Abstract
Spontaneous coronary artery dissection is an increasingly recognized cause of acute coronary syndrome in younger patients. Management remains challenging and involves weighing the benefits of revascularization with the potential to worsen the dissection. We present a case of spontaneous coronary artery dissection with the superimposed complexity of an anomalous intramural coronary artery. (Level of Difficulty: Intermediate.)
Key Words: anomalous coronary artery, intravascular ultrasonography, myocardial infarction, percutaneous coronary intervention, spontaneous coronary artery dissection
Abbreviations and Acronyms: AAOCA, anomalous aortic origin of a coronary artery; IVUS, intravascular ultrasonography; LVEF, left ventricular ejection fraction; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection
Graphical abstract
Spontaneous coronary artery dissection is an increasingly recognized cause of acute coronary syndrome in younger patients. Management remains challenging…
Presentation
A 39-year-old woman with a history of generalized anxiety was brought to the emergency department by ambulance after developing severe substernal chest pain, diaphoresis, and transient syncope.
Learning Objectives
-
•
To review the causes, epidemiology, and natural history of SCAD.
-
•
To understand the indications and strategies for revascularization in SCAD.
-
•
To identify anomalous coronary anatomy and recognize its implications for management.
The patient had felt well until participating in an aerobic exercise class on the day of presentation. She developed palpitations followed by frank syncope. Upon regaining consciousness, she noted severe substernal chest pain, and emergency medical services were called. Aspirin and sublingual nitroglycerin were administered.
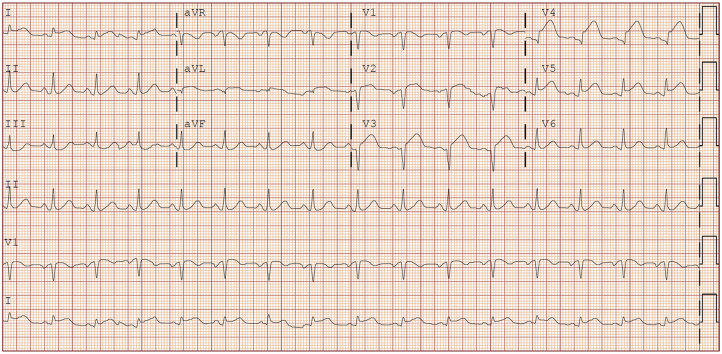
On presentation, the patient was visibly uncomfortable. Her temperature was 36.4°C, blood pressure was 104/69 mm Hg, heart rate was 97 beats/min, respiratory rate was 14 breaths/min, and oxygen saturation was 100% on 2 l/min of oxygen by nasal cannula. Lung auscultation observation was normal. She was tachycardic with otherwise normal heart sounds. There was no jugular venous distension. The extremities were warm and without edema. The remaining examination findings were normal. Electrocardiography demonstrated sinus rhythm with anteroseptal ST-segment elevations in V2 to V5 (Figure 1).
Figure 1.
Presenting Electrocardiogram
Medical History
Generalized anxiety.
Differential Diagnosis
Acute coronary syndrome, arrhythmia, stress cardiomyopathy, valvular heart disease.
Management
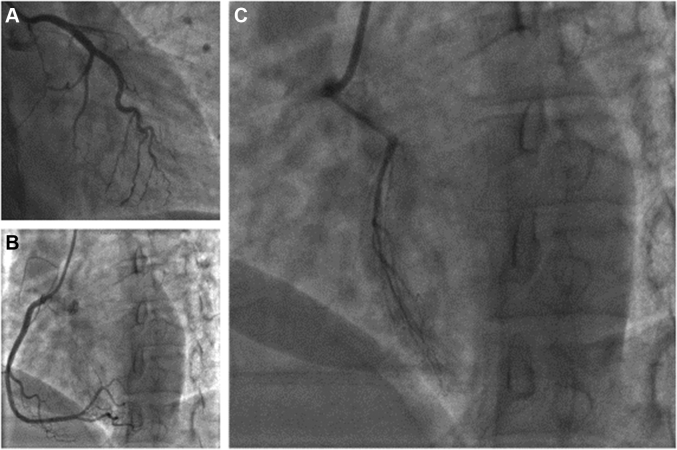
The patient underwent emergency cardiac catheterization using the radial approach. Selective coronary angiography with a 6-F Ikari left 3.5 guide catheter (Terumo Interventional Systems, Somerset, New Jersey) revealed an isolated left circumflex artery arising from the left coronary cusp and a dominant right coronary artery, both of which were free of disease (Figures 2A and 2B). The left anterior descending (LAD) artery was found to originate anomalously from the right coronary cusp and was occluded distal to a large septal perforator (Figure 2C,Videos 1 and 2). There was a filling defect proximal to the occlusion which appeared to be a dissection versus a thrombus.
