1 deficient hPDL cells were then challenged with purified dentilisin at 1 μg/mL concentration or Td-WT bacteria at an MOI of 50 followed by RNA isolation and RT-qPCR as previously described. Control shRNA hPDL cells stimulated or challenged with purified dentilisin and Td-WT bacteria resulted in the statistically significant upregulation of MMPs 2, 11, 14, 17 and 28 (Fig 9B–9F). By contrast, Sp1 deficient hPDL cells under the same conditions suppressed the upregulation of all MMP targets (Fig 9B–9F).
Fig 9. Stable Suppression of Sp1 inhibits T. denticola-stimulated upregulation of MMP 2, 11, 14, 17 and 28 in hPDL cells.
A) RT-qPCR validation of stable gene suppression using shRNA vectors targeted against Sp1 in healthy hPDL cells. Cells transduced with scrambled shRNA vectors were used as a control. Statistical significance was determined using an unpaired t-test. Bars represent ± SEM of mean values (n = 3 clones). **p < .01 versus control. B-F) RT-qPCR for MMP-2, MMP-11, MMP-14, MMP-17 and MMP-28 mRNA expression of scrambled shRNA control and Sp1 shRNA hPDL cells challenged or stimulated with purified dentilisin at a concentration of 1 μg/mL or Td-WT at an MOI of 50 for 2-hours in alpha-MEM media supplemented with 10% FBS and no antibiotics followed by a 22-hour incubation in alpha-MEM media supplemented with 10% FBS, 1% Pen/Strep and 1% Amphotericin B. The expression of each gene was normalized to that of GAPDH. Statistical significance was determined using a Two-Way ANOVA followed by post-hoc Tukey’s multiple comparisons. Bars represent mean ± SEM (n = 3). #p < .05 versus scramble control group. ##p < .01 versus scramble control group. ###p < .001 versus scramble control group. *p < .05 versus Sp1 shRNA equivalent group. **p < .01 versus Sp1 shRNA equivalent group. ***p < .001 versus Sp1 shRNA equivalent group.
Discussion
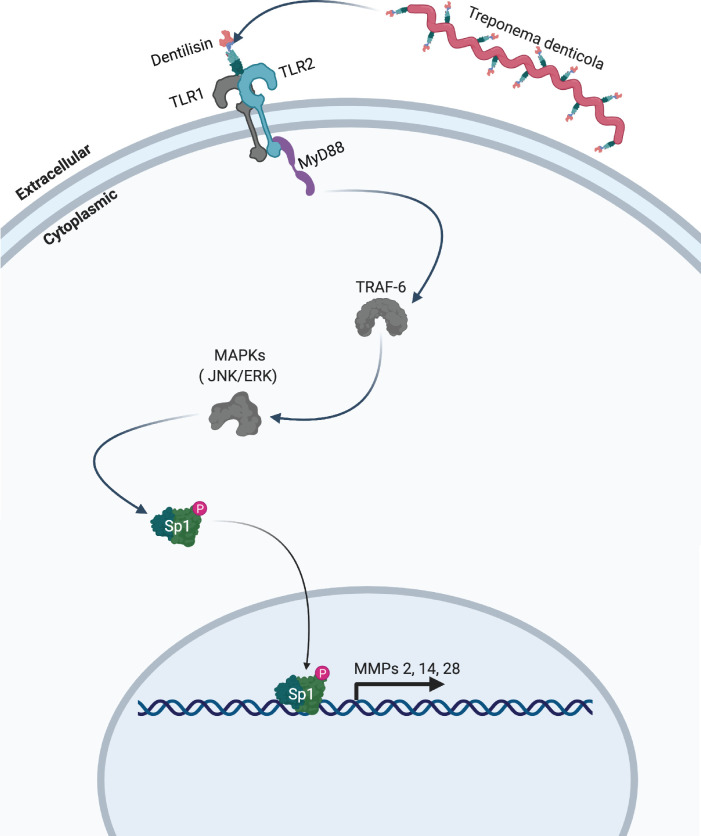
Data from this study extends our earlier reports that short term exposure to dentilisin leads to sustained upregulation of tissue destructive processes at both the transcriptional and translational levels. Specifically, we demonstrated that T. denticola surface-expressed dentilisin plays a direct role in activating TLR2/MyD88-dependent pathways resulting in the subsequent upregulation of MMPs in hPDL cells. Additionally, we have identified the transcription factor Sp1 as an important downstream target that is activated as a result of TLR2/MyD88 activation, leading to its translocation into the nucleus and mediating the upregulation of various MMPs (Fig 10). Our RNA-Seq analysis revealed specific gene expression patterns that are associated with distinct pathologic features presented clinically [16], reported in in vivo studies [85] and previous studies from our lab [39,46]. Additionally, our present data expands on this finding demonstrating T. denticola’s ability to drive a tissue-destructive gene profile after a brief exposure. While the 5-hour incubation group clustered with the control group, the 24-hour incubation group clustered away from the control group illustrating T. denticola’s ability to mediate large-scale transcriptional changes within 24-hours after infection (Fig 1B).
Fig 10. Model of proposed mechanism. T. denticola activates host expressed TLR2 receptors via surface-expressed dentilisin.
Subsequent downstream activation of MyD88 and translocation of the transcription factor Sp1 lead to the upregulation of MMPs 2, 11, 14, 17 and 28 in human periodontal ligament cells. Grey colored icons represent genes that were identified through RNA-sequencing and analysis but were not directly validated.
PDLs insert into the cementum of the tooth root through Sharpey’s fibers which are primarily comprised of fibrillar collagen [86,87]. While most collagen is degraded by collagenases such as MMP-1, MMP-8 and MMP-13, MMP-2 (gelatinase B) and MMP-14 are the predominately expressed species in human PDLs and they are able to actively degrade fibrillar collagen [88,89]. MMP proteolytic cascades can also lead to widespread periodontal tissue destruction due to cooperative MMP activation [90]. A myriad of MMP-14 substrates have been identified including complement components [91] and fibrinogens [92] as well as being a potent activator of other MMPs such as pro-enzymatic MMP-2[90]. Additionally, both MMP-2 and MMP-14 participate in the active degradation of various cytokine and chemokine substrates expressed by hPDL including IL-1β, IL-6, TNF-α, CXCL12, IL-8 and MCP protein family members [57]. Lastly, although MMPs are classically viewed as extracellular and localized to the cell surface, they have also been found in every cellular compartment [21]. Compelling evidence has revealed MMP-14 moonlighting as a transcription factor independent of its catalytic activity, regulating >100 genes associated with inflammation and bacterial clearance [21,93]. Therefore, it is possible that MMP-14 regulates other MMPs or initiates a forward feed-back loop to regulate itself. Taken together, these data suggest that dysregulation of MMP-2 and MMP-14 play a key role in modulating numerous host responses during periodontal infections.
While MMPs 2 and 14 have been linked to periodontal disease progression, MMPs 11, 17 and 28 have yet to be described in this disease context [17]. Our gene ontology analysis places all within the various collagen and ECM catabolic processes (Fig 1C). Similar to many other MMPs, clear roles for MMP-17 (also called MT4-MMP) have been described within the context of cancer [94]. However, identifying a specific role in tissue homeostasis and in other diseases has been challenging. The GPI- anchored MMP-17 has minimal catalytic activity against ECM substrates only showing affinity for fibrin/fibrinogen, gelatin and pro-TNF-alpha ligands [94,95]. To date, analysis of MMP-17 null mice has revealed a weak phenotype associated with water retention that is regulated by processes in the hypothalmus [96]. Additionally, MMP-17 mediates C-terminal processing of ADAMTS4, one of the aggrecanases that are thought to play a role in arthritis [97]. Pro-MMP-11 (also called Stromelysin-3) contains a unique pro-peptide motif for intracellular calcium activation by furin and has also demonstrated rather restricted substrate specificity [98,99]. Identified MMP-11 ECM substrates include the laminin receptor, fibronectin, elastin and the native alpha3 chain of collagen [100]. All of these are constituent ECM proteins found to be upregulated by T. denticola challenge (Fig 1C), highlighting MMP-11 as a potentially unique response regulator underpinning periodontal disease progression.
