Abstract
While published guidelines are useful in the care of patients with long QT syndrome (LQTS), it can be difficult to decide how to apply the guidelines to individual patients, particularly those with intermediate risk. We explored the diversity of opinion among 24 clinicians with expertise in LQTS. Experts from various regions and institutions were presented with 4 challenging clinical scenarios and asked to provide commentary emphasizing why they would make their treatment recommendations. All 24 authors were asked to vote on case-specific questions so as to demonstrate the degree of consensus or divergence of opinion. Of 24 authors, 23 voted and one abstained. Details of voting results with commentary are presented. There was consensus on several key points, particularly on the importance of the diagnostic evaluation and of beta-blocker use. There was diversity of opinion about the appropriate use of other therapeutic measures in intermediate-risk individuals. Significant gaps in knowledge were identified.
Keywords: long QT syndrome, beta-blocker, genetic testing, implantable cardioverter-defibrillator, left cardiac sympathetic denervation, Sudden Cardiac Death, Genetics
Introduction
Patients with long QT syndrome (LQTS) encompass a broad spectrum of ages and risk categories. While there is usually consensus about how to treat the lowest and highest risk patients, it can be difficult to decide how to apply the guidelines to individual patients with intermediate risk. This document explores the diversity of opinions among experts, from various regions and institutions, in nuanced management decisions that may not be addressed by consensus documents.
Four clinical scenarios are presented to several experts in the field, who provide a commentary emphasizing why they make their treatment recommendations and what influences their management. These discussions emphasize differing points of view as well as points of agreement (summarized in tables 1 and 2). Second, all authors are asked to vote on case-specific questions, so as to demonstrate the degree of consensus or lack thereof. After each case, a separate group of experts summarizes the key points and suggests what gaps in our knowledge should inform further investigation (summarized in table 3). Of 24 authors, 23 voted and one abstained. The voting questions and results are compiled in Figures 1–4 (full voting summary is provided in Supplemental data).
Table 1:
Points of Agreement
| A robust evaluation is crucial to avoid misdiagnosis and inappropriate treatment. |
| The QT interval must be measured accurately, excluding the U-wave. |
| Isolated long QT < 500 ms in an athlete may resolve with deconditioning. |
| Previous genetic testing conducted in a research laboratory should be repeated. |
| Baseline tests should include exercise ECG, echocardiogram (once), and Holter monitor. |
| A child with LQTS should have serial evaluations every 6–12 months. |
| A child with LQTS should receive nadolol 1–1.5 mg/kg or long-acting propranolol 2–3 mg/kg unless there is a contraindication. |
| Pregnant LQTS patients should be treated with beta-blockers regardless of symptoms |
| Post-partum period for LQT2 is a particularly vulnerable time for arrhythmic events and treatment with surveillance should be emphasized |
| ICDs implantation for primary prevention without a beta-blocker trial is rarely indicated, and evaluation of clinical indications of previously implanted devices should be thoroughly reconsidered |
Table 2:
Points of Controversy
| LCSD should be considered if beta-blocker use is not possible in a person with moderate-risk LQTS. |
| An ICD could be avoided in a patient with LQTS type 1 and QTc > 550 ms unless symptoms occur on beta-blocker. |
| A selective beta-blocker could be used if a non-selective beta-blocker was not tolerated in a patient with high-risk LQTS. |
Table 3:
Gaps in Knowledge
| What restrictions should be placed on sports participation? |
| What is the best beta-blocker initiation strategy in LQTS? |
| Is prophylactic LCSD appropriate instead of beta-blocker in low- to medium-risk patients? |
| What are the long-term risks of retained unnecessary hardware versus extraction in young patients? |
| What is the role of pacing in LQTS types 2 and 3? |
| What is the role of subcutaneous ICD in LQTS who will then not have the benefit of atrial pacing? |
| What is the best strategy for treating depression in LQTS? |
| What is the best strategy for treating LQTS in patients with asthma? |
Figure 1:
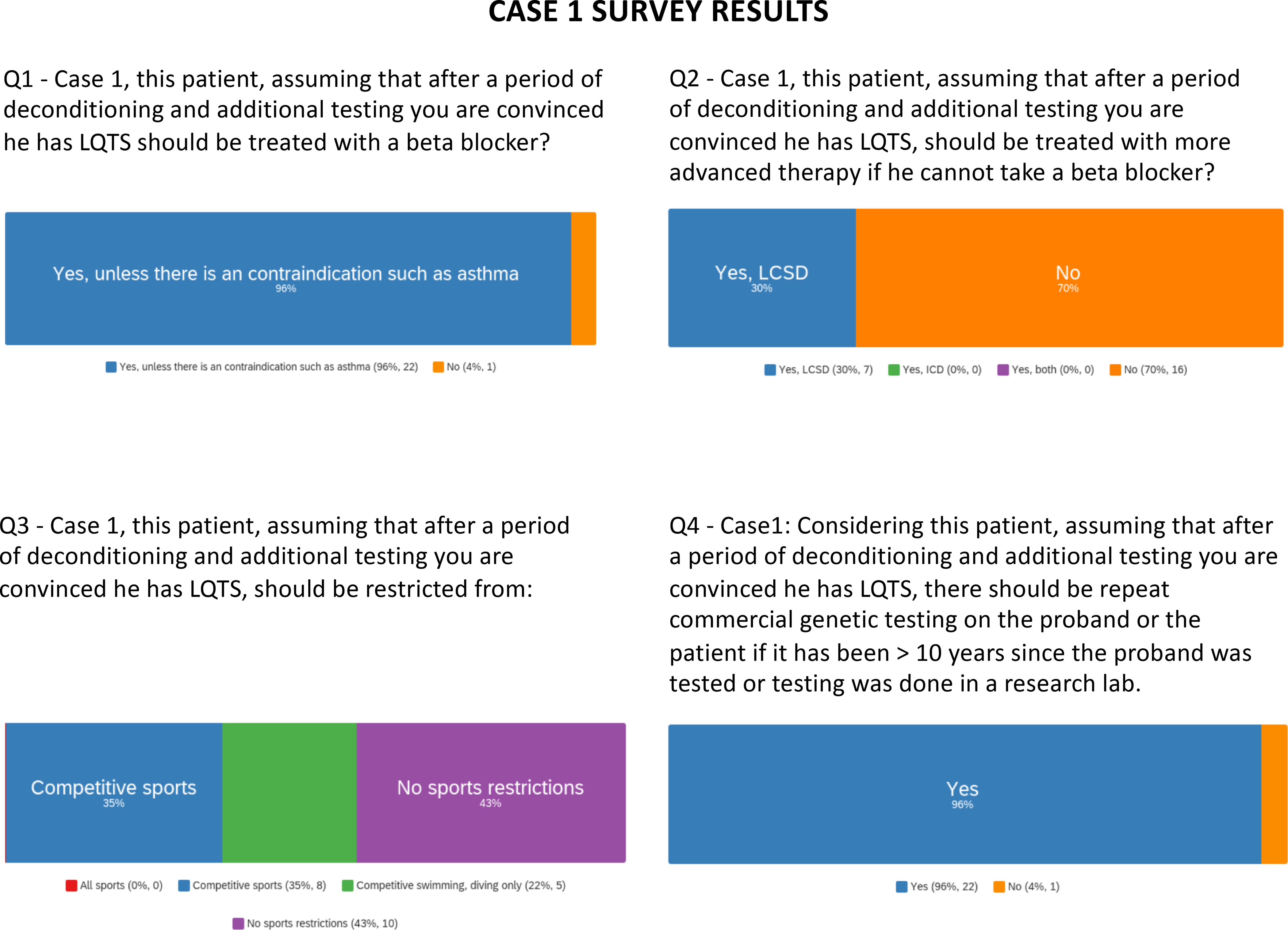
Survey results are presented for questions pertaining to case 1.