Figure 2.
Emergency Invasive Coronary Angiogram
(A) Left circumflex artery; (B) dominant right coronary artery; (C) anomalous left anterior descending artery dissected and occluded distal to the first septal perforator.
Online Video 1.
Cineangiography of left circumflex artery.
Online Video 2.
Cineangiography of right coronary / left anterior descending arteries.
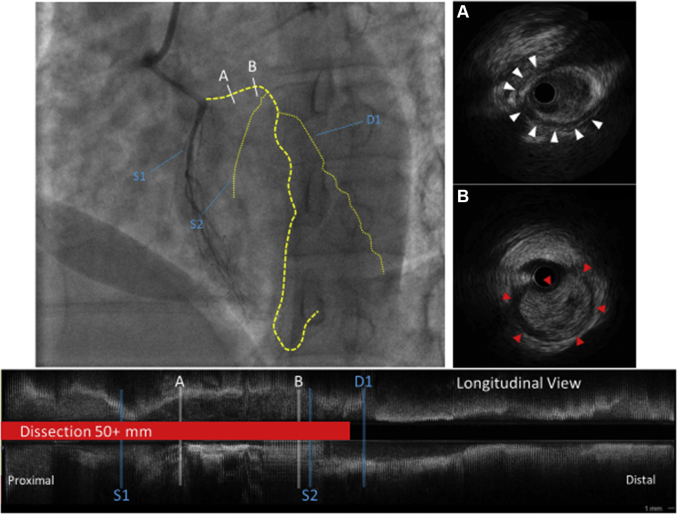
Given the worsening chest pain, persistent ST-segment elevations, and proximal nature of the occlusion, percutaneous coronary intervention (PCI) was performed. The vessel was carefully wired with a Sion Blue workhorse wire (ASAHI Intecc, Aichi, Japan). Intravascular ultrasonography (IVUS) was performed with the following findings (Figure 3): 1) dissection with intramural hematoma extending from the ostium to the first diagonal; 2) the vessel appeared intramyocardial along the dissected segment; and 3) the guidewire was in the true lumen both at major branch bifurcations and beyond the dissection.
Figure 3.
Intravascular Ultrasonography Pullback of the Left Anterior Descending Artery
(A) Representative frame of halo bands (white arrowheads) suggests possible intramyocardial course. (B) Hyperechoic blood pooling suggests intramural hematoma. (red arrowheads). Yellow dotted lines = vessel outline. D1 = first diagonal; S1 = first septal; S2 = second septal.
Direct stenting was performed to minimize vascular trauma. Drug-eluting stents were deployed 4 mm distal to the dissection up to the LAD ostium. Repeat IVUS demonstrated well-apposed and expanded stents with no hematoma propagation. Final angiography (Figure 4, Video 3) demonstrated a small residual non—flow-limiting dissection of the first diagonal, which was left alone given the potential to worsen it with further intervention.
Figure 4.