MMP-28 overexpression in vitro lead to increased MMP-2 and MMP-14 mRNA levels, constitutive MMP-2 activation, and revealed e-cadherin as a high-affinity substrate for proteasomal degradation in lung carcinoma cells [101,102]. Our data aligned with these findings as MMP-28 was the most upregulated MMP (~2-fold change) within the proteinaceous ECM gene ontology term and was not expressed in control groups, suggesting that MMP-28 may play a multifaceted role in exacerbating MMP-2 and MMP-14 mRNA upregulation while directly contributing to their constitutive activation. Taken together, the multi-dimensionality of MMPs and their varied localizations highlight their role as integral host factors that, in addition to modulating homeostatic tissue turnover, also respond to bacterial challenge through a web of complex interactions.
Spirochetes express a wide range of novel and structurally diverse lipoproteins that help define how these unique microbes interact with their environments [103,104]. All lipoproteins are acylated on the N-terminal cysteine residue, and many spirochete lipoproteins are localized to the outer membrane [55]. Compelling reports have demonstrated the importance of specific lipoprotein acyl groups as drivers of both pro and anti-inflammatory cytokine responses in various experimental infection models over the last few decades [65,103]. However, thorough characterization of acylation of T. denticola lipoproteins including dentilisin has yet to be done. Therefore, due to the availability and access to numerous other T. denticola dentilisin isogenic mutants, we decided to investigate the role of dentilisin proteolyitic activity in the context of host gene regulation, an area which has yet to be explored in oral tissues. Of the various available dentilisin mutants, we chose to utilize CF522 which expresses PrcA (one of the three lipoproteins of the dentilisin complex), but lacks PrtP, the actual protease. Planned future studies will include an otherwise native “catalytically dead” isogenic T. denticola dentilisin mutant strain carrying single base changes in each of the triad of PrtP catalytic residues (Asp203, His258, and Ser447).
In our study, stimulation with purified dentilisin lead to the upregulation of MMPs 2, 11, 14, 17 and 28. By contrast, challenging hPDL cells with isogenic Td-CF522 (which lacks the proteolytic PrtP subunit)[105] had no effect (Fig 4). Thus, while we originally hypothesized dentilisin lipid moieties to be the causative drivers of TLR2 activation, our data suggest dentilisin protease activity may be an integral factor responsible for the induction of various MMPs in oral tissues. However, the exact mechanism of action remains to be determined. We plan to pursue the issue of relative contributions of dentilisin acylation and proteolytic activity in future studies.
Although both TLR2 and TLR4 have been implicated in periodontal disease progression, various studies utilizing knockout mice and in vitro systems suggest that TLR2 plays the most direct role is in sensing various components produced by periodontal pathogens that drive alveolar bone destruction [32,34,85,106]. However, the precise roles, whether protective or destructive, played by TLR2 in periodontal inflammation and disease are poorly understood, particularly in the context of T. denticola infection. Once a bacterial ligand binds, a conformational change is thought to occur that brings the two Toll/interleukin-1 (IL-1) receptor (TIR) domains on the cytosolic face of each receptor into closer proximity, creating a new platform on which to build a signaling complex through various adaptor molecules [107]. Thus, availability of intracellular adaptors can accommodate rather distinct signaling patterns as a result of TLR activation [74]. Because MyD88 is the predominant mediator for almost all TLRs with the exception of TLR3 and TLR4, we sought out to determine its role in regulating MMP expression in PDL tissues first.
MyD88 deficient hPDL cells stimulated with Td-WT or purified dentilisin resulted in universal suppression of all MMP targets compared to the shRNA control group (Fig 5). Interestingly, while shRNA knockdown of MyD88 resulted in a statistically significant downregulation of MMP-2 in the negative control group (Fig 5B), this was not observed for any other MMP targets as well as in any TLR2 knockdown groups. Similarly, TLR2 deficiency (in hPDL cells under the same experimental conditions) was sufficient to suppress all MMP targets with the exception of MMP-11 (Fig 4). Thus, our data suggests that T. denticola utilizes a TLR2/MyD88-dependent pathway to regulate MMPs 2, 14, 17 and 28 in PDL tissues. A possible explanation for this differential response is that MMP-11 may be more highly regulated by an alternative pathway that is indirectly activated as a result of T. denticola infection or treatment with dentilisin.
ECM degradation is a key event in the early stages of periodontal disease, generating fragments that can act as danger-associated molecular patterns (DAMPs) such as fragmented aggrecan, fibronectin, biglycan, decorin and low molecular weight hyaluronic acid [108]. Our lab has readily demonstrated that dentilisin mediates MMP-2-dependent degradation of Fibronectin in patient derived hPDL cell cultures [35], with specific fragments (i.e. 29, 40, 68 and 120 kDa) capable of modulating various cellular processes including apoptosis, proliferation and proteinase expression through integrin signaling [109–113]. Dongsheng and colleagues reported that fibronectin was able to activate TLR2/4 to stimulate an innate immune response in macrophages through cross-talk between TLR2, TLR4 and integrin signaling, leading to upregulation of pro-inflammatory genes [114]. Interestingly, while the upregulation of integrins α10 and β4 associated with collagen and laminin interactions were detected, integrins responsible for sensing full-length or fragmented fibronectin did not change. These data suggest that T. denticola dentilisin-induced fibronectin fragmentation may play a role in activating and amplifying TLR2-dependent signaling resulting in the differential expression of MMPs.
Several other pathways modulate TLR-signaling, fostering tissue-specific outcomes. Synergistic cooperation between the Notch pathway and acute TLR-induced signals in the activation of canonical and non-canonical Notch target genes has been characterized as an integral pathway governing the regulation of innate and adaptive immunity [115–118]. Consistent with this, our KEGG pathway analysis revealed Notch signaling (~2-fold change) as the second most enriched pathway resulting from T. denticola challenge in hPDL cells 22-hours post-challenge (Fig 1D). However, mechanisms by which TLRs induce Notch ligand expression and indirectly activate Notch signaling are not well understood and are in many cases, contradictory [119–121].
Although inflammatory pathways include a core set of genes that are activated in response to inflammatory stimuli, they differ extensively depending on the cell and tissue type and on the intensity of the trigger [78]. Examples include nuclear factor-κB (NF-κB)[78,122], interferon-regulatory factor (IRF) proteins [123] and Kruppel-Like family transcription factors such as Sp1[124,125]. While increased Sp1 levels have been implicated as a factor in periodontal disease progression in previous studies, a thorough characterization of its role in PDL tissues has not been reported. Many MMPs, including MMPs 2, 11, 14, 17 and 28, have multiple GC boxes in their proximal promoters that bind Sp1 and potentially other GC-binding proteins [83]. For example, the Sp1 binding site in the human MMP-14 promoter is crucial in maintaining expression of this gene, since introducing mutations reduces promoter activity by approximately 90%[126]. Sp1 has also demonstrated the capacity to affect cis-regulatory elements that are highly susceptible to cell stimulation [127], potentially explaining how constitutively expressed MMPs (such as MMP-2 and MMP-14) are still subject to upregulation upon T. denticola stimulation. Swingler et al. 2010 reported that Sp1 transcription factors are acetylated in response to HDAC inhibitors, bind to the conserved GT-box of the MMP28 promoter, potentially working in conjunction with p300/CREB to upregulate mRNA expression [80].
When healthy hPDL cells were challenged with wild-type T. denticola, total Sp1 protein expression increased compared to the control group (Fig 6A). In contrast, this effect was inhibited in hPDL cells challenged with isogenic mutant Td-CF522 (Fig 6B). This led us to determine if this increase in Sp1 protein expression was combined with increased Sp1 translocation to the nucleus. To answer this question, TLR2- and MyD88-knockdown patient-derived hPDL cells lines were generated and challenged with wild-type T. denticola, Td-CF522 and purified dentilisin. shRNA-control cells stimulated with dentilisin and challenged with Td-WT resulted in Sp1 protein localizing to the nucleus, while TLR2 and MyD88 suppression under the same conditions inhibited this effect (Figs 7 and 8). Interestingly, a slight increase in cytoplasmic Sp1 was noted in shRNA control, TLR2 and MyD88 knockdown groups when challenged with the Td-CF522 isogenic mutant, suggesting that other factors expressed by T. denticola may influence Sp1 expression, whereas dentilisin preferentially regulates its post-translational activation. While shRNA-control hPDL cells stimulated with Td-WT and dentilisin resulted in the upregulation of MMPs 2, 11, 14, 17 and 28, this effect was suppressed in Sp1-deficient hPDL cells under the same conditions (Fig 9). Taken together, these data suggest that the transcription factor Sp1 is an important downstream early response gene that is post-translationally activated as a consequence of TLR2/MyD88 activation, mediating T. denticola-induced upregulation of several MMPs in hPDL cells.
