Abstract
Background
Early studies on COVID-19 identified unequal patterns in hospitalization and mortality in urban environments for racial and ethnic minorities. These studies were primarily single center observational studies conducted within the first few weeks or months of the pandemic. We sought to examine trends in COVID-19 morbidity, hospitalization, and mortality over time for minority and rural populations, especially during the U.S. fall surge.
Methods
Data were extracted from a statewide cohort of all adult residents in Indiana tested for SARS-CoV-2 infection between March 1 and December 31, 2020, linked to electronic health records. Primary measures were per capita rates of infection, hospitalization, and death. Age adjusted rates were calculated for multiple time periods corresponding to public health mitigation efforts. Comparisons across time within groups were compared using ANOVA.
Results
Morbidity and mortality increased over time with notable differences among sub-populations. Initially, hospitalization rates among racial minorities were 3–4 times higher than whites, and mortality rates among urban residents were twice those of rural residents. By fall 2020, hospitalization and mortality rates in rural areas surpassed those of urban areas, and gaps between black/brown and white populations narrowed. Changes across time among demographic groups was significant for morbidity and hospitalization. Cumulative morbidity and mortality were highest among minority groups and in rural communities.
Conclusions
The synchronicity of disparities in COVID-19 by race and geography suggests that health officials should explicitly measure disparities and adjust mitigation as well as vaccination strategies to protect those sub-populations with greater disease burden.
Introduction
The rapid spread of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has challenged health systems. As of May 5, 2021, more than 155 million individuals were infected, and over 3.2 million individuals have died from COVID-19 globally [1]. In the United States, there are over 32 million cases, 2.1 million hospitalizations, and 580,000 deaths associated with COVID-19. At the end of 2020, the US Centers for Disease Control and Prevention (CDC) reported a COVID-19-associated hospitalization rate of 326.7 per 100,000 population [2].
Data from China, Italy, the US, and other nations suggest that hospitalization and mortality are associated with age as well as gender, in which older and male populations are at higher risk of severe outcomes including death [3–5]. Moreover, early evidence in the US identified unequal patterns in hospitalization and mortality from COVID-19 in dense urban environments with respect to race and ethnicity [6, 7]. Existing studies, however, are limited with respect to scope, data, and temporality. Early evidence largely comes from single, urban center studies or regional data during the first wave [8, 9] of the COVID-19 pandemic. Subsequent waves, or surges, have not been examined. For example, it is unclear whether the same populations were impacted in fall versus the spring 2020. Furthermore, most studies examine patients after inpatient admission. There are limited studies on individuals with COVID-19 in community settings. For example, early data suggested that testing rates per capita were unequal in the US with respect to race and ethnicity [10], yet it is unclear how these rates have changed over time. Early studies from April 2020 also identified unequal infections among racial groups [11, 12]. However, these studies used pre-pandemic, area-level indicators to associate the proportion of Black residents with overall cases reported for the same area. These studies did not use data at the individual level to examine infections, hospitalizations, or deaths.
There further exists limited evidence on individuals in rural communities, with few studies that compare rural patients to those in urban settings. Nearly 1-in-5 Americans live in a rural county [13], which are often labeled as medically underserved areas. Using data from CDC gathered two years before the pandemic, Kaufman et al. [14] estimate rural residents to be at increased risk of hospitalization and death from COVID-19. A similar analysis using data from 2018 by Peters [15] concluded many rural areas are vulnerable to COVID-19 given populations with advanced age and high rates of death due to conditions such as cardiovascular disease. In a single site study in rural Georgia, individuals hospitalized with COVID-19 from March to May 2020 found that, despite a higher burden of comorbid conditions, both critical care and in-hospital mortality was lower than in New York City, as well as China and Italy [16]. A national, population-based study similarly found lower rates of COVID-19 mortality in rural areas compared to urban areas using county-level data through June 12, 2020 [17]. Given limited data from rural communities through Spring 2020, additional analyses are warranted.
No studies to date examine the resurgence of COVID-19 infections within the US during the fall, and little is known about shifts in morbidity and mortality among population groups over time. This study examines the epidemiologic trends in COVID-19 infection, hospitalization, and death rates in Indiana with a focus on health equity. The study examines a large, statewide cohort, including individuals tested by local and state health departments. It further examines care delivered at a wide array of settings, including critical access hospitals and rural county hospitals. Per capita rates, when stratified by age, race, sex, and geography, can shed light on changes in morbidity and mortality over time, as well as within and among sub-populations. Understanding patterns of COVID-19 morbidity and mortality beyond individual health systems and major metropolitan areas can inform national strategies to mitigate the ongoing spread of COVID-19, including vaccination strategies that seek to immunize based primarily on age.
Methods
Setting
The setting for this study is the State of Indiana, which is the 16th largest state in the US with respect to population density and 38th by area. The state has a growing population of 6,732,219 individuals, of which 5,063,133 (75.2%) are adults. Most residents identify as non-Hispanic White (78.4%), followed by Black or African American (9.9%), Hispanic (7.8%), and Asian (2.6%). Approximately 21.7% of residents reside in a rural county.
The State of Indiana reported its first case of COVID-19 on March 6, 2020. Similar to many locations, Indiana implemented public health interventions, including a stay-at-home order, to mitigate spread of COVID-19. On May 1, 2020, following declining rates of hospitalization for COVID-19, the Governor ended the stay-at-home order and initiated a phased re-opening plan [18]. New cases increased while hospitalizations declined into the summer, flattening until a second wave began following Labor Day. The second wave consisted of steady increases in new cases as well as hospitalizations and deaths, all of which climbed through the holidays before leveling off towards the end of the year.