Figure 4:
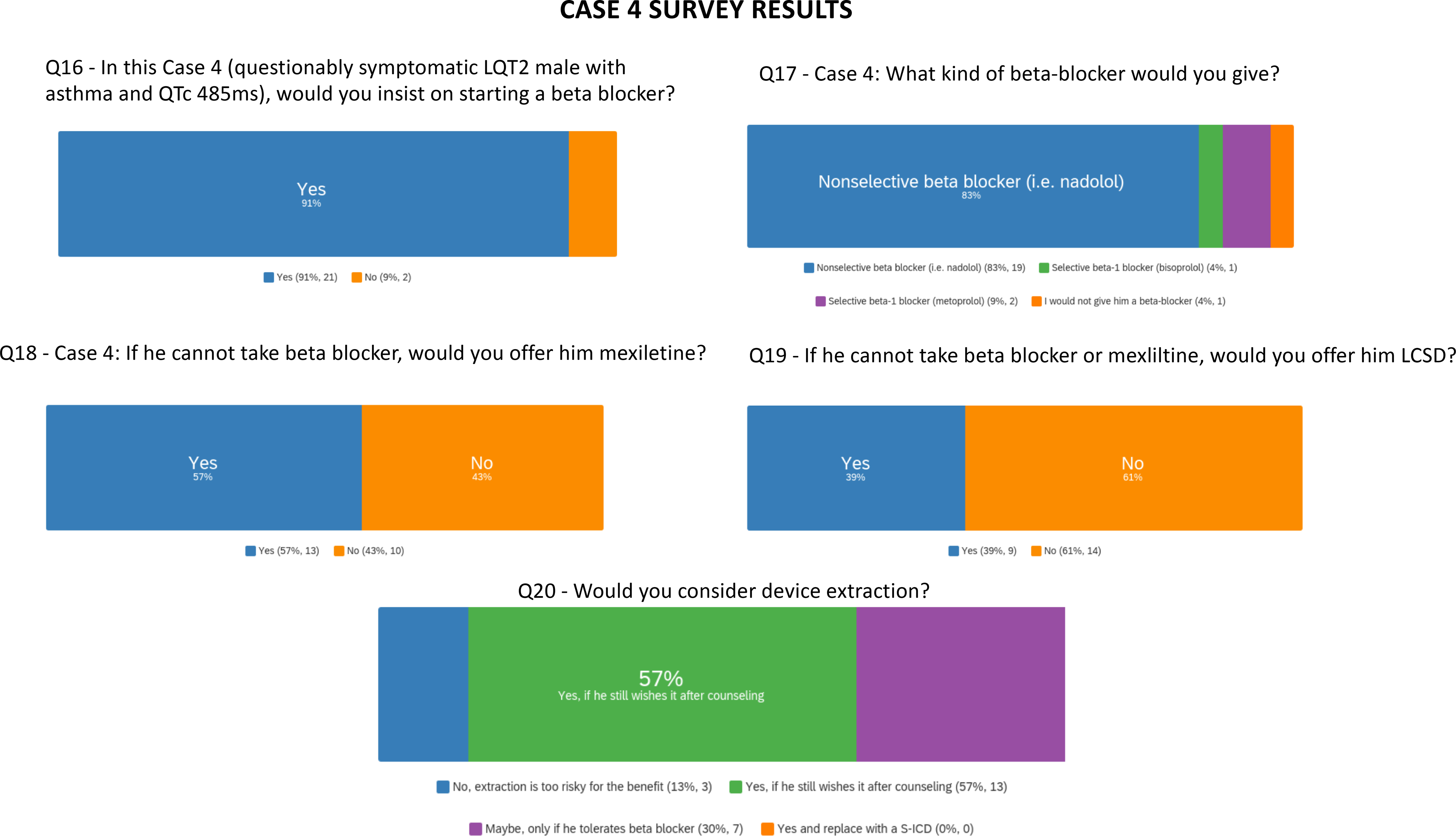
Survey results are presented for questions pertaining to case 4.
Case 1 [Experts: Etheridge (section leader), Ackerman, Schwartz, Shimizu]
A 12-year-old boy presents with family history of LQTS; the proband is LQTS genotype negative. The child has moderate QT prolongation (QTc 480 ms) and no syncope. He is an avid athlete. Would you start a beta-blocker, which one and how much? How would you counsel regarding safe sports participation? What kind of follow up and testing should he have?
Response
It is critical to be certain that a robust assessment of the proband has been undertaken and that the diagnosis is certain1. Detailed information concerning the proband and family members should be elicited, including any symptoms suggesting arrhythmic syncope, unexplained drowning, or sudden death at a young age.
Intense physical training can markedly prolong the QT interval and a period of 3–4 months of deconditioning (complete training cessation) may result in normalization of repolarization in genotype-negative subjects with a negative family and personal history2. In a study of 2000 elite athletes, the QTc was prolonged in 7 (0.4%)3, yet a QTc < 500 ms without symptoms or familial disease was unlikely to represent LQTS. Isolated QT prolongation in an athlete may represent the effect of delayed repolarization as a result of increased left ventricular mass or other effects of athleticism on remodeling of the heart’s electrical system.4
One should consider repeating genetic testing in the proband if the original was conducted in a research lab particularly because more modern and sophisticated testing capabilities can increase the yield of testing5. A genetic confirmation would be important as LQTS genotype and mutation location allow for risk stratification, impact therapy and improve prognostic capabilities6, 7.
Evaluation should also include exercise testing. The QT during and after exercise may better characterize the phenotype in this gene-negative patient. Analysis of postural and exercise changes in the QT/RR relationship, can point to a phenotype possibly consistent with LQT1, LQT2 or LQT38–10.
An echocardiogram at least once in the life of this child is indicated to assess for secondary causes of QTc prolongation such as left ventricular hypertrophy, subclinical myopathy or congenital heart defect4.
A 12-lead Holter can identify dynamic changes in the QT interval and assess for unsuspected arrhythmias. In LQT3 patients, bradycardia or sinus pauses can increase risk; knowledge of the QT at these times is important 7. Cardiac events in LQTS are strongly associated with triggers linked to inappropriate QT adaptation to changes in heart rate.