This study has potential limitations, most of which we intend to address in future studies. Because our samples are generated from a small number of human patient tissues, genetic variation may lead to heavily variegated responses. Due to a low sample size, our RNA-Seq in particular may be subject to read count bias and confounding that may have influenced our results. The Pearson’s correlation reflects the linear relationship between two variables accounting for differences in their mean and SD. The more similar the expression profiles for all transcripts are between two samples, the higher the correlation coefficient will be. As shown in S3 Fig, our samples clustered well, suggesting that dispersion effects are low across the 3 biological replicates. In addition, comprehensive analyses have shown that data sets with very low dispersions, a power of 0.8 is easily reached with very low sample size and sequencing depth [128]. While our results provide a strong support for mechanisms of action underpinning periodontal disease, other systemic factors could potentially modulate true phenotypic effects. As such, simulation of similar experimental conditions in an animal model is planned in future studies.
Studies that focus on the effects of unchecked TLR signaling and its contributions to dysregulation of tissue destructive genes in periodontal ligament connective tissues are lacking. Considering the limitations of the study, to the best of our knowledge this study is the first associating dentilisin with TLR2 activation using an in vivo isogenic mutant bacteria in conjunction with purified product from its parent strain. Additionally, we also identified potentially tissue-specific inducible MMPs that that may play novel roles in modulating host immune responses that have yet to be characterized in the context of periodontal disease.
Materials and methods
Ethics statement
Approval to conduct human subjects’ research, including protocols for the collection and use of human teeth and PDL tissue was obtained from the University of California San Francisco Institutional Review Board (#16–20204; reference #227030). Consent was not obtained due to anonymity of the samples.
Human PDL cell cultures
As described previously, the primary culture of PDL cells was conducted via the direct cell outgrowth method by isolating cells from the PDL tissue around the middle third of extracted healthy human teeth [129,130]. Cells were maintained in Minimum Eagle Medium-α (MEM-α) (Gibco, USA) augmented with 10% fetal bovine serum (FBS) (Gibco, USA), 1% penicillin/streptomycin (P/S), and 1% amphotericin B (Gibco, USA) in a Steri Cycle 370 incubator (Thermo-Fisher Scientific, USA) with a humid atmosphere containing 95% air and 5% CO2 at 37°C. Cell outgrowths were passaged before reaching confluency. Only cells passaged three to seven were used for experimentation. Cells were validated by morphological assessment and gene expression of confident biomarkers such as Periostin.
Anaerobic bacterial cultures
T. denticola ATCC 35405 (Td-WT) and its isogenic Td-CF522 dentilisin-deficient mutant Td-CF522 were grow in in Oral Treponeme Enrichment Broth (OTEB; Anaerobe Systems, USA) at 37°C) grown in tanks filled with an anaerobic gas mixture [131]. Veilonella parvula ATCC 10790 was grown under the same anaerobic conditions as T. denticola in brain heart infusion (BHI) media supplemented with 0.075% sodium thioglycolate, 0.1% Tween 80 and 1% of 85% lactic acid and pH adjusted to 6.5–6.6 using 1 M NaOH.
Purification of T. denticola dentilisin
The dentilisin protease complex was purified from the detergent phase of Triton X-114 extracts of an isogenic T. denticola mutant that lacks Msp,the dominant outer membrane protein, as described previously. Briefly, following overnight Triton X-114 treatment, cells were pelleted, the supernatants were phase-separated, the detergent phase was re-extracted twice, precipitated in acetone, then subjected to preparative SDS-PAGE electrophoresis. Fractions of interest, eluted in detergent-free buffer, were concentrated by ultrafiltration, subjected to buffer exchange into PBS containing 0.1% CHAPS {3-[(-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} and concentrated again [35]. Aliquots of purified dentilisin were stored at -80°C. Activity of purified dentilisin samples was monitored periodically, using the chromogenic substrate succinyl-L-alanyl-L-alanyl-L-prolyl-L-phenylalanine-p-nitroanilide (SAAPFNA) as described previously [50].
Bacterial challenging of hPDL cells
~9x105 healthy hPDL cells were seeded on 6 cm plates. On the next day, these cells were challenged with either T. denticola, Td-CF522 or V. parvula, as previously described by Ateia et al.[46]. Briefly, the bacteria were centrifuged at 4000 RPM for 15 minutes and the supernatant was removed. The pelleted bacterial cells were then resuspended in antibiotic-free MEM-α (without phenol red) at an optical density (OD) of 0.1 at 600 nm using a Spectramax M2 microplate spectrophotometer (Molecular Devices, USA). It has been previously established that an OD of 0.1 at 600nm is equivalent to 2.4x108 CFU/ml for T. denticola [131] and 2x108 CFU/mL for V. parvula strains [132]. Next, hPDL cells were challenged with the bacteria at 50 multiplicity of infection (MOI), whereas the control group was challenged with antibiotic-free MEM-α. Next, the cells were incubated for 2h at 37°C and 5% CO2, washed twice with Phosphate Buffer Saline (PBS) (Gibco, USA) and incubated again at 37°C and 5% CO2 overnight in antibiotic-free MEM-α. On the next day, cells were either harvested for RNA isolation, or generation of cell lysates and stored at -80°C for further investigations.
RNA-Seq
RNA was extracted from ~9.5x105 hPDL cells challenged with wild-type T. denticola (as described above using a RNeasy Mini Kit and following the manufacturers protocol (Qiagen, Germany). The quality of the extracted RNA was assessed using a Nanodrop UV-Vis Spectrophotometer (Thermo-Fisher Scientific, USA) and by calculating the percentage of RNA fragments with size > 200 bp (DV200) using an Agilent 2100 Bioanalyzer. The RNA was used for first and second strand synthesis, polyA tail bead capture, and sequencing adapter ligation using a TruSeq RNA Library Prep Kit v2 (Illumina, USA). Libraries were sent to Novogene Genomic Services (Davis, USA) for paired-end sequencing on a HiSeq 4000 instrument (150 bp paired-end reads) (Illumina, USA) and analysis. The mean gene expression across all replicates were used for data visualization. Analysis was conducted using the opensource statistical software called R [133] and figures were produced using the Grammar of Graphics Plot 2 (GGplot2) [134] software package.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Total hPDL RNA was extracted and purified using the RNeasy mini kit (Cat# 74134, Qiagen, Germany) according to the manufacturer’s instructions and quantified using a NanoDrop UV-Vis Spectrophotometer (Thermo Scientific, USA). Reverse transcription of the RNA into cDNA was then performed using the SuperScript III cDNA synthesis kit (Cat #11754050, Invitrogen, USA). Samples were analyzed on a Bio-Rad MyCycler Thermal Cycler according to manufacturers protocol. All cDNA samples were diluted to 5 ng/mL working concentrations and stored at -20°C. cDNA samples were probed for target gene expression via RT-qPCR using PowerUp SYBR Green Master Mix (Applied Biosystems, USA) and primer sequences (Table 1) on a QuantStudio 3 platform (Applied Biosystems, USA). The relative expression levels of target genes were plotted as fold change compared with untreated or negative controls. The 2-ΔΔCT method was used to determine relative change in target gene expression, and gene expression was normalized against GAPDH expression.
Table 1. RT-qPCR forward and revers primer pairs.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5’-TTGAGGTCAATGAAGGGGTC-3’ | 5’-GAAGGTGAAGGTCGGAGTCA-3’ |
| MMP-2 | 5’-TTTCCATTCCGCTTCCAGGGCAC-3’ | 5’- TCGCACACCACATCTTTCCGTCACT-3’ |
| MMP-11 | 5’ CGATGCTGCTGCTGCTGCTCCAG-3’ | 5’-TGGCGTCACATCGCTCCATACCTTTAG-3’ |
| MMP-14 | 5’ TGAGGATCTGAATGGAAATGAC-3’ | 5’-CATAAAGTTGCTGGATGCCC-3’ |
| MMP-17 | 5-CACCAAGTGGAACAAGAGGAACCT-3’ | 5-TGGTAGTACGGCCGCATGATGGAGTGTGCA-3’ |
| MMP-28 | 5’-CACCTCCACTCGATTCAGCG-3’ | 5’-AAAGCGTTTCTTACGCCTCA-3’ |
| MyD88 | 5’-TCTCTGTTCTT GAACGTGCGGACA-3’ | 5’-TTTGGCAATCCTCCTCA ATGCTGG-3’ |
| Periostin | 5′-CCAGCAGACACACCTGTTGG-3′ | 5′-CCTTGAACTTTTTTGTTGGC-3′ |
| Sp1 | 5′-TAATGGTGGTGGTGCCTTT-3′ | 5′-GAGATGATCTGCCAGCCATT-3′ |
| TLR2 | 5’-ATCCTCCAATCAGGCTTCTCT-3’ | 5’-ACACCTCTGTAGGTCACTGTTG-3’ |
Stimulation of hPDL cells using purified dentilisin
A BCA protein assay kit (CAT # 23227 Thermo Scientific, USA) was used to determine purified dentilisin sample concentrations as described above. Assessment of enzymatic activity was determined using gelatin zymography as described below. ~9.5x105 cells were seeded on 6 cm plates. Purified dentilisin was added to MEM-α media with no serum and 1% P/S at a final concentration of 1 μg/mL and added to healthy hPDL cell cultures for 2-hours. The treated cells were washed with PBS and incubated overnight in MEM-α media free of FBS and supplemented with 1% P/S. Next, the Supernatant (conditioned media) was collected and total RNA was extracted using an RNeasy Mini kit (Cat# 74134, Qiagen, Germany). The resulting samples were stored at −80°C until analyzed further in downstream applications.