Data and sources
We use data from multiple sources integrated into the Regenstrief Institute COVID-19 Dashboard [19], a data visualization tool developed in response to the pandemic that leverages clinical and administrative health data from the Indiana Network for Patient Care (INPC) [20]. The INPC is one of the nation’s largest health information networks, which includes 38 distinct health systems representing more than 100 hospitals, commercial laboratories, and physician practices across Indiana [21]. The INPC further includes COVID-19 test results from the Indiana Department of Health (IDOH), which receives test results from large commercial labs contracted for pandemic response as well as local health departments which perform strategic testing in communities identified as high risk, such as nursing homes, prisons, and homeless shelters. All testing data, regardless of source, are linked to hospitalization data as well as death records from IDOH. The combined data represent >95% of the 5 million adult residents who interact with the state’s health system.
We extracted data on all adults (age ≥18 years) tested for COVID-19 in the health system or community, as well as those diagnosed with COVID-19 during a clinical encounter. For each individual, we queried the following information from the INPC: COVID-19 test results, age, sex, race, hospitalizations up to 21 days before or after a positive COVID-19 test, and geography associated with home address. Hospitalizations before positive diagnosis were included due to delays in testing, especially at the start of the epidemic. During March and April 2020, most patients infected with the SARS-CoV-2 virus were admitted with COVID-like symptoms before testing positive. All positive cases were identified using RT-PCR tests recorded in medical records or reported to the public health department, including community-based testing efforts statewide by public health authorities. Individuals testing positive were only counted once, during the period of their first positive result. All patient addresses were geocoded using an established method [22] with rurality determined by a classification system developed by Purdue University for Indiana’s geography based on ZIP Code [23].
The fully identified dataset involving linked medical records, death records, and COVID-19 testing results was extracted and managed by the trained data analysts (LRR and ARR) as the Regenstrief Data Services team functions as the honest data broker for the INPC. These data were fully de-identified prior to release to the study team for analysis. The de-identified data used for analysis are available for reproduction and secondary use through IUPUI DataWorks, a repository for research data for faculty at Indiana University-Purdue University Indianapolis [24]. The dataset DOI is https://doi.org/10.7912/D2/26.
Data analysis
We used epidemiological methods to calculate descriptive statistics, including rates per 100,000 population, also known as per capita rates. These rates provide an objective method for comparing population characteristics when communities or groups vary in size. Denominator data for calculating per capita rates came from 2018 U.S. Census estimates. All rates were age-adjusted using American Community Survey estimates.
Statistics were calculated overall and for multiple time periods corresponding to the state’s initial lockdown and subsequent re-opening plan. We examined data from the start of the Indiana epidemic (March 6, 2020) thru the end of the stay-at-home order (April 30, 2020), referred to as Phase 1. Following the stay-at-home order, Indiana initiated a staged reopening. Each subsequent stage reopened additional sectors of the economy or expanded capacity in a given sector. Full details of each stage can be found on the Governor’s Back on Track website [18]. The reopening occurred from May 1, 2020 through September 7, 2020 (Labor Day), when the only remaining restrictions included a statewide mask ordinance and a restriction on gatherings larger than 250 people. This is referred to as Phase 2. Finally, we examined data from September 8, 2020 through December 31, 2020, referred to as Phase 3.
We further investigated differences across study Phases and per-capita rates by each of the demographic variables using analysis of variance (ANOVA). All statistical comparisons were two-sided and a p-value <0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Institutional review board approval for the study was obtained from Indiana University. The IRB further waived the requirement for informed consent as the research involved previously collected medical record data from individuals tested for COVID-19 by public health or health care providers.
Results
Through December 31, 2020, a total of 1,833,218 unique, adult Indiana residents were tested for COVID-19, which accounts for 36.2% of the statewide adult population. Of those tested, 354,539 (19.3%) unique individuals tested positive for COVID-19 infection. Among those infected, 31,352 (8.8%) were hospitalized. A total of 8,104 (0.2% of infected individuals) died either during their hospital course or at home following COVID-19 infection.
Overall COVID-19 infection and burden
Characteristics, as well as morbidity and mortality, of individuals tested for COVID-19 in Indiana are summarized in Table 1. More women than men per capita were tested and positive for COVID-19, yet men had higher hospitalizations and mortality per capita compared to women. With respect to age, testing and morbidity were highest in the younger (0–29) and older (80+) groups. Hospitalization and mortality per capita increased with age, with older (70+) populations possessing significantly higher hospitalizations and deaths per capita. With respect to race, morbidity, hospitalization, and mortality rates per capita were higher among racial minority groups, especially Native Hawaiian/Pacific Islanders, American Indian/Native Alaskans, and African Americans respectively. Individuals who did not report their race during testing or hospitalization also possessed high rates of morbidity and mortality. With respect to geography, urban residents were tested more frequently. However, per capita morbidity, hospitalization, and mortality were highest among rural populations.
