Abstract
An apical sparing pattern of longitudinal strain and positive radionuclide bone scintigraphy are believed to be specific for the diagnosis of transthyretin cardiac amyloidosis. We report on a young woman with apical sparing of longitudinal strain and positive bone scintigraphy who was found to have metastatic myocardial calcification at autopsy. (Level of Difficulty: Intermediate.)
Key Words: cardiomyopathy, echocardiography, nuclear medicine, restrictive
Abbreviations and Acronyms: ICD, implantable cardioverter-defibrillator; LV, left ventricular; LS, longitudinal strain
Graphical abstract
An apical sparing pattern of longitudinal strain and positive radionuclide bone scintigraphy are believed to be specific for the diagnosis of transthyretin…
History of Presentation
A 35-year-old African-American woman was admitted to the hospital with syncope. She was getting out of a car when she had an acute onset of palpitations and diaphoresis followed by loss of consciousness and implantable cardioverter-defibrillator (ICD) shock. She regained consciousness after a brief period of confusion and presented to the emergency department.
Learning Objectives
-
•
To create a differential diagnosis for restrictive cardiomyopathy using multimodality cardiac imaging.
-
•
To recognize the clinical presentation of metastatic myocardial calcification.
On physical examination, the patient’s temperature was 98.7°F, heart rate 97 beats/min, blood pressure 99/67 mm Hg, respirations 16 breaths/min, oxygen saturation 98% on room air, and body mass index 31 kg/m2. Cardiovascular physical examination revealed an irregularly irregular rhythm, no murmurs or gallops, jugular venous pulsation at 8 cm of water, and trace lower extremity edema. ICD pocket appearance was within normal limits.
Medical History
The patient’s pertinent medical history included end-stage renal disease due to pre-eclampsia, 2 failed renal allografts due to antibody-mediated rejection, anuria with ongoing peritoneal dialysis (with 9 total years of treatment), paroxysmal atrial fibrillation, presumed hypertrophic cardiomyopathy with placement of a primary prevention ICD 12 years ago, history of ICD shocks for ventricular tachycardia, multiple miscarriages, and bilateral pulmonary emboli.
Medications included amiodarone 100 mg twice daily, midodrine 20 mg three times daily, fludrocortisone 0.1 mg twice daily, warfarin 3 mg daily, atorvastatin 40 mg daily, levothyroxine 250 μg daily, prednisone 5 mg daily, sevelamer 1,600 mg 3 times daily, and calcitriol 0.5 μg 3 times weekly. For peritoneal dialysis, she alternated between 2.5% and 4.25% dextrose baths using 2 L exchanges over 8.5 h of total cycler therapy time.
Initial Test Results
Laboratory evaluations revealed the following: troponin I 0.12 ng/ml, N-terminal pro–B-type natriuretic peptide 250,200 pg/ml, creatinine level 16.39 mg/dl, calcium 10.8 mg/dl, phosphorus 6.5 mg/dl, intact parathyroid hormone 998 pg/ml, and reduced urea clearance with Kt/Vurea of 1.22 (K, dialyzer clearance of urea; t, dialysis time; Vurea, volume of distribution of urea).
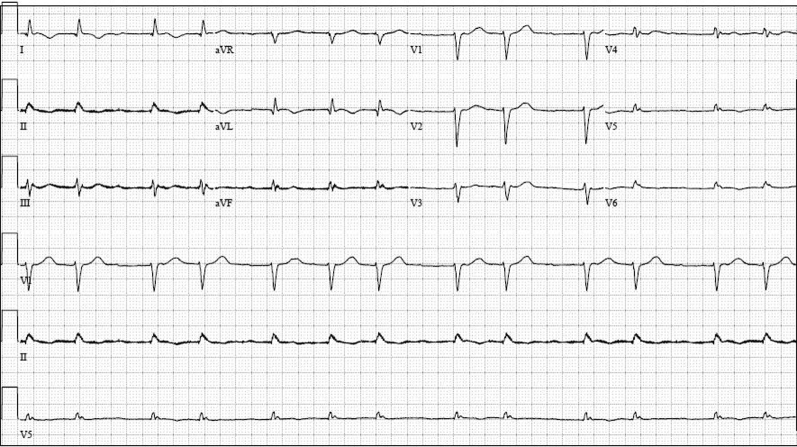
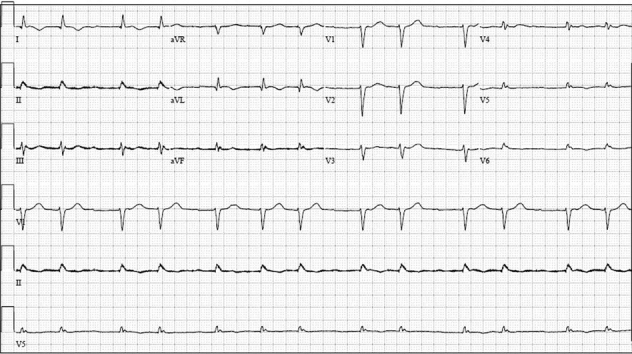
An electrocardiogram showed atrial fibrillation with low-voltage QRS and an intraventricular conduction delay (Figure 1). A transthoracic echocardiogram revealed a small, crescent-shaped left ventricular (LV) cavity with LV ejection fraction of 55% to 60% (Videos 1 and 2). Severe concentric hypertrophy was seen with a diastolic interventricular septal thickness of 2.1 cm and a posterior wall thickness of 1.8 cm. Mitral annular velocities were reduced (average e’ 0.06 m/s) with a single e’ waveform, and the mitral inflow Doppler profile was consistent with restrictive physiology (deceleration time 121 ms) (Figure 2). In addition, an apical sparing pattern of longitudinal strain (LS) was noted (Figure 3). There was no valvular or annular calcification, or pericardial effusion. Device interrogation confirmed sustained ventricular tachycardia with appropriate device therapy.