Gelatin zymography
Supernatant collected from dentilisin stimulated hPDL cells were quantified and normalized to an albumin standard using the Pierce BCA protein assay kit (Cat# 23225, Thermo Scientific, USA) according to the manufacturers protocol. Equivalent protein concentrations from samples were mixed with nonreducing sample buffer (0.25 M tris-base, 40% glycerol, 0.8% sodium dodecyl sulfate (SDS), and 0.05% bromophenol blue stain in distilled deionized water/ddH2O at pH 6.8) and loaded into 8% polyacrylamide gels that were co-polymerized with 0.4% SDS and 0.2% gelatin. Samples were electrophoretically resolved on gelatin-containing gels at 125 V for 110 min at 4°C using a Power Pack supply (Bio-Rad, USA) Next, gels were washed in a series of buffers to facilitate re-constitution of endogenous protein and stained with Coomassie Blue as previously described [46]. Briefly, Gels were then washed twice for 15 min under continuous agitation using renaturation/washing buffer (2.5% v/v Triton-X100 and 0.05 M Tris-base in ddH2O at pH 7.5) to eliminate SDS and promote the renaturation of MMP enzymes. Subsequently, gels were incubated in developing/incubation buffer (0.05MTris-base, 0.15Msodium chloride, 0.01Mcalcium chloride, and 0.02% sodium azide in ddH2O at pH 7.5) for 30 min under agitation, and then the buffer was replaced and gels incubated for 16–20 hr at 37°C. After that, gels were stained using filtered Coomassie Brilliant blue stain for 1 or 2 hr under agitation. Destaining of the gels was performed using a methanol/acetic acid destaining buffer (40% methanol and 10% acetic acid in ddH2O) until the bands on the gel appeared clear. Zymograms were scanned using an Hp Officejet 6700, and the densitometry of gelatinolytic activity represented by the clear bands was analyzed using Fiji software [135]. Brightness and contrast levels of zymogram images were slightly adjusted for publication only.
Lentiviral shRNA knockdown
~6x104 hPDL cells were seeded on 96-well plates. 48 hours later, these cells were infected with either TLR2, MyD88, or Sp1 targeted short hairpin RNA (shRNA) constructs via lentiviral particles according to the kits instructions (Cat# sc-40256-V, Cat# sc-44313-V and Cat# sc-29487-V, Santa Cruz Biotechnology, USA,). Control samples were transduced using nonspecific scrambled shRNA control constructs (Cat# sc-108060, Santa Cruz Biotechnology, USA). hPDL cells were concomitantly permeabilized using Polybrene reagent at a final concentration of 5 μg/mL (EMD Millipore, USA). Approximately 10 hours after infection, cells were washed and incubated overnight in complete MEM-α media supplemented with 10% FBS, 1% PenStrep and 1% Amphotericin B. A pol III promoter drives expression of a silencing cassette containing a puromycin resistance gene used for selection [136]. As a result, a short hairpin RNA complex is exported into the cytoplasm, processed by Dicer and assembled into the RISC complex [136]. Degradation of targeted mRNA transcripts is mediated by unwinding the siRNA duplex leading to a stable reduction in gene expression. Selection of clones with successfully integrated knockdown constructs was accomplished by treating infected hPDL cells with puromycin (Calbiochem, USA) at a final concentration of 5 μg/mL for 24-hours in supplemented MEM- α media. This media was then exchanged for supplemented MEM-α media containing 1 μg/mL puromycin until single cell colonies were isolated. Finally, cell colonies were expanded and validated via RT-qPCR and/or Western Blot.
Western blot analyses
Cell lysates generated from shRNA lentiviral infection were normalized to an albumin standard as described above. These samples were then subjected to SDS-PAGE 4–12% polyacrylamide gels (Invitrogen, USA) and transferred to polyvinylidene difluoride (PVDF) membranes (EMD Millipore, USA) using a Turbo Blot transfer system and PowerPac Basic power supply (Bio-Rad, USA). The membranes were exposed to a rabbit polyclonal anti-Sp1 (Cat# PA5-29165, Invitrogen, Carlsbad, CA, USA) primary antibody diluted 1:500 in Tris-buffered saline, 0.1% Tween 20 (TBST) solution overnight at 4°C followed by a horseradish peroxidase-conjugated mouse anti-rabbit IgG secondary antibody (Cat# sc-235, Santa Cruz, Dallas, TX, USA) diluted 1:3000 in TBST for 1 hour at room temperature with agitation. Western blots using anti-GAPDH antibodies (Cat# sc-32233, Santa Cruz Biotechnology, USA) were used to confirm equal protein loading. Blots were developed using the Super Signal West Pico kit (ThermoFischer Scientific, USA), scanned for digitization using an Officejet 6700 scanner (Hewlett-Packard, USA) and analyzed using Fiji software [135].
Confocal microscopy
Approximately 8x104 hPDL cells were seeded into 4-well glass bottom wells. Cells were challenged with purified dentilisin, wild-type T. denticola or isogenic Td-CF522 mutant bacteria as described above. Cells were washed with PBS before being fixed using 4% paraformaldehyde for 10 minutes at room temperature. Next, cells were incubated for 20 minutes with 0.1 M glycine in PBS, washed with PBS and permeabilized with 0.2% Triton X-100 in PBS for 2 minutes at room temperature. Cells were incubated in a 10% serum/PBS blocking buffer for 5 minutes at room temperature followed by a 60-minute incubation in a rabbit sourced polyclonal anti-Sp1primary antibody diluted 1:500 in 10% serum/PBS solution. Next, cells were washed 3 times with PBS followed by incubation in 10% serum/PBS blocking buffer for 1 minute at room temperature followed by incubation in anti-rabbit, mouse secondary antibody conjugated to Alexa 488 fluorophore for 30 minutes at 37°C (1:3000 dilution) and Hoescht 33342 nuclear staining (1:2000 Dilution) in 10% serum/PBS solution. Finally, cells were washed 3 times with PBS, imaged using an SP8 confocal microscope (Leica, Germany) and analyzed using Fiji [135].
Statistical analysis
Statistical analysis of differentially expressed genes in RNA-Seq data was assessed using a Kolmogorov-Smirnov test followed by Benjamini-Hochberg correction (p<0.05) using R open source software [133]. All other data was analyzed using GraphPad Prism Version 8 software (San Diego, USA). Results were evaluated by a one-way ANOVA when comparing more than two groups with a single independent variable while a two-way ANOVA was used to compare more than two groups with 2 independent variables. Western blot and gelatin zymography data was analyzed using a paired or unpaired t-test, respectively.
Supporting information
A) Top 20 downregulated Gene Ontology terms of hPDL cells challenged for 2-hours followed by a 22-hour incubation using the Reactome nomenclature. Statistical significance was assessed using a Kolmogorov-Smirnov test followed by Benjamini-Hochberg correction (p<0.05). B) Top 20 downregulated signaling pathways of hPDL cells challenged for 2-hours followed by a 22-hour incubation using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Statistical significance was assessed using a Kolmogorov-Smirnov test followed Benjamini-Hochberg correction (p<0.05).