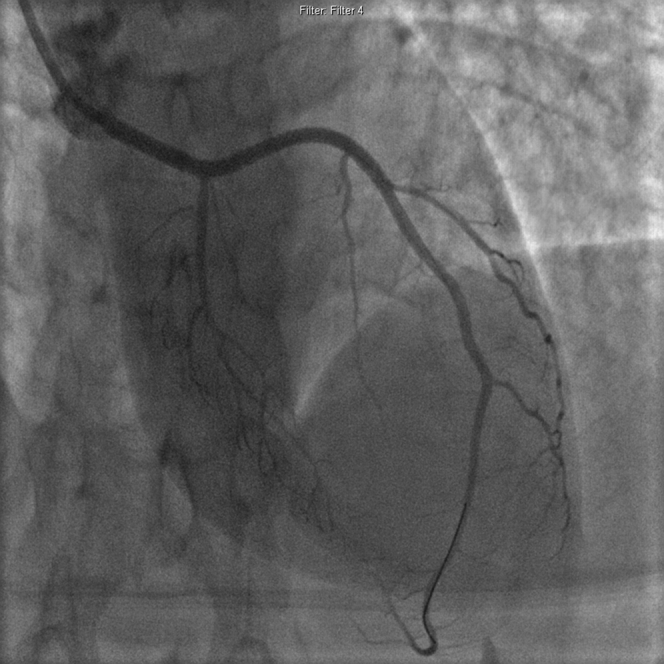
Final Angiography Post-Revascularization
Online Video 3.
Final angiography post-revascularization.
Post-intervention transthoracic echocardiography demonstrated a LV ejection fraction (LVEF) of 35%. The remainder of the hospital course was uneventful. She was discharged with dual anti-platelet therapy and a beta-blocker.
Discussion
SCAD is an under-recognized cause of acute coronary syndrome responsible for up to 35% of myocardial infarctions in women under 50 years of age (1). Recognition is contingent on understanding the demographic, as in this case. The typical patient is a younger female with no conventional atherosclerotic risk factors, who most often presents with symptoms indistinguishable from those of atherosclerotic acute coronary syndrome. The cause is generally unknown, although a fair number of patients have underlying fibromuscular dysplasia and hormonal shifts, as well as physical and emotional stress, which have been identified as possible triggers.
Diagnosis by angiography is imperfect due to its varying phenotypes. As described by Saw et al. (2), type I is characterized by arterial wall staining, type II by diffuse smoothing, and type III by its similarity to atherosclerosis. IVUS and optical coherence tomography (OCT) remain the diagnostic gold standards, but the decision to use them must be weighed against the risk of worsening or propagating the dissection. Without a clear dissection flap or mural staining, this case was an angiographic type II SCAD.
SCAD management is challenging and remains based on expert opinion. The safest catheterization access approach remains unclear, although limited retrospective data have associated radial access with higher rates of iatrogenic dissections (3). Revascularization should not be undertaken lightly in cases of SCAD. With success rates lower than 50% and the potential to worsen the disease, PCI should be reserved for refractory ischemia in cases of high-risk anatomy or instability (4). Most instances of SCAD heal spontaneously, thus, conservative management is generally preferred.
If revascularization is pursued, operators should take care to engage and wire the vessel carefully, with sparing use of contrast injections to minimize hydraulic propagation. Intravascular imaging is mandatory to verify wire position in the true lumen, vessel size, and dissection length. Interventional approaches vary, including balloon angioplasty, stenting, and fenestration with scoring balloons, although none of these are supported by substantial long-term outcome data. Stent sizing should be guided by IVUS/OCT, as late stent malapposition due to hematoma resorption has been described (5). CABG may be considered in cases with left main artery or multivessel proximal vessel involvement (1).
No randomized clinical trials exist to support the use of “conventional” therapies for coronary artery disease such as aspirin or beta-blockade. The use of such agents may be individualized based on the presence of additional indications such as left ventricular dysfunction or concurrent arrhythmias for beta-blocking agents (1).
In the present case, additional complexity was conferred by the anomalous left anterior descending artery, that is, the anomalous aortic origin of a coronary artery (AAOCA). The AAOCA is rare: a study of >120,000 adults undergoing coronary angiography suggested a prevalence of approximately 0.3% (6). Subsequent data demonstrated a prevalence of 0.17% among asymptomatic children undergoing echocardiography (7). The LAD artery arising from the right coronary cusp is an uncommon form of AAOCA (8).
Optimal management strategies for patients with AAOCA remain unclear given a lack of robust data but primarily hinge upon the decision to surgically correct the anomaly. Consensus guidelines from the American Association of Thoracic Surgeons (8) recommend considering surgical unroofing when ischemia or an interarterial and intramural arterial courses are present, which may be associated with an increased risk for sudden cardiac death. Ostial revision and reimplantation may also be considered in the absence of a significant intramural course. CABG is suboptimal, especially in the presence of a patent native vessel with substantial competitive flow (8). The use of PCI or medical therapy such as beta-blockers for AAOCA is not supported by available evidence.