Treatment
If, after thorough evaluation, the patient is believed to have LQTS, even if indeed genotype-negative LQTS, beta-blocker therapy is indicated unless there is a compelling reason against it (e.g. significant asthma). LQTS experts have long known that beta-blocker therapy reduces risk and events in LQTS11 including LQT312. Not all beta-blockers are equal and nadolol and propranolol are superior to metoprolol13, and atenolol should be avoided14. When initiating a beta-blocker in an asymptomatic patient, starting with a small dose and increasing gradually in combination with good hydration can improve tolerance. The goal beta-blocker dose is 2 −3 mg/kg/day of long-acting propranolol or divided three times a day in the short acting preparation, or 1–1.5 mg/kg/day of nadolol.
If a beta-blocker use is not possible a left cardiac sympathetic denervation (LCSD) can be considered as it has been found to be protective in high-risk patients with LQTS15. The child should undergo a yearly or twice a year evaluation with ECGs, exercise tests and possibly repeat Holter monitoring. There is lack of agreement about how often to repeat a Holter monitor. In patients incapable of performing an exercise test, annual Holter monitoring can be used to assess therapy efficacy and adherence. A Holter can be helpful if there are new symptoms. In LQT3 where events occur with rest or sleep, a Holter every 1–2 years can be used to assess for asymptomatic events. The patient should avoid medications that lengthen repolarization (www.crediblemeds.org) if possible.
Sports participation
The experts differ in their recommendations for sports participation. Some would allow full sports participation in this asymptomatic patient if compliant with therapy16, while others would limit the patient to mild-moderate exercise if this patient proves to have a LQT1 phenotype. Several experts would restrict competitive swimming and diving and voice concern about solitary exercise in a remote location. All feel that if sports are undertaken this should be under the guidance of a LQTS expert and the patient, family, and (if applicable) coach should agree about the participation. An automatic external defibrillator (AED) should be available during practices and games and the coach should know AED location and use. The patient should avoid increasing core body temperature as this can lengthen the QT17 and there should be a focus on excellent hydration.
Case 1 Summary: Aziz and Shoemaker
The patient presented in Case 1 is a genotype negative 12-year-old boy with no LQTS-related symptoms. The first “controversy” identified in the group relates to the veracity of the diagnosis of LQTS. The potential of left ventricular hypertrophy and its influence on prolonging repolarization should be appreciated in an athlete. Additionally, a thorough family history, exploration of additional phenotype (exercise stress testing, Holter monitoring, echocardiogram) and repeat genetic testing through commercial analysis are all reasonable means of clarifying the diagnosis of LQTS in this patient.
Once confirmed, experts reviewing the case shared consensus on treatment which would ultimately include beta-blockade (preferably nadolol or propranolol) and avoidance of medications that prolong the QTc. Sports participation was debated though the availability of an AED during sports was supported by all expert members.
Among the voting panel, near consensus existed for repeating genetic testing and treatment with a beta-blocker (Figure 1 and supplemental table I). Over 70% of voters would not escalate to LCSD or implantable cardioverter defibrillator (ICD) if the patient could not tolerate beta-blocker therapy. Sports participation was highly controversial, and responses were dependent on the assumption that beta-blocker therapy was tolerated. Most voters said shared-decision making was crucial with a nuanced discussion of the specific activity, its risks, and strategies for safe participation (e.g. bystanders, AEDs).
Gaps in knowledge here include management recommendations for genotype negative LQTS, sports participation/restriction, and a greater understanding of the clinical significance and management of patients with exercise-training induced QT prolongation.
Case 2 [Experts: Cerrone (section leader), Roberts, Sy, Viskin]
A 60-year-old active man struggling with depression is noted to have QT prolongation on an ECG following initiation of QT prolonging anti-depressant medications. His QTc is 570 ms and his resting heart rate 50 bpm. Genetic testing shows a disease-causing pathogenic variant in KCNQ1. He has a remote history of exertional syncope. His psychiatrist indicates multiple different anti-depressant medications have been tried unsuccessfully and insists that the current regimen be continued. Would you start a beta-blocker? Would you consider device implantation?
Response
A QTc > 550 ms occurs infrequently in LQT1, even among patients on QT-prolonging medications. Hence, it is important to first confirm that the QTc measurement is accurate; inclusion of the U-wave can lead to spuriously long values. It would also be useful to review any ECGs that preceded the initiation of QT-prolonging medications. Assuming the QTc value is accurate, it is probably his most important risk factor18, 19.
The 5-year risk of life-threatening arrhythmias in untreated LQT1 with QTc > 560 ms is estimated in the range of 5%, albeit not adjusted for age18. The history of exertional syncope is concerning, although the risk is time-dependent, being more significant for a recent event (<2 years ago) than if it was remote (>10 years ago)20. It would be important to clarify the nature of the presentation, timing and whether additional stressors were present.
Older LQTS patients are not well represented in registries, have a lower relative risk of sudden death due to LQTS compared to younger patients, and a higher risk of mortality from non-LQTS conditions; still, there remains an attenuated but still substantial risk of sudden death in patients > 40 years with LQTS and a prolonged QTc21. However, it is important to highlight that the presumed new onset prolongation of his QTc to > 550 ms secondary to medication has altered his arrhythmic substrate.
The presence of a pathogenic/likely pathogenic KCNQ1 variant identifies this patient as at lower risk compared to other genetic forms, once treatment is started. Genetic testing should be reviewed for the presence of common to intermediate prevalence QT predisposing variants, such as KCNE1-p.D85N, that are not always included in standard reports22. Cascade screening in family members is vitally important, and this patient’s benign life-course may not be the same as other affected family members.
His entire medication regimen and an electrolyte panel should be scrutinized for other reversible factors. It is imperative to highlight to both the patient and the psychiatrist that the anti-depressant has likely resulted in a problematic increase in his arrhythmic risk and, if at all possible, should be stopped or, at the very least, the doses reduced. Although the majority of antidepressants and antipsychotic drugs have been linked to QT prolongation, there are some agents that seem to have less or no effect on QT interval and the potential of switching to a safer agent should be discussed with the psychiatrist. The patient should meticulously avoid any other medications that prolong the QT interval (https://www.crediblemeds.org). One should also inform him about the effects of specific foodstuff (like grapefruit) on the QT interval and their potential proarrhythmic effects23.
Would you start a beta-blocker?
All panelists agree that beta-blocker is indicated in this case, because it is extremely effective in preventing arrhythmic events in patients with LQT1, and the benefits are likely maintained in older patients11, 18, 24. Nadolol is the first choice, starting with a low dose and then up-titrating to 1 mg/kg which should be reasonably well tolerated even considering the presence of resting bradycardia. The ‘maximal tolerated dose’ should be based on its impact on his quality of life rather than his absolute heart rate. Depression should not be viewed as a contraindication to beta-blocker therapy. Indeed, recent large-scale data that analyzed the risk of psychiatric adverse events linked to beta-blocker use suggest that depression does not occur more frequently in beta-blocker users compared to placebo25. Additionally, another study from the Danish population-based registry investigating the relation between hypertension and depression showed that beta-blocker therapy was associated with decreased rate of depression26. In case of severe side effects or poor compliance, one could try different beta-blockers and also inform him of the existence of LCSD as an alternative option.