(TIF)
Healthy hPDL cells were challenged with purified dentilisin at increasing concentrations (1, 3 and 5 μg/mL) for 2-hours followed by a 22-hour incubation in MEM-α media free of FBS and supplemented with 1% P/S. Conditioned media from these cells were used to assess the enzymatic activity of Active MMP-2 (64-kDa) and Dentilisin (98 kDa) using gelatin zymography followed by densitometry analysis using Fiji. Statistical significance was determined using a One-Way ANOVA. Bars represent ± SD of mean values (n = 3). ***p < .001 versus control.
(TIF)
The figure displays a Pearson’s plot visualizing the correlation between samples. Scale bar represents the range of the correlation coefficients (R) displayed.
(TIF)
Acknowledgments
We would like to thank Charles Le for his technical assistance recovering and maintaining Veilonella parvula bacterial stocks and Dr. Ryutaro Kuraji as creator of the artwork used as the striking image for this work.
Data Availability
The data underlying the results presented in the study are available from (https://figshare.com/collections/T_denticola_dentilisin_triggered_TLR2_MyD88_activation_upregulates_a_tissue_destructive_program_involving_MMPs_via_Sp1_in_human_oral_cells/5262821).
Funding Statement
This work was supported by funding from Research Project Grant funding from the NIH (R01 DE025225) to YLK and JCF (https://www.nih.gov/); Ruth L. Kirschstein Individual Predoctoral NRSA for MD/PhD and other Dual Degree Fellowships (F30 DE027598) to EM (https://www.nih.gov/), and the Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32DE007306) to SG (https://www.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eke P.I., et al., Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. Journal of Dental Research, 2012. 91(10): p. 914–920. doi: 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- 2.Kebschull M., Demmer R.T., and Papapanou P.N., "Gum bug, leave my heart alone!"—epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. Journal of dental research, 2010. 89(9): p. 879–902. doi: 10.1177/0022034510375281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madianos P.N., Bobetsis Y.A., and Offenbacher S., Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. Journal of Periodontology, 2013. 84(4S): p. S170–S180. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg K., et al., Periodontitis in RA—the citrullinated enolase connection. Nature Reviews Rheumatology, 2010. 6(12): p. 727–730. doi: 10.1038/nrrheum.2010.139 [DOI] [PubMed] [Google Scholar]
- 5.Karpinski T.M., Role of Oral Microbiota in Cancer Development. Microorganisms, 2019. 7(1). doi: 10.3390/microorganisms7010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dyke T.E., The management of inflammation in periodontal disease. J Periodontol, 2008. 79(8 Suppl): p. 1601–8. doi: 10.1902/jop.2008.080173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan C. and Ding A., Nonresolving Inflammation. Cell, 2010. 140(6): p. 871–882. doi: 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 8.Hasturk H. and Kantarci A., Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000, 2015. 69(1): p. 255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wescott D.C., et al., Osteogenic Gene Expression by Human Periodontal Ligament Cells under Cyclic Tension. Journal of Dental Research, 2007. 86(12): p. 1212–1216. doi: 10.1177/154405910708601214 [DOI] [PubMed] [Google Scholar]
- 10.Jiang N., et al., Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front Oral Biol, 2016. 18: p. 1–8. doi: 10.1159/000351894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokos D., Everts V., and de Vries T.J., Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. Journal of Periodontal Research, 2015. 50(2): p. 152–159. doi: 10.1111/jre.12197 [DOI] [PubMed] [Google Scholar]
- 12.Tang L., et al., Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Archives of Oral Biology, 2011. 56(10): p. 1064–1072. doi: 10.1016/j.archoralbio.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 13.Zhang P., et al., Focal adhesion kinase activation is required for TNF-alpha-induced production of matrix metalloproteinase-2 and proinflammatory cytokines in cultured human periodontal ligament fibroblasts. Eur J Oral Sci, 2015. 123(4): p. 249–53. doi: 10.1111/eos.12191 [DOI] [PubMed] [Google Scholar]
- 14.Sun Y., et al., Gram-negative periodontal bacteria induce the activation of Toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol, 2010. 81(10): p. 1488–96. doi: 10.1902/jop.2010.100004 [DOI] [PubMed] [Google Scholar]
- 15.Jönsson D., et al., The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. Journal of Periodontal Research, 2011. 46(2): p. 153–157. doi: 10.1111/j.1600-0765.2010.01331.x [DOI] [PubMed] [Google Scholar]
- 16.Sapna G., Gokul S., and Bagri-Manjrekar K., Matrix metalloproteinases and periodontal diseases. Oral Diseases, 2014. 20(6): p. 538–550. doi: 10.1111/odi.12159 [DOI] [PubMed] [Google Scholar]
- 17.Franco C., et al., Matrix Metalloproteinases as Regulators of Periodontal Inflammation. International Journal of Molecular Sciences, 2017. 18(2): p. 440. doi: 10.3390/ijms18020440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long M.E., et al., Matrix metalloproteinase 28 is regulated by TRIF- and type I IFN-dependent signaling in macrophages. Innate Immunity, 2018: p. 1753425918791024. doi: 10.1177/1753425918791024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisboa R.A., Andrade M.V., and Cunha-Melo J.R., Toll-like receptor activation and mechanical force stimulation promote the secretion of matrix metalloproteinases 1, 3 and 10 of human periodontal fibroblasts via p38, JNK and NF-kB. Archives of Oral Biology, 2013. 58(6): p. 731–739. doi: 10.1016/j.archoralbio.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 20.Li H., Xu H., and Liu S., Toll-like receptors 4 induces expression of matrix metalloproteinase-9 in human aortic smooth muscle cells. Molecular Biology Reports, 2011. 38(2): p. 1419–1423. doi: 10.1007/s11033-010-0246-4 [DOI] [PubMed] [Google Scholar]
- 21.Jobin P.G., Butler G.S., and Overall C.M., New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research, 2017. 1864(11, Part A): p. 2043–2055. doi: 10.1016/j.bbamcr.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 22.Silva N., et al., Host response mechanisms in periodontal diseases. Journal of applied oral science: revista FOB, 2015. 23(3): p. 329–355. doi: 10.1590/1678-775720140259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hienz S.A., Paliwal S., and Ivanovski S., Mechanisms of Bone Resorption in Periodontitis. Journal of immunology research, 2015. 2015: p. 615486–615486. doi: 10.1155/2015/615486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Benedetto A., et al., Periodontal Disease: Linking the Primary Inflammation to Bone Loss. Clinical and Developmental Immunology, 2013. 2013: p. 503754. doi: 10.1155/2013/503754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duran-Pinedo A.E. and Frias-Lopez J., Beyond microbial community composition: functional activities of the oral microbiome in health and disease. Microbes and Infection, 2015. 17(7): p. 505–516. doi: 10.1016/j.micinf.2015.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer-Bäumer A., et al., Periodontal pathogens and associated factors in aggressive periodontitis: results 5–17 years after active periodontal therapy. Journal of Clinical Periodontology, 2014. 41(7): p. 662–672. doi: 10.1111/jcpe.12255 [DOI] [PubMed] [Google Scholar]
- 27.Deng Z.-L., et al., Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Scientific Reports, 2017. 7(1): p. 3703. doi: 10.1038/s41598-017-03804-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solbiati J. and Frias-Lopez J., Metatranscriptome of the Oral Microbiome in Health and Disease. Journal of Dental Research, 2018. 97(5): p. 492–500. doi: 10.1177/0022034518761644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurnheer T., Belibasakis G.N., and Bostanci N., Colonisation of gingival epithelia by subgingival biofilms in vitro: role of "red complex" bacteria. Arch Oral Biol, 2014. 59(9): p. 977–86. doi: 10.1016/j.archoralbio.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 30.Fenno J.C., et al., Cytopathic Effects of the Major Surface Protein and the Chymotrypsinlike Protease of Treponema denticola. Infection and Immunity, 1998. 66(5): p. 1869–1877. doi: 10.1128/IAI.66.5.1869-1877.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakthavatchalu V., et al., Molecular characterization of Treponema denticola infection-induced bone and soft tissue transcriptional profiles. Molecular oral microbiology, 2010. 25(4): p. 260–274. doi: 10.1111/j.2041-1014.2010.00575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asai Y., Jinno T., and Ogawa T., Oral Treponemes and Their Outer Membrane Extracts Activate Human Gingival Epithelial Cells through Toll-Like Receptor 2. Infection and Immunity, 2003. 71(2): p. 717–725. doi: 10.1128/IAI.71.2.717-725.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sela M.N., Role of Treponema Denticola in Periodontal Diseases. Critical Reviews in Oral Biology & Medicine, 2001. 12(5): p. 399–413. [DOI] [PubMed] [Google Scholar]