Table 1. Characteristics and rates for Indiana residents tested for, infected with, hospitalized with, and death following infection from COVID-19 through December 31, 2020; State of Indiana.
| Characteristics | Individuals Tested for COVID-19 | COVID-19 Cases | COVID-19 Hospitalizations | COVID-19 Deaths | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | rate per capita* | N | % | rate per capita* | N | % | rate per capita* | N | % | rate per capita* | |
| Total | 1833218 | 36207.2 | 354539 | 7002.4 | 31352 | 619.2 | 8104 | 160.1 | ||||
| Gender | ||||||||||||
| Female | 1031547 | 56.3% | 39709.7 | 190632 | 53.8% | 7338.4 | 15890 | 50.7% | 611.7 | 4008 | 49.5% | 154.3 |
| Male | 790687 | 43.1% | 32071.2 | 162363 | 45.8% | 6585.6 | 15459 | 49.3% | 627.0 | 4073 | 50.3% | 165.2 |
| Age Category | ||||||||||||
| 18–19 | 73702 | 4.0% | 39707.1 | 14169 | 4.0% | 7633.6 | 130 | 0.4% | 70.0 | 2 | 0.0% | 1.1 |
| 20–29 | 357306 | 19.5% | 39038.2 | 70064 | 19.8% | 7655.0 | 1472 | 4.7% | 160.8 | 38 | 0.5% | 4.2 |
| 30–39 | 312253 | 17.0% | 37227.7 | 58867 | 16.6% | 7018.3 | 1950 | 6.2% | 232.5 | 75 | 0.9% | 8.9 |
| 40–49 | 284677 | 15.5% | 34644.3 | 59479 | 16.8% | 7238.4 | 2771 | 8.8% | 337.2 | 154 | 1.9% | 18.7 |
| 50–59 | 294838 | 16.1% | 32976.5 | 58540 | 16.5% | 6547.5 | 4731 | 15.1% | 529.1 | 408 | 5.0% | 45.6 |
| 60–69 | 258639 | 14.1% | 34580.4 | 46261 | 13.0% | 6185.2 | 6756 | 21.5% | 903.3 | 1244 | 15.4% | 166.3 |
| 70–79 | 157831 | 8.6% | 38123.8 | 27605 | 7.8% | 6667.9 | 7241 | 23.1% | 1749.1 | 2038 | 25.1% | 492.3 |
| 80+ | 93972 | 5.1% | 38238.7 | 19554 | 5.5% | 7956.8 | 6301 | 20.1% | 2564.0 | 4145 | 51.1% | 1686.7 |
| Race | ||||||||||||
| White | 1420211 | 77.5% | 32772.9 | 272622 | 76.9% | 6291.1 | 24140 | 77.0% | 557.1 | 6538 | 80.7% | 150.9 |
| African American | 164730 | 9.0% | 37075.1 | 31596 | 8.9% | 7111.2 | 4922 | 15.7% | 1107.8 | 952 | 11.7% | 214.3 |
| Asian | 29043 | 1.6% | 23872.1 | 5638 | 1.6% | 4634.2 | 353 | 1.1% | 290.2 | 54 | 0.7% | 44.4 |
| American Indian / Native Alaskan | 3961 | 0.2% | 35423.0 | 1193 | 0.3% | 10668.9 | 69 | 0.2% | 617.1 | 4 | 0.0% | 35.8 |
| Native Hawaiian / Pacific Islander | 3057 | 0.2% | 142783.7 | 783 | 0.2% | 36571.7 | 79 | 0.3% | 3689.9 | 19 | 0.2% | 887.4 |
| Other or Unknown | 212216 | 11.6% | 194470.6 | 42707 | 12.0% | 39135.9 | 1789 | 5.7% | 1639.4 | 537 | 6.6% | 492.1 |
| Geography | ||||||||||||
| Rural | 379567 | 20.7% | 34585.2 | 82276 | 23.2% | 7496.8 | 6962 | 22.2% | 634.4 | 1890 | 23.3% | 172.2 |
| Urban | 1453651 | 79.3% | 36656.1 | 272263 | 76.8% | 6865.5 | 24390 | 77.8% | 615.0 | 6214 | 76.7% | 156.7 |
*Rate per capita adjusted for age
Comparison of infection, hospitalization, and death over time
Table 2 summarizes per capita infections, hospitalizations, and deaths during the three phases of the COVID-19 epidemic in Indiana through the end of 2020. Infections, hospitalizations, and deaths per capita all increased over time. Hospitalization and death increased with age and increased across phases for all groups. There are notable differences among sub-populations.
Table 2. Comparison of per capita rates for COVID-19 infection, hospitalization and death for residents across three phases of the epidemic; State of Indiana.