Figure 1.
Electrocardiography
Electrocardiogram showed atrial fibrillation with low voltage in the limb leads and an intraventricular conduction delay.
Online Video 1.
Echocardiography–Parasternal Long-Axis View
There was severe concentric hypertrophy with a septal thickness of 2.1 cm and a posterior wall thickness of 1.8 cm.
Online Video 2.
Echocardiography–Apical 4-Chamber View
There was preserved left ventricular systolic function (left ventricular ejection fraction 55% to 60%) with a small, crescent-shaped left ventricular cavity.
Figure 2.
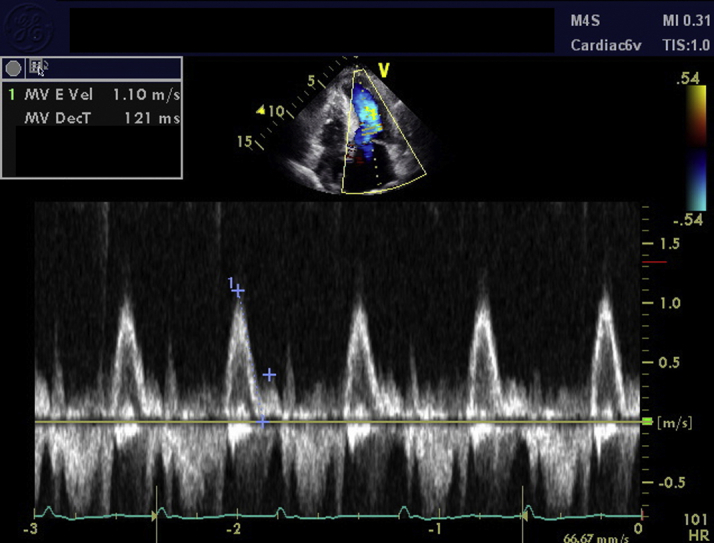
Echocardiography–Mitral Inflow Pattern
Pulse-wave Doppler at the mitral leaflet tips showed restrictive filling with an E velocity of 1.10 m/s and a deceleration time of 121 ms.
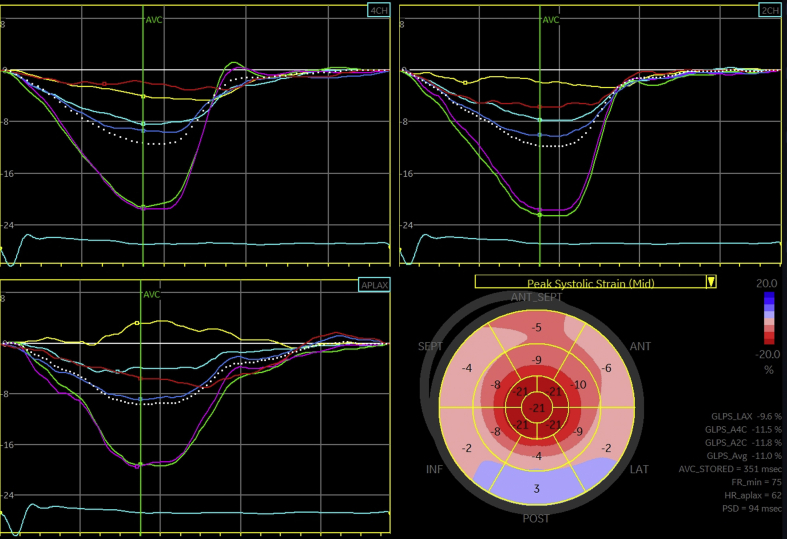
Figure 3.