- 34.Ruby J., Rehani K., and Martin M., Treponema denticola Activates Mitogen-Activated Protein Kinase Signal Pathways through Toll-Like Receptor 2. Infection and Immunity, 2007. 75(12): p. 5763–5768. doi: 10.1128/IAI.01117-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao D., et al., The Treponema denticola Chymotrypsin-Like Protease Dentilisin Induces Matrix Metalloproteinase-2-Dependent Fibronectin Fragmentation in Periodontal Ligament Cells. Infection and Immunity, 2011. 79(2): p. 806–811. doi: 10.1128/IAI.01001-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inagaki S., et al., Treponema denticola invasion into human gingival epithelial cells. Microbial Pathogenesis, 2016. 94: p. 104–111. doi: 10.1016/j.micpath.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 37.Ji Eun S. and and Youngnim C., Treponema denticola Suppresses Expression of Human β-Defensin-2 in Gingival Epithelial Cells through Inhibition of TNFα Production nd TLR2 Activation. Mol. Cells, 2010. 29(4): p. 407–412. doi: 10.1007/s10059-010-0048-5 [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto M., Ishihara K., and Okuda K., The Treponema denticola Surface Protease Dentilisin Degrades Interleukin-1β (IL-1β), IL-6, and Tumor Necrosis Factor Alpha. Infection and Immunity, 2006. 74(4): p. 2462–2467. doi: 10.1128/IAI.74.4.2462-2467.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao D., et al., Treponema denticola upregulates MMP-2 activation in periodontal ligament cells: interplay between epigenetics and periodontal infection. Archives of oral biology, 2014. 59(10): p. 1056–1064. doi: 10.1016/j.archoralbio.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godovikova V., Goetting-Minesky M.P., and Fenno J.C., Composition and localization of Treponema denticola outer membrane complexes. Infection and immunity, 2011. 79(12): p. 4868–4875. doi: 10.1128/IAI.05701-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao L., et al., Polymicrobial periodontal disease triggers a wide radius of effect and unique virome. npj Biofilms and Microbiomes, 2020. 6(1): p. 10. doi: 10.1038/s41522-020-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santiago-Rodriguez T.M., et al., Transcriptome analysis of bacteriophage communities in periodontal health and disease. BMC genomics, 2015. 16(1): p. 549–549. doi: 10.1186/s12864-015-1781-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto G., et al., The role of bacteriophages in periodontal health and disease. Future Microbiology, 2016. 11(10): p. 1359–1369. doi: 10.2217/fmb-2016-0081 [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Gao Y., and Zhao F., Phage-bacteria interaction network in human oral microbiome. Environ Microbiol, 2016. 18(7): p. 2143–58. doi: 10.1111/1462-2920.12923 [DOI] [PubMed] [Google Scholar]
- 45.Loesche W.J., The Role of Spirochetes in Periodontal Disease. Advances in Dental Research, 1988. 2(2): p. 275–283. doi: 10.1177/08959374880020021201 [DOI] [PubMed] [Google Scholar]
- 46.Ateia I.M., et al., Treponema denticola increases MMP-2 expression and activation in the periodontium via reversible DNA and histone modifications. Cellular Microbiology, 2017: p. e12815–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramseier C.A., et al., Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol, 2009. 80(3): p. 436–46. doi: 10.1902/jop.2009.080480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S., et al., Immune Response and Alveolar Bone Resorption in a Mouse Model of Treponema denticola Infection. Infection and immunity, 2008. 77: p. 694–8. doi: 10.1128/IAI.01004-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uitto V.J., et al., Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infection and immunity, 1995. 63(9): p. 3401–3410. doi: 10.1128/iai.63.9.3401-3410.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grenier D., Uitto V.J., and McBride B.C., Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infection and Immunity, 1990. 58(2): p. 347–351. doi: 10.1128/iai.58.2.347-351.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chi B., Qi M., and Kuramitsu H.K., Role of dentilisin in Treponema denticola epithelial cell layer penetration. Research in Microbiology, 2003. 154(9): p. 637–643. doi: 10.1016/j.resmic.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 52.McDowell J.V., et al., Treponema denticola: FhbB, Dentilisin, Complement Evasion and the Paradox of Factor H Cleavage, in The Pathogenic Spirochetes: strategies for evasion of host immunity and persistence, Embers M.E., Editor. 2012, Springer US: Boston, MA. p. 43–62. [Google Scholar]
- 53.Schenk M., Belisle J.T., and Modlin R.L., TLR2 Looks at Lipoproteins. Immunity, 2009. 31(6): p. 847–849. doi: 10.1016/j.immuni.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 54.Kim J., et al., Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. Journal of Bone and Mineral Research, 2013. 28(11): p. 2381–2391. doi: 10.1002/jbmr.1973 [DOI] [PubMed] [Google Scholar]
- 55.Wilson M.M. and Bernstein H.D., Surface-Exposed Lipoproteins: An Emerging Secretion Phenomenon in Gram-Negative Bacteria. Trends in Microbiology, 2016. 24(3): p. 198–208. doi: 10.1016/j.tim.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souza J.A.C.d., et al., Pam2CSK4 (TLR2 agonist) induces periodontal destruction in mice. Brazilian Oral Research, 2020. 34. doi: 10.1590/1807-3107bor-2020.vol34.0012 [DOI] [PubMed] [Google Scholar]
- 57.Van Lint P. and Libert C., Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. Journal of Leukocyte Biology, 2007. 82(6): p. 1375–1381. doi: 10.1189/jlb.0607338 [DOI] [PubMed] [Google Scholar]
- 58.Page-McCaw A., Ewald A.J., and Werb Z., Matrix metalloproteinases and the regulation of tissue remodelling. Nature reviews. Molecular cell biology, 2007. 8(3): p. 221–233. doi: 10.1038/nrm2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie Y., et al., Nuclear matrix metalloproteinases: functions resemble the evolution from the intracellular to the extracellular compartment. Cell Death Discovery, 2017. 3(1): p. 17036. doi: 10.1038/cddiscovery.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lovett D.H., et al., A Novel Intracellular Isoform of Matrix Metalloproteinase-2 Induced by Oxidative Stress Activates Innate Immunity. PLOS ONE, 2012. 7(4): p. e34177. doi: 10.1371/journal.pone.0034177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leuckfeld I., et al., Diversity of Veillonella spp. from subgingival plaque by polyphasic approach. APMIS, 2010. 118(3): p. 230–242. doi: 10.1111/j.1600-0463.2009.02584.x [DOI] [PubMed] [Google Scholar]
- 62.Sela M.N., et al., Lipoproteins of Treponema denticola: their effect on human polymorphonuclear neutrophils. J Periodontal Res, 1997. 32(5): p. 455–66. doi: 10.1111/j.1600-0765.1997.tb00558.x [DOI] [PubMed] [Google Scholar]
- 63.Sobocińska J., et al., Protein Palmitoylation and Its Role in Bacterial and Viral Infections. Frontiers in Immunology, 2018. 8(2003). doi: 10.3389/fimmu.2017.02003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doz E., et al., Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J Biol Chem, 2007. 282(36): p. 26014–25. doi: 10.1074/jbc.M702690200 [DOI] [PubMed] [Google Scholar]
- 65.Nguyen M.-T., et al., Lipid moieties on lipoproteins of commensal and non-commensal staphylococci induce differential immune responses. Nature Communications, 2017. 8(1): p. 2246. doi: 10.1038/s41467-017-02234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Complementary Tolls in the periodontium- how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host.pdf. [DOI] [PMC free article] [PubMed]
- 67.Schenkein H.A. and Berry C.R., Activation of Complement by Treponema denticola. Journal of Dental Research, 1991. 70(2): p. 107–110. doi: 10.1177/00220345910700020201 [DOI] [PubMed] [Google Scholar]