| Characteristics | COVID-19 Cases | COVID-19 Related Hospitalization | COVID-19 Related Deaths | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase 1 (rate per capita) | Phase 2 (rate per capita) | Phase 3 (rate per capita) | Phase 1 (rate per capita) | Phase 2 (rate per capita) | Phase 3 (rate per capita) | Phase 1 (rate per capita) | Phase 2 (rate per capita) | Phase 3 (rate per capita) | |
| Total | 402.8 | 1375.2 | 5224.4 | 84.6 | 133.3 | 402.5 | 41.5 | 41.1 | 77.5 |
| Gender | |||||||||
| Female | 419.4 | 1421.9 | 5497.2 | 80.3 | 134.5 | 398.2 | 39.8 | 41.9 | 72.6 |
| Male | 377.3 | 1308.1 | 4900.3 | 89.1 | 132.1 | 406.8 | 42.5 | 40.1 | 82.6 |
| p = 0.0035* | p = 0.0004* | p = 0.02* | |||||||
| Age Category | |||||||||
| 18–19 | 83.5 | 2003.1 | 5547.0 | 5.4 | 23.2 | 42.0 | 0.0 | 0.0 | 0.2 |
| 20–29 | 265.2 | 1858.7 | 5531.4 | 15.6 | 46.5 | 98.9 | 1.1 | 1.5 | 1.5 |
| 30–39 | 370.3 | 1377.3 | 5270.7 | 35.2 | 62.1 | 135.9 | 2.3 | 2.7 | 3.9 |
| 40–49 | 426.8 | 1379.4 | 5432.3 | 58.2 | 89.9 | 190.1 | 5.1 | 5.7 | 7.9 |
| 50–59 | 416.2 | 1144.5 | 4986.8 | 88.7 | 119.0 | 322.0 | 13.3 | 12.0 | 20.4 |
| 60–69 | 396.7 | 1016.1 | 4772.5 | 126.1 | 181.2 | 598.2 | 43.7 | 39.8 | 82.8 |
| 70–79 | 480.7 | 1084.1 | 5103.4 | 199.5 | 330.7 | 1221.0 | 116.9 | 125.4 | 250.0 |
| 80+ | 1026.6 | 1501.5 | 5428.7 | 323.5 | 502.1 | 1743.2 | 446.8 | 436.6 | 803.3 |
| p < .0001* | p = 0.016* | p = 0.14* | |||||||
| Race | |||||||||
| White | 287.2 | 1068.1 | 4935.9 | 62.4 | 109.2 | 386.5 | 35.3 | 36.5 | 79.1 |
| African American | 926.4 | 1816.3 | 4368.5 | 285.8 | 287.6 | 536.1 | 89.1 | 63.0 | 62.1 |
| Asian | 433.2 | 922.2 | 3278.8 | 51.0 | 87.1 | 152.1 | 14.8 | 10.7 | 18.9 |
| American Indian / Native Alaskan | 3559.3 | 2012.2 | 5097.5 | 232.5 | 152.0 | 241.5 | 8.9 | 0.0 | 26.8 |
| Native Hawaiian / Pacific Islander | 2942.6 | 10462.4 | 23166.7 | 747.3 | 747.3 | 2195.2 | 186.8 | 280.2 | 420.4 |
| Other or Unknown | 2607.1 | 12557.2 | 23971.6 | 188.8 | 549.8 | 904.5 | 137.5 | 184.2 | 170.4 |
| p = 0.02* | p = 0.04* | p = 0.22* | |||||||
| Geography | |||||||||
| Rural | 355.9 | 1232.6 | 5908.2 | 51.6 | 124.5 | 459.7 | 24.7 | 43.7 | 103.8 |
| Urban | 415.8 | 1414.6 | 5035.2 | 93.7 | 135.8 | 386.6 | 46.1 | 40.4 | 70.2 |
| p = 0.01* | p = 0.02* | p = 0.19* | |||||||
Note: p values for demographic groups and for the three phases were calculated using ANOVA.
*p values indicate comparisons over the three phases.
a Phase 1 (March–April, 2020)
b Phase 2 (May 1, 2020 –September 7, 2020)
c Phase 3 (September 8, 2020 –December 31, 2020)
COVID-19 infection rates (morbidity)
Infection rates increased for both men and women over the study period. The rates were higher among females, which increased from 419.4 per 100,00 (phase 1) to 5497.2 per 100,000 (phase 3). At the start of the pandemic, young adults (18 to 19 years) had the lowest infection rates at 83.5 per 100,000 (phase 1), which increased later to 5547.0 per 100,000 (phase 3). The oldest adults (≥80 years) possessed the highest infection rates across the three phases, increasing from 1026.6 per 100,000 (phase 1) to 5428.7 per 100,000 (phase 3). With respect to race, rates during Phase 1 were lowest among Whites (287.2 per 100,000), while African American (926.4 per 100,000) and American Indian/Native Alaskans (3559.3 per 100,000) had higher rates. Infection rates significantly increased by phase for all demographic characteristics like sex (p = 0.0035), age-group (p < .0001), race (p = 0.02), and geography (p = 0.01) during the study period.
COVID-19 hospitalization rates
Hospitalization rates for men increased from 89.1 per 100,000 (phase 1) to 406.8 per 100,000 (phase 3), and they were higher among men in phase 1 and phase 3. Hospitalization rates were highest among the oldest adults (≥80 years) across the three phases with a low of 323.5 per 100,000 (phase 1) to 1743.2 per 100,000 (phase 3). Rates were lowest among young adults (18 to 19 years) 5.4 per 100,00 (phase 1), which increased to 42.0 per 100,000 (phase 3). Rates were highest for Native Hawaiian/Pacific Islanders (747.3 per 100,000; phase 1 and 2) and among African-Americans (285.8 per 100,000; phase 1), which increased to 2195.2 per 100,000 (phase 3) and 536.1 per 100,000 (phase 3), respectively. For rural and urban populations, hospitalization rates were almost 2 times higher in the urban population 93.7 per 100,000 during Phase 1. However, hospitalization rates increased in the rural population from 51.6 per 100,000 (phase 1) to 459.7 per 100,000 population (phase 3). Hospitalization rates were significantly different across the phases by sex (p = 0.0004), age-group (p = 0.016), race (p = 0.04), and geography (p = 0.02).
COVID-19 mortality rates
Mortality rates increased for both men and women during the study period. For men, mortality increased from 42.5 per 100,000 (phase 1) to 82.6 per 100,000 (phase 3), while for women rates increased from 39.8 per 100,000 (phase 1) to 72.6 per 100,000 (phase 3). Mortality progressively increased among adults 60 years and older, with the highest rates observed among the oldest adults (≥ 80 years), increasing from 446.8 per 100,000 (phase 1) to 803.3 per 100,000 during Phase 3. Mortality was highest among Native Hawaiian/Pacific Islanders, which increased from 186.8 per 100,000 (phase 1) to 420.4 per 100,000 (phase 3). Mortality among African-Americans progressively reduced from 89.1 per 100,000 (phase 1) to 62.1 per 100,000 (phase 3), while among Whites it increased from 35.3 per 100,000 (phase 1) to 79.1 per 100,000 (phase 3). Mortality remained low in the rural population in the early phase, 24.7 per 100,000 (phase 1), but increased to 103.8 per 100,000 in Phase 3. In urban areas, mortality increased from 46.1 per 100,000 population (phase 1) to 70.2 per 100,000 (phase 3). Mortality rates across the demographics by phase differed only for sex (p = 0.02), and rates were not statistically significant for other demographic characteristics.