Echocardiography–Strain Imaging
Speckle-tracking longitudinal strain analysis was performed (EchoPAC, GE Healthcare, Chicago, Illinois). Relative apical sparing of longitudinal strain was seen with a ratio of 1.0.
Differential Diagnosis
The imaging findings of low-voltage QRS, increased LV wall thickness, restrictive Doppler echocardiography, and apical sparing pattern of LS are suspicious for an infiltrative cardiomyopathy, particularly cardiac amyloidosis. Although wild-type transthyretin cardiac amyloidosis affects older individuals, light chain amyloidosis and hereditary transthyretin cardiac amyloidosis are diagnostic possibilities in this case. Cardiac sarcoidosis is another diagnostic consideration, especially in the setting of frequent ventricular arrhythmias and the patient’s race. Other causes of LV hypertrophy include hypertensive heart disease, hypertrophic cardiomyopathy, Fabry disease, and metabolic disease (i.e., Friedreich ataxia).
Investigations and Management
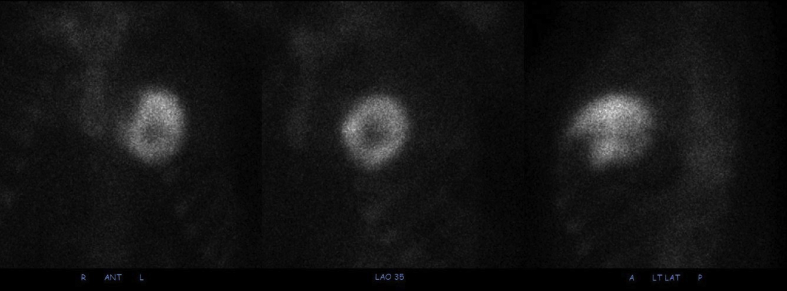
Due to clinical suspicion for cardiac amyloidosis, the patient underwent technetium pyrophosphate bone scintigraphy. This procedure revealed grade 3 myocardial uptake, consistent with the diagnosis of transthyretin cardiac amyloidosis (Figure 4). Serum protein electrophoresis did show an immunoglobulin G kappa monoclonal protein, although this was believed to be nonspecific in the setting of renal failure. Cardiac magnetic resonance imaging was not pursued due to renal failure and the inability to administer gadolinium contrast. Confirmatory endomyocardial biopsy was aborted after the operator was unable to obtain venous access to the heart despite significant effort. Unfortunately, the patient experienced a pulseless electrical activity cardiac arrest during the hospital stay and died.
Figure 4.
Radionuclide Bone Scintigraphy
There was diffusely increased radiotracer uptake throughout the myocardium of the right and left ventricles, with no uptake in the apex. This corresponded to a visual score of 3, indicating myocardial uptake greater than bone.
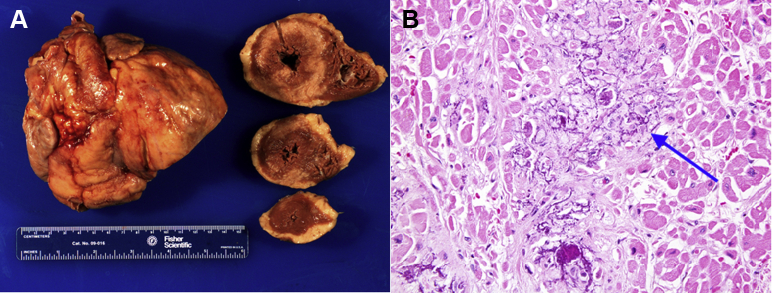
At autopsy, the patient was found to have multi-organ widespread calcium deposition, findings consistent with metastatic calcification. The severity of calcium deposition was most extensive in the heart. On gross examination, the heart was rigid and heavy (640 g; normal range 250 to 300 g). A white, firm, waxy infiltration was identified, most obvious in the LV wall (Figure 5A). On microscopic examination, the myocardium showed interstitial fibrosis and extensive calcium deposition. The calcification exhibited a chicken-wire pattern, a finding consistent with primary calcium deposition as opposed to secondary dystrophic calcification after myocardial injury (e.g., myocardial infarction) (Figure 5B). Congo Red staining was negative for amyloidosis.
Figure 5.
Autopsy Findings of the Heart
(A) Gross cardiac specimens, with cross-sections of the left ventricle and right ventricle from mid-base to apex. (B) High-magnification light microscopy image of the right atrium. The basophilic granular calcium deposition surrounds cardiomyocytes in a “chicken-wire” pattern (blue arrow) and causes damage of cardiomyocytes, as indicated by loss of nuclei, and interstitial fibrosis. Hematoxylin and eosin, 40× objective.