Would you consider device implantation?
Three experts advised against device implantation despite the marked QT prolongation. Although a QTc >500 ms has been associated with increased risk of life-threatening arrhythmias in LQTS, beta-blockers are particularly effective for LQT1, to the point that even patients who present with cardiac arrest in the absence of therapy may be safely treated only with beta-blockers24, as long as they are compliant with the medication and avoid QT-prolonging drugs. The patient’s age, beta-blocker-naïve status and remote nature of previous syncope are mitigating factors27, 28. An ICD would be indicated if the patient experiences recurrent syncope on beta-blocker 27. The option of long-term monitoring with an implantable loop recorder (ILR) which could provide information of HR response to beta-blockers and adjudicate the underlying rhythm with symptoms, could be discussed if short-term monitoring is unrevealing.
One expert would consider an ICD, since there are limited data available for LQT1 patients with QTc values > 550 ms11, 18, 24, 29. Furthermore, among LQT1 patients who suffer breakthrough events despite beta-blockade, QT-prolonging medications are a known contributing factor24. Assuming his QTc values remained > 550 ms and his anti-depressant regimen could not be adjusted (which should only be considered acceptable in exceptional circumstances), one could offer invasive therapy and discuss both an LCSD and a dual chamber ICD, the latter of which would additionally allow for intentional atrial pacing for rate stabilization.
Case 2 Summary (Cutler and Roden)
This case raises several key questions: how does age at LQTS diagnosis impact sudden death risk? Does the magnitude of QT prolongation (>550 ms here) increase sudden death risk independent of age? How can we manage patients who have comorbid conditions or drug therapies that may also prolong QT?
The panelists observe that although the risk of sudden death in LQTS decreases with age, particularly in men, the severe QT prolongation (>550 ms) in this case was worrisome and the consensus was that at a minimum this patient should be treated with a non-selective beta-blocker. More advanced therapies would be considered if there is breakthrough syncope/ventricular arrhythmia on beta-blocker therapy. The need for any QT prolonging medications should be carefully evaluated and these should only be continued if alternatives are unavailable and the indication is compelling. There is a role for shared decision making in evaluating these risks and benefits.
Figure 2 shows the distribution of expert opinion from the entire expert panel regarding management decisions relevant to Case 2. There was overall agreement that this patient should be started on non-selective beta-blocker therapy at the initial patient visit. If a non-selective beta-blocker is not tolerated, 50% of experts would consider a selective beta-blocker vs 36% who would escalate management (e.g. to ICD or LCSD). Finally, there was again divergence of opinion regarding escalation of therapy if QT interval remained prolonged after initiation of beta-blocker therapy. Supplemental Table II provides a summary of expert comments relevant to Case 2.
Figure 2:
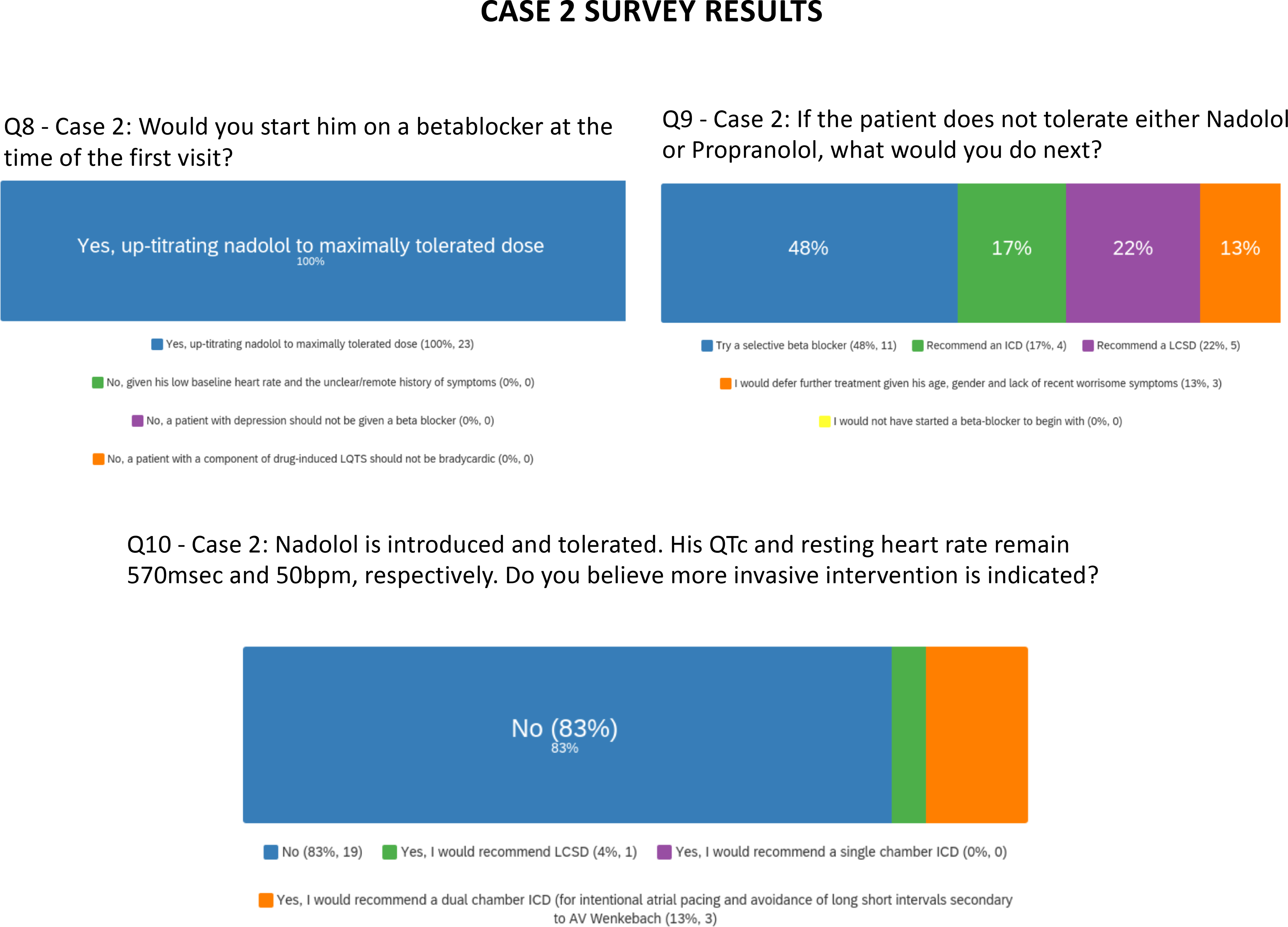
Survey results are presented for questions pertaining to case 2.
