Abstract
An 18-year-old primigravida woman underwent emergent percutaneous balloon stent repair of a severe coarctation of the aorta, allowing her to undergo an uneventful remaining pregnancy and the delivery of a healthy baby. Her case also demonstrates the association between maternal coarctation and fetal Shone complex. (Level of Difficulty: Beginner.)
Key Words: balloon expanded stent, coarctation of the aorta, pregnancy, Shone complex
Abbreviations and Acronyms: COA, coarctation of the aorta
Graphical abstract
An 18-year-old primigravida woman underwent emergent percutaneous balloon stent repair of a severe coarctation of the aorta, allowing her to undergo…
Coarctation of the aorta (COA) is usually diagnosed in childhood, but 20% of cases do not present until adult life (1). Patients with COA are increasingly reaching child-bearing age, and pregnancy-related cardiovascular changes place additional stress on coarctation physiology (2). Unrepaired COA in pregnancy can be life-threatening for both mother and fetus. Maternal complications include cerebral infarction, aortic dissection, aortic rupture, congestive heart failure, hypertensive crisis, and infective endocarditis. Fetal complications include growth retardation and premature birth with increased risk for placental ischemia or abruption (3,4).
Learning Objectives
-
•
To understand the risks of unrepaired COA to the mother and to the fetus.
-
•
To identify indications for intervention in cases of COA during pregnancy.
-
•
To appreciate that more research is needed to determine the preferred invasive intervention for emergent COA repair during pregnancy.
When medical management with typical antihypertensive drugs fails to decrease the coarctation gradient, invasive intervention may be indicated (4). Management of COA is dependent upon the patient’s age, size (weight, length, and width of the coarctation), and coarctation characteristics. Surgical resection is recommended for neonates and young children. Transcatheter treatment is preferred for adults and children weighing more than 25 kg (5). Additional cardiac defects may alter intervention strategy.
Presentation
An 18-year-old primigravid woman presented at 11 weeks gestation with unrepaired COA and severe, nonradiating substernal chest pain for 2 days. COA had been diagnosed when she was 3 years old but had been lost to follow-up for 3 years prior to the current presentation. Her chest pain was associated with dyspnea which worsened with exertion. Other symptoms included orthopnea, palpitations, and lightheadedness. Four extremity blood pressure values included 128/76 mm Hg in the right upper extremity; 133/79 mm Hg in the left upper extremity; 117/78 mm Hg in the right lower extremity; and 114/74 mm Hg in the left lower extremity. On examination, a grade 3/6 systolic ejection murmur was heard under her left scapula, and her lower extremity pulses were difficult to palpate.
Medical History
COA of the aorta had been diagnosed when the patient was 3 years old as bicuspid aortic valve and anxiety.
Differential Diagnosis
Chest pain in a pregnant patient with history of COA raises concerns for aortic aneurysm with dissection, coronary artery dissection, pulmonary embolism, pericarditis, or myocardial infarction. The presence of an arm-leg blood pressure gradient suggests possible severe COA or arterial occlusion from thrombosis or a mass. Noncardiovascular causes of chest pain include musculoskeletal chest wall pain, gastroesophageal reflux, pneumonia, and anxiety.
Investigations
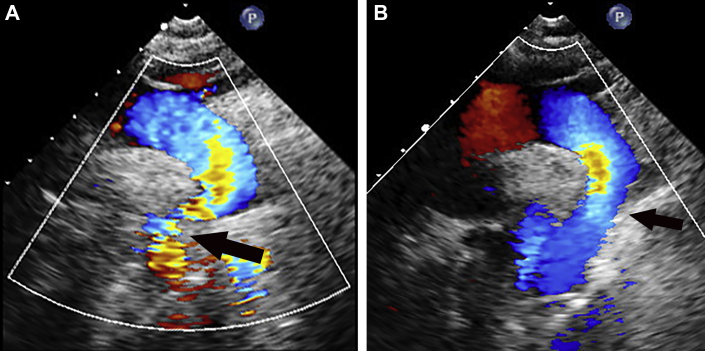
Complete blood count and chemistry assay results were unremarkable. Troponin level was undetectable. Electrocardiography showed normal sinus rhythm without ST-segment changes or T-wave abnormalities. Chest radiography demonstrated cardiomegaly with a mildly tortuous aorta. Echocardiography demonstrated a dilated ascending aorta and transverse arch with increased flow velocity in the descending aorta despite attenuated abdominal aortic pulsations and antegrade diastolic runoff consistent with significant coarctation (Figure 1). Biventricular function was normal.
Figure 1.
Suprasternal Echocardiographic Color Doppler Images Before and After Intervention
(A) Prior to stent placement, the ascending aorta and transverse arch were dilated and aliased color flow across the coarctation site (arrow) with a peak velocity of 3.5 m/s. (B) Flow is significantly improved across the stented area, with a peak velocity of 1.5 m/s.
Management
Emergent cardiac catheterization was performed with the patient under general anesthesia with lead shielding of the uterus. Significant distal aortic arch narrowing was confirmed with the following measurements: transverse arch: 17.1 mm; COA site: 8.5 mm; and descending aorta: 17.6 mm. Pre-intervention peak-to-peak gradient was 15 mm Hg. A Palmaz 3110 stent (Cordis Corp., Warren, New Jersey) on an 18- × 4-mm delivery balloon was implanted to relieve the arch obstruction (Figure 2). The residual gradient through the descending aorta after stent placement was reduced to 0 mm Hg.
Figure 2.