Fig 1 summarizes per capita rates for hospitalizations and mortality, stratified by race, in each phase. Hospitalizations were higher for African Americans in all phases. Mortality was higher for African Americans in the first two phases. Morbidity and mortality were highest in every phase among those who either reported their race as Other or did not disclose their race at the time of testing or treatment.
Fig 1. Per capita rates for hospitalizations and deaths due to COVID-19 among adults in Indiana, stratified by race, during three distinct time periods between March 1 and December 31, 2020.
State of Indiana.
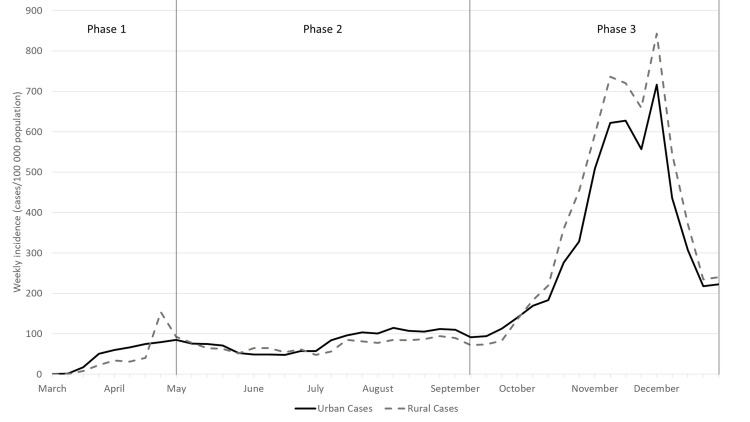
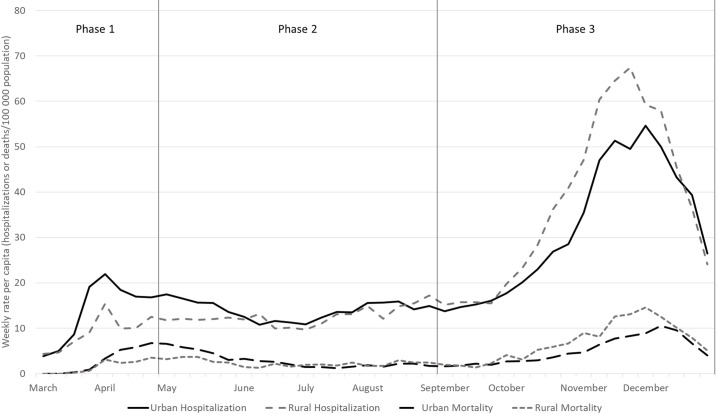
Morbidity and hospitalizations were higher for urban populations during the first two phases. Deaths were higher among urban populations only in Phase 1. Over time, per capita morbidity (Fig 2) and negative outcomes, hospitalization and mortality (Fig 3), shifted to rural populations. Morbidity shifted in Phase 3 following the conclusion of the state’s re-opening plan. Higher per capita hospitalization and mortality among rural populations began towards the end of Phase 2 then accelerated in Phase 3.
Fig 2. Weekly COVID-19 incidence, defined as cases per 100,000 population, among adults in Indiana, stratified by urban versus rural county of residence between March 1 and December 31, 2020.
State of Indiana.
Fig 3. Weekly rates per capita for hospitalizations and deaths due to COVID-19 among adults in Indiana, stratified by urban versus rural county of residence between March 1 and December 31, 2020.
Discussion
Among a statewide cohort of individuals tested for COVID-19, we examined epidemiological trends in testing, infection, hospitalization, and death rates across three time periods corresponding to mitigation efforts by public health authorities. Infections due to the SARS-CoV-2 virus increased over time, yet the impact of COVID-19 was not even across sub-populations. Following the initial lockdown in the spring, the gap between White and African American morbidity and mortality narrowed, although burden remained significantly higher among African American populations. As summer turned into fall, burden among rural communities increased and surpassed urban communities through the end of the year. These trends reveal a synchronicity of disparities, or simultaneous set of inequities in outcomes among distinct populations impacted by COVID-19. In other words, Black and Brown populations as well as Rural populations experienced unequal burden and hospitalization due to COVID-19 at the same time. Synchronicities in disparities have implications for continued mitigation of disease spread as well as vaccination strategies.
There are many similarities in the Indiana trends with prior studies as well as national trends. Rates per capita for hospitalization and death increase with age [4, 6, 25–27]. Furthermore, hospitalization and death per capita was greater among men versus women [26, 27], even though women experienced greater morbidity. Moreover, burden of COVID-19 within the African American community overall was much greater than its proportional composition of the state’s population [7, 12]. Per capita hospitalization among African Americans grew and remained highest among all sub-populations throughout the pandemic. Burden among American Indian/Native Alaskan and Native Hawaiian/Pacific Islander populations were also among the highest overall and during most time periods, a trend observed nationally [28].