In conclusion, Case 2 highlights important knowledge gaps related to age-dependent sudden death risk, the modulating impact of the extent of QT prolongation, and the dilemma that arises when patients with LQTS need QT-prolonging drugs for other serious conditions.
Case 3 [Experts: Wilde (section leader), Lubitz, Krahn, Priori]
A 29-year-old woman is found to have LQT2 with QTc 500 ms on family screening. She has not had syncope. She is 10 weeks pregnant with her first child. She has mild chronic asthma for which she uses a rescue inhaler 1–2 times monthly. What testing and treatment would you offer at this time? Would you start a beta-blocker? Would you recommend LCSD? Would you recommend ICD?
Response
Evaluation
Risk stratification in LQTS is a sensitive issue and it is obviously important to avoid serious arrhythmias potentially leading to sudden cardiac death. In particular the QTc interval and the symptom status are of relevance. Family history, not provided for this case, seems unimportant. A recent study including over 1700 LQTS patients (551 with LQT2) aimed to create an evidence-based risk classification scheme to personalize the quantification of risk18. With the characteristics given (i.e. QTc of 500 ms, asymptomatic) her 5-year risk of a lethal arrhythmic event off therapy would be 4.5% (95% CI: 1.5–7.4). Pregnancy is not a high risk period for the mother, unlike the post-partum period that is high risk, especially for LQT2 women30.
Beta-blocker therapy and considerations for pregnancy
There is no doubt that beta-blocker therapy reduces her risk significantly, and that should be the first line of therapy to be initiated. Nadolol or propranolol, both non-selective beta-blockers, are the preferable beta-blockers13, 18. However, with her mild chronic asthma, nonselective beta-blockers might lead to unacceptable side effects. The experts diverge on treatment options: some favor starting with low-dose non-selective beta-blocker and advancing the dose as tolerated. Others would start with metoprolol which, similar to propranolol, has not been shown to be detrimental during pregnancy31, and which can be dosed to a higher level. In addition, asthma may worsen during the pregnancy, usually in the 3rd trimester. If worsening occurs within 4–6 weeks on a non-selective beta-blocker, switching to the beta-1 selective beta-blockers metoprolol or bisoprolol would be appropriate. Atenolol should not be used in LQTS and early reports suggested the possibility of atenolol-associated intrauterine growth retardation.31.
Further investigation ideally includes ambulatory cardiac rhythm monitoring, and on a discretionary basis an exercise test, to identifying any high-risk subclinical arrhythmias (non-sustained polymorphic ventricular tachycardia) and assess peak heart rate.
Potassium and magnesium levels should be measured and optimized if needed. Beta-blockers should be started as soon as possible, after the above investigations or immediately after a baseline ECG with evident QTc prolongation, and additional therapy is not needed at this point in time. A repeat exercise test to demonstrate reduction in peak heart rate is recommended, with potential titration of beta-blocker in the third trimester as the volume of distribution in the mother increases. In the months after birth, the risk for LQT2 women is increased, so that is an important period for strict medication adherence and symptom surveillance. In addition, beta-blockers are expressed in breast milk, so education and awareness of the effects and the very small risk of infant hypoglycemia is important in counselling and managing both mother and baby. QT-prolonging drugs should be avoided at any time during her further life (www.crediblemeds.org and related smartphone app).
Genetic counseling and evaluation of the baby
The patient should be informed that her child will have a 50% chance of inheriting the same LQT2 mutation. There is a small chance that he or she will develop a phenotype in utero or shortly after birth, which can be monitored with standard fetal assessment32. Maternal blood for circulating fetal cells can be tested to determine the gene status of the fetus, but this is typically deferred to the post-partum period. An umbilical cord blood sample could be stored in order to obtain the genotype of the child.
The risk for arrhythmias with hemodynamic compromise at labor and delivery for the mother is estimated to be at a moderate level, mandating additional surveillance including involvement of a pregnancy heart team with expertise in inherited arrhythmia syndromes with careful heart rhythm monitoring and some experts additionally prefer an intravenous line.
Case 3 Summary (Kaufman and Perez)
This case is challenging because the patient has high-risk features; LQT2 in a woman soon to enter the risky post-partum period, with QTc 500 ms but asymptomatic. The situation is further confounded by her diagnosis of asthma requiring intermittent use of a rescue inhaler. The published guidelines27, 33 recommend beta-blocker therapy if tolerated, with intensification of therapy to LSCD or ICD in “high-risk” patients when beta-blocker is poorly tolerated. The clinician is left to determine how high the risk is in an individual patient, and when the benefits of aggressive treatment outweigh the risks.
The primary commentators agreed on treating with non-selective beta-blocker, but if her asthma worsened, they would consider switching to either metoprolol or bisoprolol. Most would start treatment after baseline ambulatory monitoring and exercise testing, if these were timely. The commentators recommended special surveillance during delivery, did not mandate Caesarian section, and discussed the need for fetal monitoring and genetic counseling.
Among the voting panel, all recommended beta-blocker therapy, with 15 waiting until timely completion of appropriate baseline investigations and with 7 favoring medication initiation immediately after the 12-lead ECG. None of the experts recommended consideration of LSCD or ICD (Figure 3 and Supplemental Table III). While most (19) experts agreed on vaginal birth with close monitoring, 2 favored Caesarian section and one commented that plans for future pregnancies could be factored in. It is notable that data from multiple LQTS registries have not pointed to a high risk for the mother at the time of delivery.
