Dear Editor,
In 2021, a global pandemic caused by a novel coronavirus (COVID-19) continues to be a major health threat. In the United States, nearly 33.5 million people have tested positive for COVID-19 and over 602,400 patients have died from complications related to COVID-19 as of early July 2021 [1]. The pandemic crisis is stressing the healthcare systems creating unprecedented challenges in providing timely oncologic care. Multiple studies have demonstrated that COVID-19 has resulted in delayed cancer care [2, 3].
A recent high-quality meta-analysis concluded that cancer treatment delay is associated with increased mortality in various malignancies, but valid data on cervical cancer remain scant [4]. Given that the majority of women with early-stage cervical cancer are treated surgically with hysterectomy, we examined the association between hysterectomy wait-time and oncologic outcomes for women with micro-invasive cervical cancer.
This retrospective observational study examined women with stage IA squamous, adenocarcinoma, and adenosquamous carcinomas of the uterine cervix diagnosed from 2004 to 2015 in the National Cancer Database. All women underwent primary hysterectomy. Cases with no wait-time were excluded due to the assumption of occult malignancy. Associations between surgical wait-time, defined as time interval from cancer diagnosis to hysterectomy, and oncologic outcomes including surgical-pathological factors (pathological parametrical invasion, nodal metastasis, and lympho-vascular space invasion) and all-cause mortality were examined [5].
A generalized linear regression model was used to assess the association between wait-time and pathologic characteristics. Binary logistic regression and Cox proportional hazards regression models with restricted cubic spline transformation of surgery wait-time were used to assess the non-linear associations between outcome measures, adjusting for other patient and tumor characteristics. The Columbia University Institutional Review Board deemed exempted this study due to the use of publicly available data.
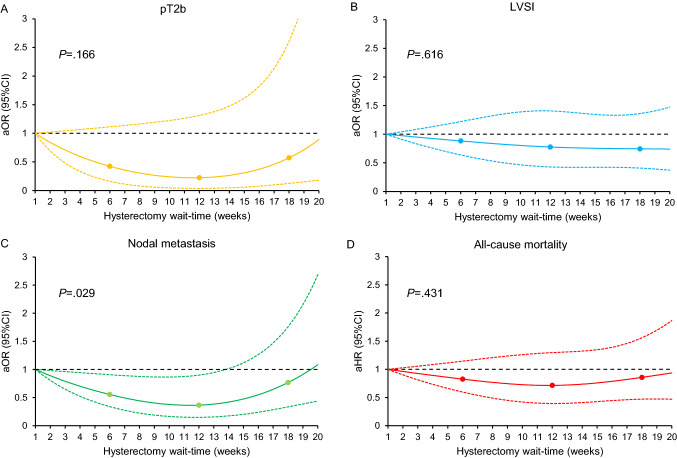
A total of 2732 women were examined. The median age was 43 (IQR 36–52) years. Squamous histology (n = 1792, 65.5%) and stage IA1 disease (n = 1185, 43.4%) were the most frequent tumor characteristics. The median hysterectomy wait-time was 6 (IQR 4–9) weeks. Non-Hispanic Black and Hispanic patients, and uninsured and Medicaid insurance were independently associated with longer hysterectomy wait-time in multivariable analysis (all, P < 0.001; Table 1). Longer hysterectomy wait-time was not associated with increased risks of pathological parametrial involvement, regional lymph node metastasis, or lympho-vascular space invasion (Fig. 1A–C). The median follow-up was 4.5 (IQR 2.2–7.2) years, and 136 (5.0%) deaths occurred. Longer hysterectomy wait-time was not associated with all-cause mortality risk (P = 0.431; Fig. 1D).
Table 1.
Multivariable model for hysterectomy wait-time in micro-invasive cervical cancer
| No. (%) | Mean (SD) | Estimated parameters (beta) (95% CI)§ | |
|---|---|---|---|
| No. patients | 2732 (100.0) | ||
| Age | |||
| < 40 | 1018 (37.3) | 7.2 (4.9) | Referent |
| 40–49 | 907 (33.2) | 7.3 (4.6) | 0.29 (− 0.84, 1.41) |
| 50–59 | 458 (16.8) | 7.9 (5.1) | 0.76 (− 0.39, 1.92) |
| 60–69 | 260 (9.5) | 7.9 (5) | 0.87 (− 0.38, 2.11) |
| 70–79 | 70 (2.6) | 7.2 (4.6) | 0.13 (− 1.56, 1.83) |
| ≥ 80 | 19 (0.7) | 5.1 (2.8) | − 1.74 (− 4.17, 0.68) |
| Race/ethnicity | |||