While data in this study share much in common with prior studies, there are several unique characteristics that distinguish our work. First, the study uses a statewide, community dataset of individuals tested for COVID-19 linked to electronic medical records. Testing data includes results from hospital-based, commercial, and public health laboratories creating a comprehensive source measuring testing per capita. Furthermore, our data source contains statewide hospitalization and death data from 38 distinct health systems. Second, the study measures burden of disease and outcomes during the fall surge, something few studies to date have reported. Stratification by phase is also unique, allowing comparison of burden and outcomes over time. These methods would not be possible without a robust electronic data infrastructure in Indiana aided by a 16+ year health information exchange [29] that partnered with the state health department, county health departments, and health care systems in response to the COVID-19 pandemic [20]. This multi-sector approach aligns with the vision set forth by the Public Health 3.0 framework described by DeSalvo et al. [30, 31].
Although prior studies document racial disparities, especially during the initial phase of the pandemic, this study presents data on racial disparities in COVID-19 morbidity, hospitalization, and mortality over time. Rates, adjusted for population size, clearly show significant burden on African American populations during each phase of the pandemic. Although deaths per capita were lower than other racial groups in the fall surge, the cumulative mortality is 50% higher than mortality among White populations. Moreover, burden among Native Hawaiian/Pacific Islander populations, despite accounting for a small percentage of the population, is nearly 10 times that of Whites. Among those who did not disclose or reported their race as ‘Other,’ hospitalization and death rates are 2–4 times higher than Whites. It is not unreasonable to assume that many Hispanics may be in that group as they may not wish to disclose demographic details and this minority group has been shown to have higher rates of hospitalizations and mortality in national studies [32]. Therefore, we conclude that while racial disparities narrowed later in the pandemic, especially as burden shifted from urban to rural communities, cumulative burden on racial minorities from COVID-19 are severe. This burden exacerbates existing health disparities, necessitating action as the nation attempts to both mitigate further disease spread and protect vulnerable populations through vaccination.
Another distinguishing feature of this analysis is a focus on rural communities. Earlier studies [14, 15, 17] only provided information on risk or observed an initial low impact in rural communities. A brief report on COVID-19 incidence thru October 2020 from the U.S. Centers for Disease Control and Prevention [33] revealed that morbidity shifted nationally from urban to rural areas beginning in late summer. These trends are mirrored in this study. Yet this study further provides evidence on shifting hospitalizations and mortality, which both surpassed urban rates per capita. However, these differences were not statistically significant.
Rural morbidity is of great concern, however, as many rural areas are medically underserved. Hospitals in rural areas may possess few ICU beds, and they may lack the staff necessary to handle an influx of COVID-19 cases [34]. During the fall surge, several rural hospitals in Indiana reached capacity, necessitating transfers to urban centers, which can be more than 2 hours away from the resident’s home. This placed additional burden on urban hospitals already managing increased workload and burden from local residents infected with COVID-19. The situation further caused a response from public health in which elective procedures were reduced by order of the Governor, placing financial strain on both rural and urban facilities. More attention is needed on the impact of COVID-19 in rural areas, combined with reasonable policies to support rural mitigation strategies and equitable distribution of vaccines to rural populations.
Limitations
This observational study has several limitations worth noting. Observational clinical data (e.g., “real-world evidence”), from which much of our findings are derived, is known to have potential biases [35]. First, a significant number of race classifications were reported as Other or Unknown. Similarly, the dataset could not identify ethnicity, as these data are also missing for many patients. Medical records as well as other health information systems, must improve the capture rates for race and ethnicity to enable large scale measurement of health disparities so public health can work with health systems to ensure health for all persons [36, 37]. Second, these data represent hospitalization and mortality among individuals from one state. The patterns observed in Indiana may not generalize to all geographic regions of the U.S. or other countries. These patterns are more applicable to states or regions where the population has similar demographic or rural characteristics.
Public health implications
This study offers several implications for public health in the wake of the COVID-19 pandemic. First, trends demonstrate a flattening of the curve following the initial stay-at-home order from public health authorities. As the state re-opened, morbidity and mortality increased during subsequent phases. This suggests aggressive mitigation for a longer period of time may be necessary for stronger disease control and prevention. Moreover, sub-population differences highlight the need for more nuanced mitigation policies, such as data-driven approaches that target high-risk groups, that can evolve over time.
As public health attempts to mitigate disease spread going forward, additional attention should be paid to racial minority and rural populations. Testing increased per capita among racial minority groups in Indiana, enabling better detection of morbidity. Equitable testing was not sufficient for stemming hospitalization due to COVID-19. Mortality decreased among minority groups in the latter phases, yet this might be attributable to improved clinical management rather than contact tracing and isolation which our data did not measure. With respect to rural populations, morbidity, hospitalization, and mortality steadily increased over time, suggesting perhaps rural health departments struggled with mitigation strategies or rural populations ignored mask ordinances, restrictions on social gatherings, and/or other public health interventions. Anecdotally, we observed complaints from several rural county health officers that local authorities would not enforce ordinances and that residents overtly refused to comply with many policies. More research is necessary to confirm these observations and support the development of more robust mitigation policies.
Strategies to vaccinate against COVID-19 need to explicitly address racial disparities. Poorer health outcomes among racial minorities is often attributable to lack of health care access [38], including preventative medicine and vaccination. In a majority White state, we achieved equity in testing. This means we can achieve equity in vaccination. However, current policies focus on age and comorbid conditions to drive decisions about which populations should receive vaccines first. While age places individuals at higher risk of hospitalization and death, this study demonstrates that racial minorities and rural populations also should be prioritized given their higher morbidity and mortality. If health departments are serious about addressing social determinants and racial disparities, they must factor these phenomena into vaccination plans.
