Abstract
Dyspnea in the postpartum period can be a symptom of a wide range of causes spanning normal pregnancy to life-threatening pathology. We describe a case of acute postpartum heart failure with preserved systolic function in the absence of pre-eclampsia or prior cardiovascular disease. (Level of Difficulty: Beginner.)
Key Words: cardiomyopathy, diastolic heart failure, pregnancy
Abbreviations and Acronyms: BNP, B-type natriuretic peptide; LV, left ventricular; PPCM, peripartum cardiomyopathy
Graphical abstract
Dyspnea in the postpartum period can be a symptom of a wide range of causes spanning normal pregnancy to life-threatening pathology…
History of Presentation
A 37-year-old pregnant woman was admitted at 39 weeks 2 days for elective induction of labor during her first pregnancy, which was otherwise uncomplicated. Because of arrest of dilation, she underwent uncomplicated cesarean delivery with an estimated 1 liter blood loss, and she received 2 liters intravenous crystalloid.
Learning Objectives
-
•
To identify acute postpartum heart failure with preserved systolic function and the differential diagnoses.
-
•
To review the symptoms, examination, laboratory values, and imaging parameters, including left atrial strain imaging, which may distinguish peripartum heart failure from the normal spectrum of findings in pregnancy.
She was readmitted 5 days after delivery for worsening dyspnea with minimal exertion, lower extremity edema, orthopnea, and palpitations. She denied having these symptoms before or during her pregnancy. Her physical examination was notable for blood pressure of 119/76 mm Hg, conversational dyspnea, bilateral rales, lower extremity edema, and jugular venous distention. B-type natriuretic peptide (BNP) was 324 pg/ml, and other laboratory tests did not show evidence of myocardial injury, renal failure, transaminase elevation, thrombocytopenia, coagulopathy, or proteinuria. An electrocardiogram demonstrated normal sinus rhythm. Computed tomography with pulmonary embolism protocol revealed pulmonary edema and small bilateral pleural effusions.
Past Medical History
Before her pregnancy, she had stage II obesity (34.2 kg/m2) and uterine fibroids but had no other prior medical history.
Differential Diagnosis
There is a broad differential for dyspnea and edema within the first month postpartum including acute decompensated heart failure, specifically peripartum cardiomyopathy (PPCM); spontaneous coronary artery dissection; pulmonary embolism; amniotic fluid embolism; pulmonary hypertension; pre-eclampsia; HELLP syndrome; and renal failure.
Investigations
A transthoracic echocardiogram demonstrated normal left ventricular (LV) size and systolic function with ejection fraction of 65% and normal LV global longitudinal strain (Table 1). There was no LV hypertrophy, but there was biatrial enlargement (left atrial volume index = 52 ml/m2) and moderate tricuspid regurgitation with an estimated right ventricular systolic pressure of 49 mm Hg. Because of concern for pulmonary hypertension, she underwent right heart catheterization, which demonstrated biventricular pressure overload with normal cardiac output and pulmonary vascular resistance (right atrial pressure = 15 mm Hg, pulmonary artery pressure = 38/22 mm Hg, mean pulmonary artery pressure = 30 mm Hg, pulmonary capillary wedge pressure = 22 mm Hg, Fick cardiac output = 6.4 l/min, and thermodilution cardiac output = 5.6 l/min).
Table 1.
Echocardiographic Parameters at Presentation and Follow-Up
| At Presentation | At 2-Week Follow-Up | |
|---|---|---|
| Left atrial volume index | 52 ml/m2 | 32 ml/m2 |
| Estimated right ventricular systolic pressure | 49 mm Hg | 25 mm Hg |
| Septal E’ | 8.6 cm/s | 7.9 cm/s |
| Lateral E’ | 11.9 cm/s | 12.5 cm/s |
| Septal E/e’ | NA | 7.9 |
| Lateral E/e’ | NA | 5 |
| E/A | NA | 1.1 |
| Left atrial reservoir strain | −30% | −39% |
| Left atrial contraction strain | −6% | −10% |
| Left atrial conduit strain | −25% | −30% |
| Left ventricular internal diastolic dimension | 47 mm | 47 mm |
| Intraventricular septum diastolic dimension | 8 mm | 8 mm |
| Posterior wall diastolic dimension | 8 mm | 8 mm |
| Relative wall thickness | 0.34 | 0.34 |
| Left ventricular mass index | 64 g/m2 | 67 g/m2 |
| Left ventricular ejection fraction | 65% | 55% |
| Left ventricular global longitudinal strain | −19.4% | −20.6% |
NA = not applicable.
Management
The findings were consistent with heart failure with preserved ejection fraction. Further analysis of her echocardiogram revealed abnormal left atrial strain imaging consistent with diastolic dysfunction. The patient had resolution of symptoms after diuresis and was discharged on oral diuretics.
Discussion
This case highlights the existence of an infrequently described entity—acute postpartum heart failure with preserved systolic function. Because normal pregnancy and PPCM can also present with dyspnea and peripheral edema, distinguishing among these diagnoses is essential.