Figure 3:
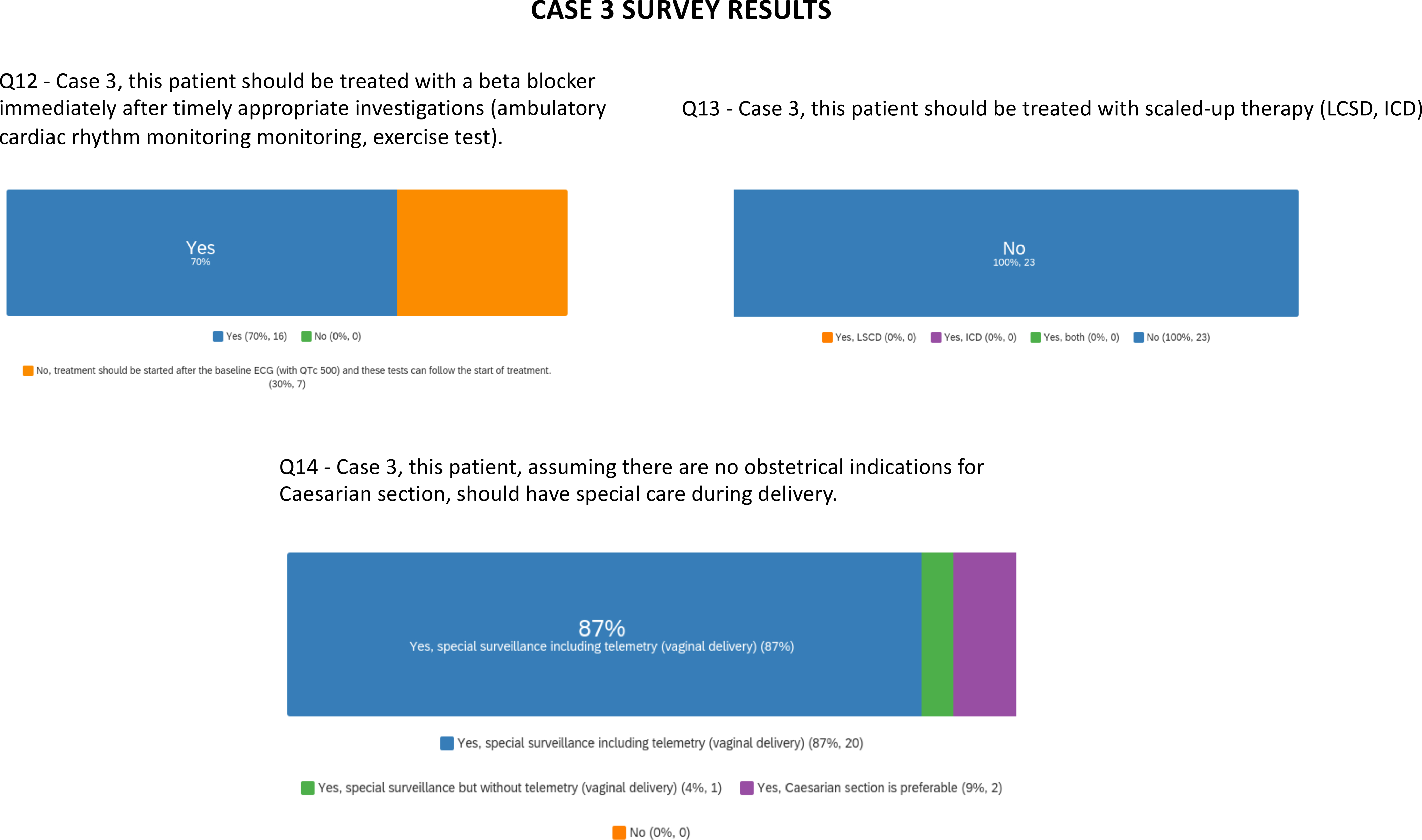
Survey results are presented for questions pertaining to case 3.
Case 3 highlights the complexity of managing LQTS in pregnancy. Gaps in knowledge include evidence for birth plan procedures and beta-blocker tolerability in pregnant persons.
Case 4: [Experts: Towbin (section leader), Behr, Schulze-Bahr, Zareba]
A 24-year-old male suffered a non-exertional near syncopal spell at age 15 years. His past medical history was notable only for intermittent asthma. His ECG at that time showed QTc 490 ms and he was diagnosed with LQTS. A disease-causing variant was identified in KCNH2 (i.e., LQT2). An electrophysiologist implanted a single chamber ICD but failed to start beta-blocker. The patient has never had syncope, repeat near syncope, nor appropriate shocks from his device. He is seeing you for the first time and requests ICD removal. His QTc today is 485 ms and he still has not taken a beta-blocker. What other data would you request? Would you start a beta-blocker? Would you consider device extraction?
Response
General considerations
This patient’s history has several important features including a single non-exertional near syncopal event at the age of 15 years but no history of any cardiovascular-related illness before or in the 9 years since the “event”. He has never had actual syncope or repeat near syncope and he has not had any appropriate shocks, even though he has not received beta-blocker therapy.
The experts agreed that this LQT2 patient with near syncope at the age of 15 years should have been treated with beta-blockers and there was no indication for an ICD. With only intermittent asthma, beta-blockers should be tried with caution as clinical practice indicates that such patients usually tolerate beta-blockers, which could be administered at a lower dose if needed. Furthermore, use of beta-blockers decreases the risk of cardiac events that could be augmented by beta-2 agonists34, 35. There is a significant difference between the risks of cardiac events between LQT2 males and females after the age of 15 years with females showing over 3-fold higher risk of cardiac events and 2.5-fold higher risk of cardiac arrest or sudden cardiac death than males6, 36, 37. Incremental risk in adult LQT2 males over the lifespan is very low especially with QTc below 500 ms37.
Additional Data
Additional data would help the experts to evaluate this case, including what the specific pathogenic variant in KCNH2 is and where it is located, as some variants and regions (pore region) of the channel are associated with more severe disease and worse outcomes36.
In addition, they would like to know what the T-wave morphology looked like at rest as notching or flattened T-waves are associated with more aggressive forms of LQTS10, 38–40, and further, how the T-wave responds to exercise (normalization is a good prognostic indicator)10.
Complete phenotyping information would be useful, including resting and exercise-associated QTc and the QTc change, and whether there are PVCs.
Finally, they would want to know the family history as well as the working and living environment to determine if there are potential triggers for arrhythmia initiation, like loud noise. They wish to know the severity of the asthma, whether brittle or not, and if this has resolved as he has become older. They would inquire about a family history with cascade screening (if not done before), although family history is of less significance than patient’s personal history of cardiac events41.
Beta-blocker
The panel of experts agreed that they would initiate beta-blocker therapy while he still has a functional ICD42. Recognizing that he has a history of asthma, they would follow him closely for respiratory symptoms. He should take the beta-blocker just before going to bed (as late as possible to avoid the potential of arousal in the AM after awakening and avoiding a triggered arrhythmia). They would use nadolol first or bisoprolol as second line (especially if the asthma is still active)13, 43, 44. Long-acting metoprolol twice daily could be a possible second-line therapy although limitations of metoprolol are acknowledged13, 44. If none of these are tolerated, mexiletine would be a potential therapy45. Efficacy would be assessed by limitation of maximal heart rate with exercise, and QTc shortening at rest or during exercise. In addition, excessive bradycardia, defined by age46, would result in discontinuation of the beta-blocker.
Device Extraction Considerations
The panel agreed to consider device extraction if the medical therapy demonstrated efficacy and no untoward side effects developed. They would strongly recommend avoidance of QT prolonging medications/beta-mimetics. In fact, the patient did not appear to meet ICD implant criteria initially47. Near syncope with QTc < 550 ms, but especially < 500 ms, is not an indication for an ICD, especially without beta-blocker therapy. Syncope on beta-blocker therapy could qualify this patient even with his QTc of 485 ms.
If medical therapy was not tolerated or was not efficacious but the patient still wanted the ICD extracted following careful counselling about the risks of extraction, then this would be his choice and some experts found this acceptable.
One expert suggested possible LCSD with suitable counseling, particularly if there were other markers suggestive of risk as described above, e.g. pore-localizing variant, late-coupled ventricular ectopic complexes during exercise ECG testing, or follow-up QTc measurements exceeded 500 ms and/or syncope. However, the other 3 experts did not perceive an indication for LCSD48.
Case 4 Summary (Chung and Eckhardt)
Controversial issues in this case include: 1) whether a patient without LQTS-associated symptoms, with asthma and LQT2 should be started on a beta-blocker, if so which one; 2) if the ICD was or remains indicated, and 3) whether the ICD should be extracted.