- 68.Ruby J., et al., Activation of the Innate Immune System by Treponema denticola Periplasmic Flagella through Toll-like Receptor 2. Infection and Immunity, 2017. doi: 10.1128/IAI.00573-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asai Y., et al., Bacterial Fimbriae and Their Peptides Activate Human Gingival Epithelial Cells through Toll-Like Receptor 2. Infection and Immunity, 2001. 69(12): p. 7387. doi: 10.1128/IAI.69.12.7387-7395.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graves D.T., et al., The Use of Rodent Models to Investigate Host-Bacteria Interactions Related to Periodontal Diseases. Journal of clinical periodontology, 2008. 35(2): p. 89–105. doi: 10.1111/j.1600-051X.2007.01172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hajishengallis G., Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol, 2015. 15(1): p. 30–44. doi: 10.1038/nri3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajishengallis G., Wang M., and Liang S., Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae. J Immunol, 2009. 182(11): p. 6690–6. doi: 10.4049/jimmunol.0900524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin M.S., et al., Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell, 2007. 130(6): p. 1071–1082. doi: 10.1016/j.cell.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 74.Li J., Lee D., and Madrenas J., Evolving Bacterial Envelopes and Plasticity of TLR2-Dependent Responses: Basic Research and Translational Opportunities. Frontiers in Immunology, 2013. 4(347). doi: 10.3389/fimmu.2013.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y., et al., Chlamydial Lipoproteins Stimulate Toll-Like Receptors 1/2 Mediated Inflammatory Responses through MyD88-Dependent Pathway. Frontiers in microbiology, 2017. 8: p. 78–78. doi: 10.3389/fmicb.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Makkawi H., et al., Porphyromonas gingivalis Stimulates TLR2-PI3K Signaling to Escape Immune Clearance and Induce Bone Resorption Independently of MyD88. Front Cell Infect Microbiol, 2017. 7: p. 359. doi: 10.3389/fcimb.2017.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsson L., et al., Interleukin-10 Genotypes of the −1087 Single Nucleotide Polymorphism Influence Sp1 Expression in Periodontitis Lesions. Journal of Periodontology, 2011. 82(9): p. 1376–1382. doi: 10.1902/jop.2011.100623 [DOI] [PubMed] [Google Scholar]
- 78.Medzhitov R. and Horng T., Transcriptional control of the inflammatory response. Nature Reviews Immunology, 2009. 9(10): p. 692–703. doi: 10.1038/nri2634 [DOI] [PubMed] [Google Scholar]
- 79.Li Z., et al., Differential regulation of MMPs by E2F1, Sp1 and NF-kappa B controls the small cell lung cancer invasive phenotype. BMC Cancer, 2014. 14: p. 276. doi: 10.1186/1471-2407-14-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swingler Tracey E., et al., <em>MMP28</em> gene expression is regulated by Sp1 transcription factor acetylation. Biochemical Journal, 2010. 427(3): p. 391. [DOI] [PubMed] [Google Scholar]
- 81.Illman S.A., Keski-Oja J., and Lohi J., Promoter characterization of the human and mouse epilysin (MMP-28) genes. Gene, 2001. 275(1): p. 185–194. doi: 10.1016/s0378-1119(01)00664-3 [DOI] [PubMed] [Google Scholar]
- 82.Wierstra I., Sp1: Emerging roles—Beyond constitutive activation of TATA-less housekeeping genes. Biochemical and Biophysical Research Communications, 2008. 372(1): p. 1–13. doi: 10.1016/j.bbrc.2008.03.074 [DOI] [PubMed] [Google Scholar]
- 83.Clark I.M., et al., The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol, 2008. 40(6–7): p. 1362–78. doi: 10.1016/j.biocel.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 84.Wang T., Lafuse W.P., and Zwilling B.S., NFκB and Sp1 Elements Are Necessary for Maximal Transcription of Toll-like Receptor 2 Induced by Mycobacterium avium</em>. The Journal of Immunology, 2001. 167(12): p. 6924. doi: 10.4049/jimmunol.167.12.6924 [DOI] [PubMed] [Google Scholar]
- 85.Barreiros D., et al., MMP2 and MMP9 are Associated with Apical Periodontitis Progression and Might be Modulated by TLR2 and MyD88. Brazilian Dental Journal, 2018. 29: p. 43–47. doi: 10.1590/0103-6440201801731 [DOI] [PubMed] [Google Scholar]
- 86.Aaron J.E., Periosteal Sharpey’s fibers: a novel bone matrix regulatory system? Frontiers in endocrinology, 2012. 3: p. 98–98. doi: 10.3389/fendo.2012.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho S.P., et al., The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials, 2007. 28(35): p. 5238–5245. doi: 10.1016/j.biomaterials.2007.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jabłońska-Trypuć A., Matejczyk M., and Rosochacki S., Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of Enzyme Inhibition and Medicinal Chemistry, 2016. 31(sup1): p. 177–183. doi: 10.3109/14756366.2016.1161620 [DOI] [PubMed] [Google Scholar]
- 89.Ohuchi E., et al., Membrane Type 1 Matrix Metalloproteinase Digests Interstitial Collagens and Other Extracellular Matrix Macromolecules*. Journal of Biological Chemistry, 1997. 272(4): p. 2446–2451. doi: 10.1074/jbc.272.4.2446 [DOI] [PubMed] [Google Scholar]
- 90.Checchi V., et al., The Role of Matrix Metalloproteinases in Periodontal Disease. International Journal of Environmental Research and Public Health, 2020. 17: p. 4923. doi: 10.3390/ijerph17144923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hwang I.K., et al., A proteomic approach to identify substrates of matrix metalloproteinase-14 in human plasma. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 2004. 1702(1): p. 79–87. doi: 10.1016/j.bbapap.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 92.Hiller O., et al., Matrix Metalloproteinases Collagenase-2, Macrophage Elastase, Collagenase-3, and Membrane Type 1-Matrix Metalloproteinase Impair Clotting by Degradation of Fibrinogen and Factor XII*. Journal of Biological Chemistry, 2000. 275(42): p. 33008–33013. [DOI] [PubMed] [Google Scholar]
- 93.Fingleton B., Matrix metalloproteinases as regulators of inflammatory processes. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research, 2017. 1864(11, Part A): p. 2036–2042. doi: 10.1016/j.bbamcr.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 94.Itoh Y., Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biology, 2015. 44–46: p. 207–223. doi: 10.1016/j.matbio.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 95.English W.R., et al., Membrane Type 4 Matrix Metalloproteinase (MMP17) Has Tumor Necrosis Factor-α Convertase Activity but Does Not Activate Pro-MMP2. Journal of Biological Chemistry, 2000. 275(19): p. 14046–14055. [DOI] [PubMed] [Google Scholar]
- 96.Srichai M.B., et al., Membrane-Type 4 Matrix Metalloproteinase (MT4-MMP) Modulates Water Homeostasis in Mice. PLOS ONE, 2011. 6(2): p. e17099. doi: 10.1371/journal.pone.0017099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao G., et al., ADAMTS4 (Aggrecanase-1) Activation on the Cell Surface Involves C-terminal Cleavage by Glycosylphosphatidyl Inositol-anchored Membrane Type 4-Matrix Metalloproteinase and Binding of the Activated Proteinase to Chondroitin Sulfate and Heparan Sulfate on Syndecan-1. Journal of Biological Chemistry, 2004. 279(11): p. 10042–10051. doi: 10.1074/jbc.M312100200 [DOI] [PubMed] [Google Scholar]
- 98.Pei D. and Weiss S.J., Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature, 1995. 375(6528): p. 244–7. doi: 10.1038/375244a0 [DOI] [PubMed] [Google Scholar]
- 99.Illman S.A., et al., The mouse matrix metalloproteinase, epilysin (MMP-28), is alternatively spliced and processed by a furin-like proprotein convertase. Biochemical Journal, 2003. 375(Pt 1): p. 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu P., et al., Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harbor perspectives in biology, 2011. 3(12): p. doi: 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Illman S.A., Lohi J., and Keski-Oja J., Epilysin (MMP-28)–structure, expression and potential functions. Experimental Dermatology, 2008. 17(11): p. 897–907. doi: 10.1111/j.1600-0625.2008.00782.x [DOI] [PubMed] [Google Scholar]
- 102.Illman S.A., et al., Epilysin (MMP-28) induces TGF-β mediated epithelial to mesenchymal transition in lung carcinoma cells. Journal of Cell Science, 2006. 119(18): p. 3856. doi: 10.1242/jcs.03157 [DOI] [PubMed] [Google Scholar]