In normal pregnancy, the plasma volume increases approximately 50%, and systemic vascular resistance decreases by the same magnitude with the peak of this adaptation occurring by the end of the first trimester. Cardiac output similarly experiences increments through a combination of increased stroke volume and heart rate. During normal pregnancy, up to 80% of women will have peripheral edema, and on echocardiography mild 4-chamber dilation is present (1). Importantly, despite these changes, in women without known cardiovascular disease, BNP levels increase but remain within the normal range throughout pregnancy. Natriuretic peptide levels are recommended to assess cardiovascular risk in pregnancy, and BNP levels <100 pg/ml have a strong negative predictive value for cardiovascular events in pregnancy (1,2). However, BNP may be nonspecifically elevated in approximately one third of pregnant women with known cardiovascular disease in the absence of clinical heart failure (2).
Given the disproportionate magnitude of symptoms, the timing of presentation, and the abnormal BNP in our patient, echocardiography was pursued to evaluate for possible PPCM. PPCM is characterized as an idiopathic nonischemic cardiomyopathy with LV systolic dysfunction occurring toward the end of pregnancy or in the months following (1). In prospective cohorts, many patients have complete recovery of LV systolic function (47% to 72%); however, a significant proportion develop persistent severe or end-stage cardiomyopathy (13% to 15%) (3,4). Because our patient had normal systolic function but significantly elevated pulmonary pressures on echocardiography, right heart catheterization was performed. Because the first symptoms of pulmonary arterial hypertension may be encountered in pregnancy, right heart catheterization is recommended by guidelines to confirm this diagnosis when clinical uncertainty exists (1). In our patient, right heart catheterization was instead indicative of pulmonary hypertension secondary to left-sided pressure overload.
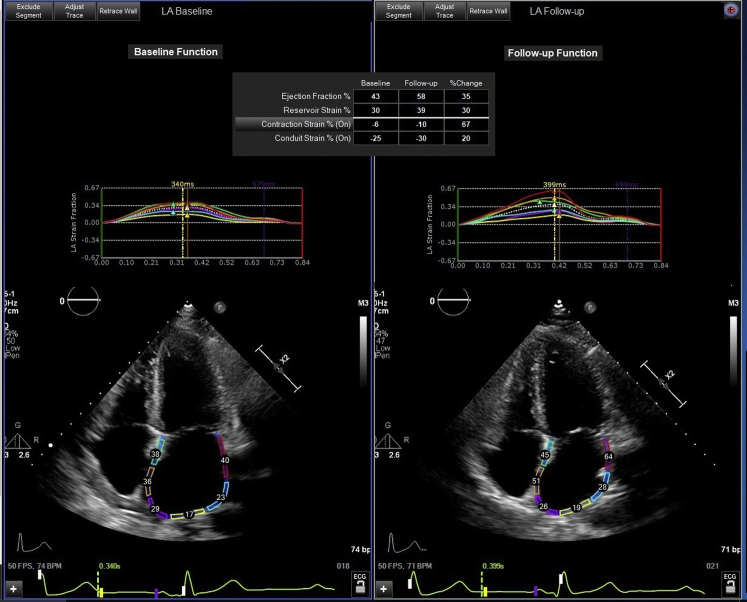
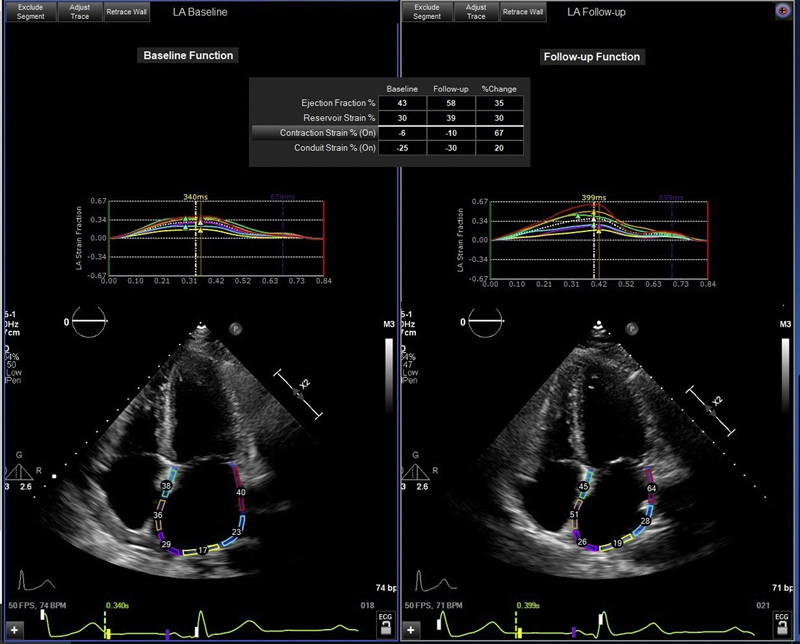
When systolic dysfunction is not present, acute heart failure at the end of pregnancy may be due to left ventricular diastolic dysfunction in the setting of pre-eclampsia, eclampsia, and severe gestational or pre-existing hypertension (1). Diastolic dysfunction is present in nearly one-half of women with pre-eclampsia and persists in one-half of pre-eclamptic women in long-term follow-up (5). In addition, 40% of women with pre-eclampsia develop hypertension in the 2-year period after delivery (5). However, our patient did not have hypertension, pre-eclampsia, or LV hypertrophy. An echocardiogram showed a normal LV global longitudinal strain pattern. On baseline evaluation, our patient did not have adequate pulsed wave Doppler imaging of the mitral valve flow. Therefore, to further assess diastolic dysfunction in our patient, left atrial strain imaging was retrospectively evaluated. This analysis revealed reductions in the left atrial reservoir, conduit, and contraction strain on echocardiography during heart failure that improved in follow-up (Table 1, Figure 1). Left atrial strain is a novel marker of diastolic dysfunction and has not been previously demonstrated to resolve along with symptomatic improvement (6). A limitation of this analysis is that normal values of left atrial strain in otherwise healthy pregnant patients have yet to be well established.