Discussion
Extraskeletal calcium deposition is common in patients on maintenance dialysis, with mitral annular calcification and aortic valve sclerosis being the most common sites of cardiac calcification (1). Massive myocardial calcification is a rare autopsy finding, although it is more common in anuric patients receiving long-term peritoneal dialysis and concomitant warfarin therapy. Sudden cardiac death is common in the setting of metastatic myocardial calcification (2, 3, 4, 5, 6). Although most cases are diagnosed at autopsy, dual-energy chest radiography and chest computed tomography imaging have both shown utility for diagnosis (2,7). In our patient, abnormal myocardial attenuation was seen on noncontrast computed tomography scans as early as 7 years before her death.
Our case details the electrocardiographic and echocardiographic findings of metastatic myocardial calcification, which overlap significantly with those seen in cardiac amyloidosis. These include low-voltage QRS, increased LV wall thickness, low mitral annular tissue Doppler velocities, and a restrictive diastolic filling pattern. Importantly, our patient had an apical sparing pattern of LS with relative apical LS of 1.0, calculated as (average LS of apical segments)/(average LS of basal segments + average LS of mid-segments) (8). Relative apical LS ≥1.0 has been reported to have high diagnostic accuracy for cardiac amyloidosis among patients with increased LV wall thickness (8). Alternative causes of an apical sparing pattern have not been well characterized. To our knowledge, this report is the first of an apical sparing pattern of LS in metastatic myocardial calcification.
The study patient had also tested markedly positive on radionuclide bone scintigraphy. A large multicenter study found >99% sensitivity and 82% specificity of radionuclide bone scintigraphy for transthyretin cardiac amyloidosis, with light chain cardiac amyloidosis patients accounting for nearly all of the false-positive findings (9). In our patient, the markedly positive test result was likely due to high myocardial calcium content. The diagnostic performance of radionuclide bone scintigraphy for cardiac amyloidosis in dialysis patients is not known, and clinicians should be aware that positive radionuclide bone scintigraphy in this population may indicate primary myocardial calcification and not transthyretin cardiac amyloidosis. This is particularly important as radionuclide bone scintigraphy increasingly takes on a primary role in the diagnostic algorithm for cardiac amyloidosis in general practice and clinical trial settings.
Conclusions
Metastatic myocardial calcification presents in dialysis patients as a restrictive cardiomyopathy with high cardiac arrhythmia burden. Cardiac imaging findings may mimic those of transthyretin cardiac amyloidosis and include apical sparing of longitudinal strain and high-grade myocardial uptake on radionuclide bone scintigraphy. Clinicians should maintain a high index of suspicion for metastatic myocardial calcification in the appropriate clinical context.
Footnotes
This study was supported by The Foundation for Barnes-Jewish Hospital. Dr. Miller has served as a consultant for Fresenius Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, see the online version of this paper.
References
- 1.Kerr D.N.S. Hypercalcemia and metastatic calcification. Cardiovasc Res. 1997;36:293–297. doi: 10.1016/s0008-6363(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 2.Matsui M., Okayama S., Takitsume A. Heart failure associated with metastatic myocardial calcification in a hemodialysis patient with progressive calcification of the hand. Cardiorenal Med. 2012;2:251–255. doi: 10.1159/000343497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isotalo P.A., Halil A., Green M., Tang A., Lach B., Veinot J.P. Metastatic calcification of the cardiac conduction system with heart block: an under-reported entity in chronic renal failure patients. J Forensic Sci. 2000;45:1335–1338. [PubMed] [Google Scholar]
- 4.Cao V., Brickner L. Myocardial calcification in a patient with end-stage renal disease. J Hosp Med. 2009;4:E16. doi: 10.1002/jhm.457. [DOI] [PubMed] [Google Scholar]
- 5.Na J.Y. A heart of stone: an autopsy case of massive myocardial calcification. Forensic Sci Med Pathol. 2018;14:102–105. doi: 10.1007/s12024-017-9936-8. [DOI] [PubMed] [Google Scholar]
- 6.Okada M., Kyakuno M., Imamura J., Nakamura T., Takahara S. An autopsy case of sudden death in renal transplant recipient. Clin Transplant. 2002;16(Suppl 8):58–61. doi: 10.1034/j.1399-0012.16.s8.3.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanders C., Frank M.S., Rostand S.G., Rutsky E.A., Barnes G.T., Fraser R.G. Metastatic calcification of the heart and lungs in end-stage renal disease: detection and quantification by dual-energy digital chest radiography. AJR Am J Roentgenol. 1987;149:881–887. doi: 10.2214/ajr.149.5.881. [DOI] [PubMed] [Google Scholar]
- 8.Phelan D., Collier P., Thavendiranathan P. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 9.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]