Experts reviewing the case agreed that beta-blockers should be started (with some variance in the type and timing) and that he does not have clear indications for ICD implantation by today’s guidelines and consensus statements. A male age 15–40 years with QTc <500 ms and no prior syncope is a lower risk patient particularly because he was beta-blocker naïve at implant. Case reviewers also thought that information on the specific mutation and phenotyping might be useful, including T wave morphology, response to exercise, and family history.
Within the entire expert panel participating in the voting questions, almost all agreed that a non-selective beta-blocker was preferred, one would give bisoprolol and one would not give the beta-blocker (Figure 4 and Supplemental Table IV). If he could not take a beta-blocker, the group diverged in opinion on use of mexiletine and in offering LCSD. Device extraction responses were nuanced in both the reviewing panel and voting panel and largely qualified with additional comments, highlighting the role of shared decision making and balancing risks of extraction.
Gaps in knowledge here include the lack of randomized trials of beta-blockers, mexiletine, prophylactic LCSD, and ICDs in LQTS with reliance on retrospective observational studies. Likewise risks of retained unnecessary hardware vs. extraction in young patients is unclear.
Conclusions
Expert consensus statements provide guidelines for treatment of patients with LQTS. However, the clinician must apply these guidelines with care and good judgement to avoid over- or under-treating patients. The initial evaluation is critical for establishing a diagnosis and assessing individual risk. Once the diagnosis (and more than trivial risk) has been established with confidence, most patients will benefit from beta-blocker therapy, with careful selection of particular beta-blocker and dose. Although it is important to counsel against use of QT-prolonging medications, it is not infrequent for LQTS patients to have other serious illness with indication for use of QT-prolonging agents; in such patients, therapy must be individualized to optimize outcomes. Invasive therapies such as LCSD or ICD implantation are indicated in the rare patient with substantial risk despite beta-blocker therapy and/or with significant contraindication to its use.
Supplementary Material
Disclosures:
Dr. Eckhardt is supported by NIH R01 HL139738-01 and R01 HL128598-01; she is funded in part for this project by the Gary and Marie Weiner Professor in Cardiovascular Medicine Research. Dr. Ackerman is supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program. Dr. Lubitz is supported by NIH grant 1R01HL139731 and American Heart Association 18SFRN34250007. Dr. Schwartz is partially supported by the Italian Ministry of Health grant RF-2016-02361501
Non-standard abbreviations and acronyms:
- LQTS
long QT syndrome
- LCSD
left cardiac sympathetic denervation
- AED
automatic external defibrillator
- ICD
implantable cardioverter defibrillator
Footnotes
Supplemental Material:
References:
- 1.Taggart NW, Haglund CM, Tester DJ, Ackerman MJ. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115:2613–20. [DOI] [PubMed] [Google Scholar]
- 2.Dagradi F, Spazzolini C, Castelletti S, Pedrazzini M, Kotta MC, Crotti L, Schwartz PJ. Exercise Training-Induced Repolarization Abnormalities Masquerading as Congenital Long QT Syndrome. Circulation. 2020;142:2405–2415. [DOI] [PubMed] [Google Scholar]
- 3.Basavarajaiah S, Wilson M, Whyte G, Shah A, Behr E, Sharma S. Prevalence and significance of an isolated long QT interval in elite athletes. Eur Heart J. 2007;28:2944–9. [DOI] [PubMed] [Google Scholar]
- 4.Tanriverdi H, Kaftan HA, Evrengul H, Dursunoglu D, Turgut G, Kilic M. QT dispersion and left ventricular hypertrophy in athletes: relationship with angiotensin-converting enzyme I/D polymorphism. Acta Cardiol. 2005;60:387–93. [DOI] [PubMed] [Google Scholar]
- 5.Medlock MM, Tester DJ, Will ML, Bos JM, Ackerman MJ. Repeat long QT syndrome genetic testing of phenotype-positive cases: prevalence and etiology of detection misses. Heart Rhythm. 2012;9:1977–82. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu W, Moss AJ, Wilde AA, Towbin JA, Ackerman MJ, January CT, Tester DJ, Zareba W, Robinson JL, Qi M, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. [DOI] [PubMed] [Google Scholar]
- 8.Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol. 2010;3:120–5. [DOI] [PubMed] [Google Scholar]
- 9.Adler A, van der Werf C, Postema PG, Rosso R, Bhuiyan ZA, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Halkin A, et al. The phenomenon of “QT stunning”: the abnormal QT prolongation provoked by standing persists even as the heart rate returns to normal in patients with long QT syndrome. Heart Rhythm. 2012;9:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S, Horie M. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838–44. [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–23. [DOI] [PubMed] [Google Scholar]
- 12.Wilde AA, Moss AJ, Kaufman ES, Shimizu W, Peterson DR, Benhorin J, Lopes C, Towbin JA, Spazzolini C, Crotti L, et al. Clinical Aspects of Type 3 Long-QT Syndrome: An International Multicenter Study. Circulation. 2016;134:872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chockalingam P, Crotti L, Girardengo G, Johnson JN, Harris KM, van der Heijden JF, Hauer RN, Beckmann BM, Spazzolini C, Rordorf R, et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: higher recurrence of events under metoprolol. J Am Coll Cardiol. 2012;60:2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackerman MJ, Priori SG, Dubin AM, Kowey P, Linker NJ, Slotwiner D, Triedman J, Van Hare GF, Gold MR. Beta-blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: Are all beta-blockers equivalent? Heart Rhythm. 2017;14:e41–e44. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–33. [DOI] [PubMed] [Google Scholar]
- 16.Ackerman MJ, Zipes DP, Kovacs RJ, Maron BJ. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 10: The Cardiac Channelopathies: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2424–2428. [DOI] [PubMed] [Google Scholar]
- 17.Amin AS, Herfst LJ, Delisle BP, Klemens CA, Rook MB, Bezzina CR, Underkofler HA, Holzem KM, Ruijter JM, Tan HL, et al. Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome. J Clin Invest. 2008;118:2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzanti A, Maragna R, Vacanti G, Monteforte N, Bloise R, Marino M, Braghieri L, Gambelli P, Memmi M, Pagan E, et al. Interplay Between Genetic Substrate, QTc Duration, and Arrhythmia Risk in Patients With Long QT Syndrome. J Am Coll Cardiol. 2018;71:1663–1671. [DOI] [PubMed] [Google Scholar]