Acknowledgments
The authors thank Jack VanSchaik and Connor McAndrews, MS, of the Regenstrief Institute for their assistance with the data management for this analysis. We further acknowledge the broader COVID-19 Dashboard Team, part of the Regenstrief Data Services group, for their daily efforts to gather, link, and publish data on COVID-related infections, hospitalizations, and deaths for communities across Indiana. Data management during the pandemic has been challenging, and they have risen to every challenge. The authors further thank Nir Menachemi, PhD, of the IU Fairbanks School of Public Health for his feedback on an early draft of this manuscript.
Data Availability
All data used in this study are available from the IUPUI DataWorks repository (https://doi.org/10.7912/D2/26).
Funding Statement
BED receives funding from the U.S. Agency for Healthcare Research and Quality (R21HS025502) to study health information exchange. The Regenstrief Institute as well as the IU Fairbanks School of Public Health received funds from the State of Indiana and Marion County Public Health Department to support COVID-19 response and mitigation, including disease surveillance and outcomes measurement. The funders did not have influence over the findings or this publication.
References
- 1.Center for Systems Science and Engineering. COVID-19 Dashboard Baltimore: Johns Hopkins University; 2020 [cited 2020 Jul 19]. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- 2.Centers for Disease Control and Prevention US. COVIDView: A Weekly Surveillance Summary of U.S. COVID-19 Activity—Report for Week 52 Atlanta, GA: Department of Health and Human Services, U.S.; 2020. [updated Dec 26; cited 2021 Jan 5]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-04-2021.pdf. [Google Scholar]
- 3.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clinical Infectious Diseases. 2020. doi: 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner FS, McCulloch DJ, Atluri V, Blain M, McGuffin SA, Nalla AK, et al. Clinical Features and Outcomes of 105 Hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis. 2020. Epub 2020/05/24. doi: 10.1093/cid/ciaa632 ; PubMed Central PMCID: PMC7314181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. Jama. 2020;323(16):1574–81. Epub 2020/04/07. doi: 10.1001/jama.2020.5394 ; PubMed Central PMCID: PMC7136855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–9. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19—Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545–50. Epub 2020/05/08. doi: 10.15585/mmwr.mm6918e1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doidge JC, Gould DW, Ferrando-Vivas P, Mouncey PR, Thomas K, Shankar-Hari M, et al. Trends in Intensive Care for Patients with COVID-19 in England, Wales and Northern Ireland. Am J Respir Crit Care Med. 2020. Epub 2020/12/12. doi: 10.1164/rccm.202008-3212OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Halem K, Bruyndonckx R, van der Hilst J, Cox J, Driesen P, Opsomer M, et al. Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: a retrospective cohort study. BMC Infect Dis. 2020;20(1):897. Epub 2020/11/29. doi: 10.1186/s12879-020-05605-3 ; PubMed Central PMCID: PMC7691970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan TQ, Kullar R, Swartz TH, Mathew TA, Piggott DA, Berthaud V. Location Matters: Geographic Disparities and Impact of Coronavirus Disease 2019. J Infect Dis. 2020;222(12):1951–4. Epub 2020/09/18. doi: 10.1093/infdis/jiaa583 ; PubMed Central PMCID: PMC7543383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMaggio C, Klein M, Berry C, Frangos S. Black/African American Communities are at highest risk of COVID-19: spatial modeling of New York City ZIP Code-level testing results. Ann Epidemiol. 2020;51:7–13. Epub 2020/08/23. doi: 10.1016/j.annepidem.2020.08.012 ; PubMed Central PMCID: PMC7438213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. Epub 2020/05/19. doi: 10.1016/j.annepidem.2020.05.003 ; PubMed Central PMCID: PMC7224670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davoodi NM, Healy M, Goldberg EM. Rural America’s Hospitals are Not Prepared to Protect Older Adults From a Surge in COVID-19 Cases. Gerontol Geriatr Med. 2020;6:2333721420936168. Epub 2020/07/21. doi: 10.1177/2333721420936168 ; PubMed Central PMCID: PMC7343358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman BG, Whitaker R, Pink G, Holmes GM. Half of Rural Residents at High Risk of Serious Illness Due to COVID-19, Creating Stress on Rural Hospitals. J Rural Health. 2020;36(4):584–90. Epub 2020/07/01. doi: 10.1111/jrh.12481 ; PubMed Central PMCID: PMC7361543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters DJ. Community Susceptibility and Resiliency to COVID-19 Across the Rural-Urban Continuum in the United States. The Journal of rural health: official journal of the American Rural Health Association and the National Rural Health Care Association. 2020;36(3):446–56. Epub 2020/06/16. doi: 10.1111/jrh.12477 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah P, Owens J, Franklin J, Mehta A, Heymann W, Sewell W, et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52(7):354–60. Epub 2020/07/06. doi: 10.1080/07853890.2020.1791356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim SA, Chen HF. Deaths From COVID-19 in Rural, Micropolitan, and Metropolitan Areas: A County-Level Comparison. J Rural Health. 2021;37(1):124–32. Epub 2020/11/07. doi: 10.1111/jrh.12533 . [DOI] [PubMed] [Google Scholar]
- 18.Holcomb E. Back on Track Indiana: Governor Holcomb’s Roadmap to Safely Reopen Indiana Indianapolis, IN: State of Indiana; 2020 [cited 2020 Jul 4]. Available from: https://backontrack.in.gov/.
- 19.Regenstrief Institute. Regenstrief COVID-19 Dashboard Indianapolis: Regenstrief Institute, Inc.; 2020 [cited 2020 Jun 10]. Available from: https://www.regenstrief.org/covid-dashboard/.