The patient’s presentation represents a combination of 2 uncommon pathologies, neither of which are well understood. The case raises questions about cause and therapy that highlight the lack of evidence guiding management in either condition. Was the intramyocardial course of the LAD unrelated, or was the dissection a result of traumatic arterial compression? Will the result be durable, given the higher restenosis rates in stents for atherosclerosis within myocardial bridges? There is a strong need for reliable data to help guide decision making in both pathologies, a need highlighted by this rare patient presenting with both simultaneously.
Follow-Up
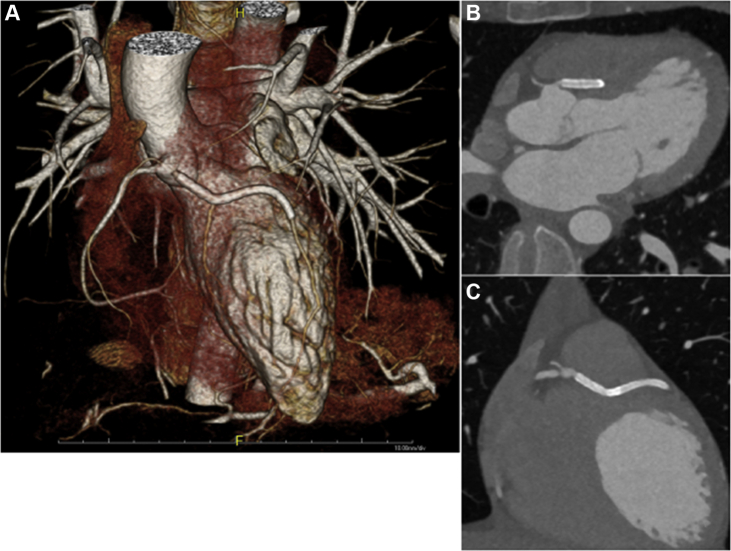
One month later, the patient reported resolution of her symptoms and resumption of normal activity. The LVEF improved to 42%, and no evidence of fibromuscular dysplasia, collagen vascular disease, or autoimmunity was noted. Follow up CT coronary angiography (Figure 5) confirmed the anomalous intramyocardial course of the LAD.
Figure 5.
Follow-Up Computed Tomography Coronary Angiography
(A) 3-Dimensional reconstruction demonstrates stented region and origin off the right coronary cusp. (B and C) The intramyocardial course of the left anterior descending artery.
Conclusions
This unusual case highlights the lack of established management recommendations in patients with SCAD, as well as AAOCA, and the decision making challenges with few data to guide therapy. Although conservative management is generally preferred with SCAD, intervention may be unavoidable in some cases, particularly with unstable ischemia. Angiographically-guided PCI for SCAD can be suboptimal and should be supplemented with intravascular imaging. To the best of the present authors’ knowledge, this is the first reported case of SCAD in a congenitally anomalous coronary artery.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Hayes S.N., Kim E.S.H., Saw J. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saw J., Mancini G.B., Humphries K. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87:E54–E61. doi: 10.1002/ccd.26022. [DOI] [PubMed] [Google Scholar]
- 3.Prakash R., Starovoytov A., Heydari M., Mancini G.B., Saw J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. J Am Coll Cardiol Intv. 2016;9:1851–1853. [Google Scholar]
- 4.Tweet M.S., Eleid M.F., Best P.J. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 5.Lempereur M., Fung A., Saw J. Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovasc Diagn Ther. 2015;5:323–329. doi: 10.3978/j.issn.2223-3652.2015.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka O., Hobbs R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheter Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 7.Davis J.A., Cecchin F., Jones T.K., Portman M.A. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37:593–597. doi: 10.1016/s0735-1097(00)01136-0. [DOI] [PubMed] [Google Scholar]
- 8.Brothers J.A., Frommelt M.A., Jaquiss R.D.B., Myerburg R.J., Fraser C.D., Tweddell J.S. Expert consensus guidelines: Anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153:1440–1457. doi: 10.1016/j.jtcvs.2016.06.066. [DOI] [PubMed] [Google Scholar]