Angiographic Images Before and After Intervention
Lateral anterior oblique angiographic images demonstrate the coarctation before (A) and after (B) stent placement. Note the significant change in aortic caliber and the lack of aortic wall injury.
The patient chose to continue the pregnancy after being counseled about the uncertainty of the outcome and her options of termination, surgical intervention, catheterization, and medical management. The patient was maintained on aspirin and clopidogrel therapy. She was monitored closely with frequent ultrasonograms, and the remainder of her pregnancy was uneventful. Delivery proceeded without bleeding complications.
Discussion
This case demonstrates successful percutaneous repair of COA during pregnancy by using a balloon expandable stent. Management of COA during pregnancy is complicated by heightened procedural risks to the pregnant woman and her fetus. For aortic procedures, high estrogen levels can impact aortic remodeling, predisposing the aorta to dissection and rupture (3,6). However, if residual arch gradient is low, outcomes of further pregnancies are favorable, with rates of preeclampsia similar to those of the general population (4).
Invasive intervention of aortic COA is usually reserved for gradients of ≥20 mm Hg (7). Gradients <20 mm Hg should be considered for intervention when superimposed over heart structural defects, hypertension, heart failure, or a life-threatening symptom profile as seen in this patient (7). Another point of consideration is the use of fluoroscopy. Ideally, radiation should be avoided in pregnancy as there is no safe dose, but in life-threatening situations, such as significant COA, the use of diagnostic examinations to preserve the life of the mother can outweigh the risks. Specific precautions should be taken to limit fetal exposure to radiation, including limiting fluoroscopic time and positioning of the beam to avoid positioning the fetus in a direct line (8).
When invasive intervention for COA is indicated, treatment options include balloon angioplasty alone, transcatheter stent implantation, and surgical coarctectomy (4). To the best of the present authors’ knowledge, only one other transcatheter stent has been successfully placed during pregnancy for treatment of COA (3). Although further evidence is needed to demonstrate a safety profile of stent placement in pregnant patients for severe COA, the present case provides a second example of successful implantation with a good outcome.
Mothers with congenital heart disease, such as COA, have an increased risk of having children with congenital heart disease (6). Although the present patient’s child during this presentation did not have cardiac abnormalities, she later had 2 children with Shone complex, which consists of left heart obstructive lesions that may include COA, supramitral stenosing ring, valvular aortic stenosis, and subaortic stenosis. Left-sided obstructive lesions may occur in 10% to 20% of first-degree relatives of patients with COA, in whom the ERBB4 and NOTCH1 genes are implicated in left ventricular outflow tract abnormalities (1,9). Thus, a fetal echocardiogram should be performed at 18 to 22 weeks gestation in pregnant women with a history of COA (10). Early detection can lead to improved outcomes for neonates by allowing specialists to be present in care prior to and immediately following birth.
Follow-up
The present patient is now a 24-year-old G5P3 healthy woman. In addition to the child discussed, she has had 2 living children with Shone complex and 2 miscarriages. Fetal echocardiography was performed for each pregnancy, and the patient received genetic counseling but declined further genetic testing.
Conclusions
Although further research is needed to determine the safest interventions, management of this case demonstrated a successful stent implantation for treatment of severe COA in pregnancy. Pregnant patients with COA may benefit from genetic testing and fetal echocardiographic screening for early detection and intervention of cardiac defects in their offspring.
Footnotes
No external funding was provided for this work. Dr. Hoyer is a proctor for St. Jude Medical; and is a consultant for Gore Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Beauchesne L.M., Connolly H.M., Ammash N.M., Warnes C.A. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol. 2001;38:1728–1733. doi: 10.1016/s0735-1097(01)01617-5. [DOI] [PubMed] [Google Scholar]
- 2.Thilen U., Olsson S.B. Pregnancy and heart disease: a review. Eur J Obstet Gynecol Reprod Biol. 1997;75:43–50. doi: 10.1016/s0301-2115(97)00188-7. [DOI] [PubMed] [Google Scholar]
- 3.Assaidi A., Sbragia P., Fraisse A. Transcatheter therapy for aortic coarctation with severe systemic hypertension during pregnancy. Catheter Cardiovasc Interv. 2013;82:556. doi: 10.1002/ccd.24404. [DOI] [PubMed] [Google Scholar]
- 4.Saeed S.A., Dean S., Degiovanni J.V. Not pre-eclampsia. J Royal Society of Med. 2001;94:351–353. doi: 10.1177/014107680109400712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dijkema E.J., Leiner T., Grotenhuis H.B. Diagnosis, imaging and clinical management of aortic coarctation. Heart. 2017;103:1148–1155. doi: 10.1136/heartjnl-2017-311173. [DOI] [PubMed] [Google Scholar]
- 6.Canobbio M.M., Warnes C.A., Alboulhosn J. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e50–e87. doi: 10.1161/CIR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 7.Forbes T.J., Kim D.W., Du W. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC. J Am Coll Cardiol. 2011;58:2664–2674. doi: 10.1016/j.jacc.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Tomà P., Bartoloni A., Salerno S. Protecting sensitive patient groups from imaging using ionizing radiation: effects during pregnancy, in fetal life and childhood. Radiol Med. 2019;124:1–9. doi: 10.1007/s11547-019-01034-8. [DOI] [PubMed] [Google Scholar]
- 9.McBride K.L., Zender G.A., Fitzgerald-Butt S.M. Association of common variants in ERBB4 with congenital left ventricular outflow tract obstruction defects. Birth Defects Res. 2011;91:162. doi: 10.1002/bdra.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donofrio M.T., Moon-Grady A.J., Hornberger L.K. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014 May 27;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]