- 20.Dixon BE, Grannis SJ, McAndrews C, Broyles AA, Mikels-Carrasco W, Wiensch A, et al. Leveraging Data Visualization and a Statewide Health Information Exchange to Support COVID-19 Surveillance and Response: Application of Public Health Informatics. J Am Med Inform Assoc. 2021;(In Press). Epub 2021/01/23. doi: 10.1093/jamia/ocab004 ; PubMed Central PMCID: PMC7928924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overhage JM. The Indiana Health Information Exchange. In: Dixon BE, editor. Health Information Exchange: Navigating and Managing a Network of Health Information Systems. 1 ed. Waltham, MA: Academic Press; 2016. p. 267–79. [Google Scholar]
- 22.Comer KF, Grannis S, Dixon BE, Bodenhamer DJ, Wiehe SE. Incorporating geospatial capacity within clinical data systems to address social determinants of health. Public Health Rep. 2011;126 Suppl 3:54–61. Epub 2011/08/13. doi: 10.1177/00333549111260S310 ; PubMed Central PMCID: PMC3150130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purdue Extension. Geographic Classifications West Lafayette, Indiana: Purdue University; 2019 [cited 2020 Aug 22]. Available from: https://pcrd.purdue.edu/ruralindianastats/geographic-classifications.php.
- 24.Dixon BE, Grannis SJ, Lembcke LR, Roberts AR, Embi PJ. Indiana COVID-19 Testing, Morbidity, and Mortality Population Trends Study Indianapolis, Indiana, USA: IUPUI University Library; 2021. [cited 2021 May 21]. Version 1:[Available from: http://hdl.handle.net/11243/40. [Google Scholar]
- 25.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80(6):656–65. Epub 2020/04/14. doi: 10.1016/j.jinf.2020.03.041 ; PubMed Central PMCID: PMC7151416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA internal medicine. 2020;180(7):1–11. Epub 2020/03/14. doi: 10.1001/jamainternmed.2020.0994 ; PubMed Central PMCID: PMC7070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. Epub 2020/02/28. doi: 10.1016/S2213-2600(20)30079-5 ; PubMed Central PMCID: PMC7102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Center for Immunization and Respiratory Diseases, Division of Viral Diseases. Health Equity Considerations and Racial and Ethnic Minority Groups Atlanta: Centers for Disease Control and Prevention,; 2020 [updated 24 Jul; cited 2020 Aug 29]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html#fn1.
- 29.Dixon BE. What is Health Information Exchange? In: Dixon BE, editor. Health Information Exchange: Navigating and Managing a Network of Health Information Systems. Waltham: Academic Press; 2016. p. 3–20. [Google Scholar]
- 30.DeSalvo KB, Wang YC, Harris A, Auerbach J, Koo D, O’Carroll P. Public Health 3.0: A Call to Action for Public Health to Meet the Challenges of the 21st Century. Preventing chronic disease. 2017;14:E78. doi: 10.5888/pcd14.170017 PMC5590510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeSalvo KB, O’Carroll PW, Koo D, Auerbach JM, Monroe JA. Public Health 3.0: Time for an Upgrade. Am J Public Health. 2016;106(4):621–2. Epub 2016/03/10. doi: 10.2105/AJPH.2016.303063 ; PubMed Central PMCID: PMC4816012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romano SD, Blackstock AJ, Taylor EV, El Burai Felix S, Adjei S, Singleton CM, et al. Trends in Racial and Ethnic Disparities in COVID-19 Hospitalizations, by Region—United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(15):560–5. Epub 2021/04/16. doi: 10.15585/mmwr.mm7015e2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.COVID-19 Stats: COVID-19 Incidence,* by Urban-Rural Classification(†)—United States, January 22-October 31, 2020(§). MMWR Morb Mortal Wkly Rep. 2020;69(46):1753. Epub 2020/11/20. doi: 10.15585/mmwr.mm6946a6 ; PubMed Central PMCID: PMC7676636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett C. Will COVID-19 Trigger More Rural Hospital Closures? Indianapolis: Side Effects Public Media; 2020. [updated Oct 12; cited 2020 Oct 12]. Available from: https://indianapublicmedia.org/news/will-covid-19-trigger-more-rural-hospital-closures.php. [Google Scholar]
- 35.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther. 2018;35(11):1763–74. Epub 2018/10/26. doi: 10.1007/s12325-018-0805-y ; PubMed Central PMCID: PMC6223979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasnain-Wynia R, Van Dyke K, Youdelman M, Krautkramer C, Ivey SL, Gilchick R, et al. Barriers to collecting patient race, ethnicity, and primary language data in physician practices: an exploratory study. J Natl Med Assoc. 2010;102(9):769–75. Epub 2010/10/07. doi: 10.1016/s0027-9684(15)30673-8 . [DOI] [PubMed] [Google Scholar]
- 37.Bergdall A, Asche S, Schneider N, Kerby T, Margolis K, Sperl-Hillen J, et al. CB3-01: Comparison of Ethnicity and Race Categorization in Electronic Medical Records and by Self-report. Clinical Medicine & Research. 2012;10(3):172. doi: 10.3121/cmr.2012.1100.cb3-01 [DOI] [Google Scholar]
- 38.Alcendor DJ. Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US. J Clin Med. 2020;9(8). Epub 2020/08/06. doi: 10.3390/jcm9082442 ; PubMed Central PMCID: PMC7466083. [DOI] [PMC free article] [PubMed] [Google Scholar]