- 103.Kelesidis T., The Cross-Talk between Spirochetal Lipoproteins and Immunity. Frontiers in Immunology, 2014. 5(310). doi: 10.3389/fimmu.2014.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zückert W.R., Protein Secretion in Spirochetes. Microbiology spectrum, 2019. 7(3): p. doi: 10.1128/microbiolspec.PSIB-0026-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Godovikova V., et al., Treponema denticola PrcB Is Required for Expression and Activity of the PrcA-PrtP (Dentilisin) Complex. Journal of Bacteriology, 2010. 192(13): p. 3337–3344. doi: 10.1128/JB.00274-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burns E., et al., Cutting Edge: TLR2 Is Required for the Innate Response to Porphyromonas gingivalis: Activation Leads to Bacterial Persistence and TLR2 Deficiency Attenuates Induced Alveolar Bone Resorption. The Journal of Immunology, 2006. 177(12): p. 8296. doi: 10.4049/jimmunol.177.12.8296 [DOI] [PubMed] [Google Scholar]
- 107.O’Neill L.A. and Bowie A.G., The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol, 2007. 7(5): p. 353–64. doi: 10.1038/nri2079 [DOI] [PubMed] [Google Scholar]
- 108.Piccinini A.M. and Midwood K.S., DAMPening Inflammation by Modulating TLR Signalling. Mediators of Inflammation, 2010. 2010: p. 672395. doi: 10.1155/2010/672395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai R., et al., Disease-associated fibronectin matrix fragments trigger anoikis of human primary ligament cells: p53 and c-myc are suppressed. Apoptosis, 2005. 10(3): p. 503–512. doi: 10.1007/s10495-005-1880-5 [DOI] [PubMed] [Google Scholar]
- 110.Kapila Y.L., Kapila S., and Johnson P.W., Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol, 1996. 15(4): p. 251–61. doi: 10.1016/s0945-053x(96)90116-x [DOI] [PubMed] [Google Scholar]
- 111.Hwang H.S., et al., Fibronectin fragment-induced expression of matrix metalloproteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res Ther, 2015. 17: p. 320. doi: 10.1186/s13075-015-0833-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kapila Y.L., Lancero H., and Johnson P.W., The response of periodontal ligament cells to fibronectin. J Periodontol, 1998. 69(9): p. 1008–19. doi: 10.1902/jop.1998.69.9.1008 [DOI] [PubMed] [Google Scholar]
- 113.Huynh Q.N., et al., Specific fibronectin fragments as markers of periodontal disease status. J Periodontol, 2002. 73(10): p. 1101–10. doi: 10.1902/jop.2002.73.10.1101 [DOI] [PubMed] [Google Scholar]
- 114.Fei D., et al., Fibronectin (FN) cooperated with TLR2/TLR4 receptor to promote innate immune responses of macrophages via binding to integrin β1. Virulence, 2018. 9(1): p. 1588–1600. doi: 10.1080/21505594.2018.1528841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsaouli G., et al., Molecular Mechanisms of Notch Signaling in Lymphoid Cell Lineages Development: NF-κB and Beyond. Adv Exp Med Biol, 2020. 1227: p. 145–164. doi: 10.1007/978-3-030-36422-9_10 [DOI] [PubMed] [Google Scholar]
- 116.Gentle M.E., et al., Noncanonical Notch Signaling Modulates Cytokine Responses of Dendritic Cells to Inflammatory Stimuli. The Journal of Immunology, 2012. 189(3): p. 1274. doi: 10.4049/jimmunol.1103102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gamrekelashvili J., et al., Notch and TLR signaling coordinate monocyte cell fate and inflammation. Elife, 2020. 9. doi: 10.7554/eLife.57007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vanderbeck A. and Maillard I., Notch signaling at the crossroads of innate and adaptive immunity. J Leukoc Biol, 2020. [DOI] [PubMed] [Google Scholar]
- 119.Foldi J., et al., Autoamplification of Notch Signaling in Macrophages by TLR-Induced and RBP-J–Dependent Induction of Jagged1. The Journal of Immunology, 2010. 185(9): p. 5023. doi: 10.4049/jimmunol.1001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Q., et al., Notch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor κB activation. J Biol Chem, 2012. 287(9): p. 6208–17. doi: 10.1074/jbc.M111.310375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palaga T., et al., Notch signaling is activated by TLR stimulation and regulates macrophage functions. European Journal of Immunology, 2008. 38(1): p. 174–183. doi: 10.1002/eji.200636999 [DOI] [PubMed] [Google Scholar]
- 122.Smale S.T. and Natoli G., Transcriptional control of inflammatory responses. Cold Spring Harb Perspect Biol, 2014. 6(11): p. a016261. doi: 10.1101/cshperspect.a016261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taniguchi T., et al., IRF Family of Transcription Factors as Regulators of Host Defense. Annual Review of Immunology, 2001. 19(1): p. 623–655. doi: 10.1146/annurev.immunol.19.1.623 [DOI] [PubMed] [Google Scholar]
- 124.Zhao J.-G., et al., Antimicrobial activity-specific to Gram-negative bacteria and immune modulation-mediated NF-κB and Sp1 of a medaka β-defensin. Developmental & Comparative Immunology, 2009. 33(4): p. 624–637. doi: 10.1016/j.dci.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 125.Brightbill H.D., et al., A Prominent Role for Sp1 During Lipopolysaccharide- Mediated Induction of the IL-10 Promoter in Macrophages. The Journal of Immunology, 2000. 164(4): p. 1940. doi: 10.4049/jimmunol.164.4.1940 [DOI] [PubMed] [Google Scholar]
- 126.Lohi J., et al., Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene, 2000. 242(1): p. 75–86. doi: 10.1016/s0378-1119(99)00549-1 [DOI] [PubMed] [Google Scholar]
- 127.Bao J., et al., Integrated high-throughput analysis identifies super enhancers associated with chemoresistance in SCLC. BMC Medical Genomics, 2019. 12(1): p. 67. doi: 10.1186/s12920-019-0520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ching T., Huang S., and Garmire L.X., Power analysis and sample size estimation for RNA-Seq differential expression. RNA (New York, N.Y.), 2014. 20(11): p. 1684–1696. doi: 10.1261/rna.046011.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marchesan J.T., et al., Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review. Arch Oral Biol, 2011. 56(10): p. 933–43. doi: 10.1016/j.archoralbio.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanaka K., et al., Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Archives of Oral Biology, 2011. 56(4): p. 380–388. doi: 10.1016/j.archoralbio.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 131.Fenno J.C., Laboratory maintenance of Treponema denticola. Curr Protoc Microbiol, 2005. Chapter 12: p. Unit 12B.1. doi: 10.1002/9780471729259.mc12b01s00 [DOI] [PubMed] [Google Scholar]
- 132.Pustelny C., et al., Contribution of Veillonella parvula to Pseudomonas aeruginosa-mediated pathogenicity in a murine tumor model system. Infection and immunity, 2015. 83(1): p. 417–429. doi: 10.1128/IAI.02234-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Team, R.C., 2017, in R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- 134.Wickham H., ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York, 2009. [Google Scholar]
- 135.Schindelin J., et al., Fiji: an open-source platform for biological-image analysis. Nature Methods, 2012. 9(7): p. 676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singer O. and Verma I.M., Applications of lentiviral vectors for shRNA delivery and transgenesis. Current gene therapy, 2008. 8(6): p. 483–488. doi: 10.2174/156652308786848067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Top 20 downregulated Gene Ontology terms of hPDL cells challenged for 2-hours followed by a 22-hour incubation using the Reactome nomenclature. Statistical significance was assessed using a Kolmogorov-Smirnov test followed by Benjamini-Hochberg correction (p<0.05). B) Top 20 downregulated signaling pathways of hPDL cells challenged for 2-hours followed by a 22-hour incubation using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Statistical significance was assessed using a Kolmogorov-Smirnov test followed Benjamini-Hochberg correction (p<0.05).
(TIF)
Healthy hPDL cells were challenged with purified dentilisin at increasing concentrations (1, 3 and 5 μg/mL) for 2-hours followed by a 22-hour incubation in MEM-α media free of FBS and supplemented with 1% P/S. Conditioned media from these cells were used to assess the enzymatic activity of Active MMP-2 (64-kDa) and Dentilisin (98 kDa) using gelatin zymography followed by densitometry analysis using Fiji. Statistical significance was determined using a One-Way ANOVA. Bars represent ± SD of mean values (n = 3). ***p < .001 versus control.
(TIF)
The figure displays a Pearson’s plot visualizing the correlation between samples. Scale bar represents the range of the correlation coefficients (R) displayed.
(TIF)
Data Availability Statement
The data underlying the results presented in the study are available from (https://figshare.com/collections/T_denticola_dentilisin_triggered_TLR2_MyD88_activation_upregulates_a_tissue_destructive_program_involving_MMPs_via_Sp1_in_human_oral_cells/5262821).