- 19.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs JB, Peterson DR, Moss AJ, McNitt S, Zareba W, Goldenberg I, Qi M, Robinson JL, Sauer AJ, Ackerman MJ, et al. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. Jama. 2006;296:1249–54. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg I, Moss AJ, Bradley J, Polonsky S, Peterson DR, McNitt S, Zareba W, Andrews ML, Robinson JL, Ackerman MJ, et al. Long-QT syndrome after age 40. Circulation. 2008;117:2192–201. [DOI] [PubMed] [Google Scholar]
- 22.Giudicessi JR, Roden DM, Wilde AAM, Ackerman MJ. Classification and Reporting of Potentially Proarrhythmic Common Genetic Variation in Long QT Syndrome Genetic Testing. Circulation. 2018;137:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chorin E, Hochstadt A, Granot Y, Khoury S, Schwartz AL, Margolis G, Barashi R, Viskin D, Ghantous E, Schnapper M, et al. Grapefruit juice prolongs the QT interval of healthy volunteers and patients with long QT syndrome. Heart Rhythm. 2019;16:1141–1148. [DOI] [PubMed] [Google Scholar]
- 24.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, Crotti L, Piippo K, Lupoglazoff JM, Villain E, et al. High efficacy of beta-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures”. Circulation. 2009;119:215–21. [DOI] [PubMed] [Google Scholar]
- 25.Riemer TG, Villagomez Fuentes LE, Algharably EAE, Schafer MS, Mangelsen E, Furtig MA, Bittner N, Bar A, Zaidi Touis L, Wachtell K, et al. Do beta-Blockers Cause Depression?: Systematic Review and Meta-Analysis of Psychiatric Adverse Events During beta-Blocker Therapy. Hypertension. 2021:HYPERTENSIONAHA12016590. [DOI] [PubMed] [Google Scholar]
- 26.Kessing LV, Rytgaard HC, Ekstrom CT, Torp-Pedersen C, Berk M, Gerds TA. Antihypertensive Drugs and Risk of Depression: A Nationwide Population-Based Study. Hypertension. 2020;76:1263–1279. [DOI] [PubMed] [Google Scholar]
- 27.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, Gasparini M, Wilde AA, Knops RE, Denjoy I, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–82. [DOI] [PubMed] [Google Scholar]
- 29.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. Jama. 2004;292:1341–4. [DOI] [PubMed] [Google Scholar]
- 30.Khositseth A, Tester DJ, Will ML, Bell CM, Ackerman MJ. Identification of a common genetic substrate underlying postpartum cardiac events in congenital long QT syndrome. Heart Rhythm. 2004;1:60–4. [DOI] [PubMed] [Google Scholar]
- 31.Roston TM, van der Werf C, Cheung CC, Grewal J, Davies B, Wilde AAM, Krahn AD. Caring for the pregnant woman with an inherited arrhythmia syndrome. Heart Rhythm. 2020;17:341–348. [DOI] [PubMed] [Google Scholar]
- 32.Cuneo BF, Kaizer AM, Clur SA, Swan H, Herberg U, Winbo A, Rydberg A, Haugaa K, Etheridge S, Ackerman MJ, et al. Mothers with long QT syndrome are at increased risk for fetal death: findings from a multicenter international study. Am J Obstet Gynecol. 2020;222:263 e1–263 e11. [DOI] [PubMed] [Google Scholar]
- 33.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 34.Rosero SZ, Zareba W, Moss AJ, Robinson JL, Hajj Ali RH, Locati EH, Benhorin J, Andrews ML. Asthma and the risk of cardiac events in the Long QT syndrome. Long QT Syndrome Investigative Group. Am J Cardiol. 1999;84:1406–11. [DOI] [PubMed] [Google Scholar]
- 35.Thottathil P, Acharya J, Moss AJ, Jons C, McNitt S, Goldenberg I, Zareba W, Kaufman E, Qi M, Robinson JL, et al. Risk of cardiac events in patients with asthma and long-QT syndrome treated with beta(2) agonists. Am J Cardiol. 2008;102:871–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, et al. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–9. [DOI] [PubMed] [Google Scholar]
- 37.Kutyifa V, Daimee UA, McNitt S, Polonsky B, Lowenstein C, Cutter K, Lopes C, Zareba W, Moss AJ. Clinical aspects of the three major genetic forms of long QT syndrome (LQT1, LQT2, LQT3). Ann Noninvasive Electrocardiol. 2018;23:e12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JL, Schwartz PJ, Towbin JA, Vincent GM, Lehmann MH. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation. 1995;92:2929–34. [DOI] [PubMed] [Google Scholar]
- 39.Platonov PG, McNitt S, Polonsky B, Rosero SZ, Kutyifa V, Huang A, Moss AJ, Zareba W. Risk Stratification of Type 2 Long-QT Syndrome Mutation Carriers With Normal QTc Interval: The Value of Sex, T-Wave Morphology, and Mutation Type. Circ Arrhythm Electrophysiol. 2018;11:e005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaglio M, Couderc JP, McNitt S, Xia X, Moss AJ, Zareba W. A quantitative assessment of T-wave morphology in LQT1, LQT2, and healthy individuals based on Holter recording technology. Heart Rhythm. 2008;5:11–8. [DOI] [PubMed] [Google Scholar]
- 41.Kimbrough J, Moss AJ, Zareba W, Robinson JL, Hall WJ, Benhorin J, Locati EH, Medina A, Napolitano C, Priori S, et al. Clinical implications for affected parents and siblings of probands with long-QT syndrome. Circulation. 2001;104:557–62. [DOI] [PubMed] [Google Scholar]
- 42.Goldenberg I, Bradley J, Moss A, McNitt S, Polonsky S, Robinson JL, Andrews M, Zareba W, International LRI. Beta-blocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: implications for patient management. J Cardiovasc Electrophysiol. 2010;21:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu-Zeitone A, Peterson DR, Polonsky B, McNitt S, Moss AJ. Efficacy of different beta-blockers in the treatment of long QT syndrome. J Am Coll Cardiol. 2014;64:1352–8. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg C, Padfield GJ, Al-Sabeq B, Adler A, Yeung-Lai-Wah JA, Kerr CR, Deyell MW, Andrade JG, Bennett MT, Yee R, et al. Experience with bisoprolol in long-QT1 and long-QT2 syndrome. J Interv Card Electrophysiol. 2016;47:163–170. [DOI] [PubMed] [Google Scholar]
- 45.Bos JM, Crotti L, Rohatgi RK, Castelletti S, Dagradi F, Schwartz PJ, Ackerman MJ. Mexiletine Shortens the QT Interval in Patients With Potassium Channel-Mediated Type 2 Long QT Syndrome. Circ Arrhythm Electrophysiol. 2019;12:e007280. [DOI] [PubMed] [Google Scholar]
- 46.Zareba W, Moss AJ, le Cessie S, Locati EH, Robinson JL, Hall WJ, Andrews ML. Risk of cardiac events in family members of patients with long QT syndrome. J Am Coll Cardiol. 1995;26:1685–91. [DOI] [PubMed] [Google Scholar]
- 47.Biton Y, Rosero S, Moss AJ, Goldenberg I, Kutyifa V, McNitt S, Polonsky B, Baman JR, Zareba W. Primary prevention with the implantable cardioverter-defibrillator in high-risk long-QT syndrome patients. Europace. 2019;21:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dusi V, De Ferrari GM, Pugliese L, Schwartz PJ. Cardiac Sympathetic Denervation in Channelopathies. Front Cardiovasc Med. 2019;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