Figure 1.
Left Atrial Strain Imaging
Abnormal left atrial strain in the setting of postpartum heart failure with preserved systolic function, which improved on follow-up assessment.
Acute heart failure in pregnancy without systolic dysfunction in the absence of pre-eclampsia, eclampsia, or hypertension has been infrequently described (7, 8, 9). As in our patient, in these case descriptions, heart failure generally occurred in the first week after delivery and resolved in follow-up. However, in the largest series of peripartum heart failure without systolic dysfunction, 50% of patients continued to have class II to III heart failure symptoms in long-term follow-up (9).
Given the paucity of data regarding this entity, further study is warranted to address several questions. It is uncertain whether the described case represents the mildest part of the PPCM spectrum or is instead a distinct form of diastolic heart failure. Heart failure with preserved ejection fraction is itself a heterogenous entity that requires thorough evaluation and phenotyping. Similar to peripartum cardiomyopathy, vascular dysfunction and increased levels of oxidative stress at the end of pregnancy may contribute to impaired myocardial functioning. Multiparity is a potential risk factor for diastolic dysfunction, and, therefore, understanding this pathophysiology may also provide insight into sex differences in the risk for heart failure with preserved ejection fraction (10). The risk factors for heart failure with preserved systolic function in pregnancy are also unclear. Our patient may have been predisposed to heart failure because of advanced maternal age and obesity, which are 2 features that are increasingly common in pregnant patients. In addition, the incidence of recurrent heart failure or other cardiovascular diseases in future pregnancies for our patient is unknown. Finally, because PPCM and pre-eclampsia carry long-term risks for heart failure and hypertension outside of pregnancy, it will be important to determine whether postpartum heart failure with preserved systolic function implies a long-term risk for diastolic dysfunction or other cardiovascular disease.
Follow-Up
At the 2-week follow-up, the patient had resolution of symptoms and was discontinued from diuretics. A transthoracic echocardiogram at this time demonstrated resolution of all prior abnormalities and demonstrated normal diastolic function by all parameters (Table 1).
Conclusions
Postpartum heart failure can occur in the absence of systolic dysfunction. Echocardiographic measures of diastolic dysfunction such as left atrial strain imaging can be helpful in the diagnosis of post-partum heart failure with preserved ejection fraction. Future research should address the prevalence and long-term outcomes of this frequently overlooked diagnosis.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 2.Tanous D., Siu S.C., Mason J. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol. 2010;56:1247–1253. doi: 10.1016/j.jacc.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 3.Haghikia A., Podewski E., Libhaber E. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNamara D.M., Elkayam U., Alharethi R. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy) J Am Coll Cardiol. 2015;66:905–914. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melchiorre K., Sutherland G.R., Liberati M., Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–715. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 6.Thomas L., Marwick T.H., Popescu B.A., Donal E., Badano L.P. Left atrial structure and function, and left ventricular diastolic dysfunction. J Am Coll Cardiol. 2019;73:1961–1977. doi: 10.1016/j.jacc.2019.01.059. [DOI] [PubMed] [Google Scholar]
- 7.Wells G.L., Little W.C. Peripartum cardiomyopathy presenting as diastolic heart failure. Congest Hear Fail. 2008;14:52–54. doi: 10.1111/j.1751-7133.2008.07378.x. [DOI] [PubMed] [Google Scholar]
- 8.Rogers F.J., Cooper S. Peripartum heart failure caused by left ventricular diastolic dysfunction. J Am Osteopath Assoc. 2010;110:87–90. [PubMed] [Google Scholar]
- 9.Afonso L., Arora N.P., Mahajan N. Comparison of patients with peripartum heart failure and normal (≥55%) versus low (<45%) left ventricular ejection fractions. Am J Cardiol. 2014;114:290–293. doi: 10.1016/j.amjcard.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S.R., Herrington D.M., Vladutiu C.J. Higher number of live births is associated with left ventricular diastolic dysfunction and adverse cardiac remodelling among US Hispanic/Latina women: results from the Echocardiographic Study of Latinos. Open Heart. 2017;4 doi: 10.1136/openhrt-2016-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]