| Non-Hispanic: White | 1803 (66.0) | 6.8 (4.5) | Referent |
| Non-Hispanic: Black | 305 (11.2) | 8.7 (5.5) | 1.21 (0.60, 1.81)** |
| Hispanic | 356 (13.0) | 9.2 (5.5) | 1.23 (0.64, 1.82)** |
| Non-Hispanic: Other | 109 (4.0) | 8.0 (4.7) | 0.58 (− 0.34, 1.50) |
| Unknown | 159 (5.8) | 7.2 (4.9) | 0.49 (− 0.27, 1.25) |
| Insurance status | |||
| Not insured | 176 (6.4) | 9.5 (5.9) | 2.09 (1.33, 2.84)** |
| Private | 1656 (60.6) | 6.7 (4.3) | Referent |
| Medicaid | 539 (19.7) | 8.7 (5.6) | 1.49 (1.01, 1.97)** |
| Medicare | 240 (8.8) | 7.4 (4.9) | 0.52 (-0.28, 1.32) |
| Other Government | 44 (1.6) | 7.2 (4.5) | 0.53 (-0.86, 1.93) |
| Unknown | 77 (2.8) | 8.6 (4.8) | 1.58 (0.50, 2.67)* |
| Neighborhood average household income | |||
| < $40,227 | 583 (21.3) | 8.3 (5.2) | Referent |
| $40,227—$50,353 | 640 (23.4) | 7.3 (4.6) | − 0.47 (− 1.03, 0.09) |
| $50,354—$63,332 | 636 (23.3) | 7.0 (4.7) | − 0.65 (− 1.26, − 0.04)* |
| ≥ $63,333 | 837 (30.6) | 7.1 (4.8) | − 0.29 (− 0.99, 0.41) |
| Not available | 36 (1.3) | 8.9 (5.5) | 3.92 (− 0.22, 8.05) |
| Neighborhood education level | |||
| ≤ 17.6% | 702 (25.7) | 8.4 (5.3) | Referent |
| 10.9%–17.5% | 753 (27.6) | 7.4 (4.8) | − 0.12 (− 0.65, 0.41) |
| 6.3%–10.8% | 708 (25.9) | 6.8 (4.5) | − 0.48 (− 1.10, 0.13) |
| < 6.3% | 538 (19.7) | 6.7 (4.5) | − 0.66 (− 1.38, 0.07) |
| Not available | 31 (1.1) | 8.7 (5.4) | − 4.20 (− 8.67, 0.28) |
| Urban/Rural | |||
| Metropolitan | 2,227 (81.5) | 7.5 (4.9) | Referent |
| Urban | 384 (14.1) | 6.8 (4.3) | − 0.59 (− 1.14, − 0.05)* |
| Rural | 46 (1.7) | 6.3 (4.6) | − 0.87 (− 2.25, 0.50) |
| Unknown | 75 (2.7) | 8.5 (5.6) | 0.99 (− 0.12, 2.11) |
| Charlson/Deyo comorbidity | |||
| 0 | 2,397 (87.7) | 7.4 (4.8) | Referent |
| 1 | 285 (10.4) | 7.4 (4.8) | − 0.37 (− 0.95, 0.21) |
| 2 | 50 (1.8) | 8.1 (5.6) | − 0.02 (− 1.35, 1.31) |
| Year of diagnosis | |||
| 2004 | 139 (5.1) | 7.8 (5.3) | Referent |
| 2005 | 170 (6.2) | 7.8 (5.3) | 0.14 (− 0.91, 1.18) |
| 2006 | 177 (6.5) | 7.1 (4.6) | − 0.52 (-1.56, 0.51) |
| 2007 | 182 (6.7) | 7.5 (4.9) | − 0.13 (− 1.16, 0.89) |
| 2008 | 224 (8.2) | 7.2 (4.7) | − 0.33 (− 1.32, 0.65) |
| 2009 | 284 (10.4) | 7.7 (4.7) | 0.08 (− 0.87, 1.02) |
| 2010 | 261 (9.6) | 7.0 (4.7) | − 0.60 (− 1.57, 0.37) |
| 2011 | 251 (9.2) | 7.3 (4.8) | − 0.27 (− 1.24, 0.71) |
| 2012 | 238 (8.7) | 7.6 (5.2) | − 0.16 (− 1.14, 0.83) |
| 2013 | 256 (9.4) | 7.3 (4.7) | − 0.33 (− 1.30, 0.65) |
| 2014 | 286 (10.5) | 7.1 (4.7) | − 0.49 (− 1.44, 0.47) |
| 2015 | 264 (9.7) | 7.4 (4.9) | − 0.11 (− 1.08, 0.87) |
| Histology | |||
| Squamous cell | 1,792 (65.6) | 7.6 (5) | Referent |
| Adenocarcinoma | 873 (32.0) | 6.9 (4.5) | − 0.23 (− 0.63, 0.18) |
| Adenosquamous | 67 (2.5) | 7.0 (5.4) | − 0.36 (− 1.51, 0.78) |
| Clinical Stage IA | |||
| IA1 | 1185 (43.4) | 7.4 (4.8) | Referent |
| IA2 | 449 (16.4) | 7.4 (4.9) | 0.16 (− 0.35, 0.68) |
| IA NOS | 1098 (40.2) | 7.3 (4.9) | 0.01 (− 0.39, 0.41) |
| Grade | |||
| Well | 595 (21.8) | 7.4 (4.8) | Referent |
| Moderate | 822 (30.1) | 7.3 (4.7) | − 0.44 (− 0.94, 0.07) |
| Poorly | 323 (11.8) | 7.2 (4.9) | − 0.64 (− 1.29, 0.01) |
| Unknown | 992 (36.3) | 7.6 (5) | − 0.20 (− 0.68, 0.29) |
| Facility location | |||
| Eastern | 344 (12.6) | 7.8 (4.9) | Referent |
| South | 449 (16.4) | 7.0 (4.4) | − 0.64 (− 1.31, 0.04) |
| Midwest | 647 (23.7) | 7.1 (4.6) | − 0.67 (− 1.30, − 0.05)* |
| West | 274 (10.0) | 8.8 (5.5) | 0.75 (− 0.01, 1.50) |
| Unknown | 1018 (37.3) | 7.2 (4.9) | Non-estimated |
| Facility type | |||
| Community cancer program | 88 (3.2) | 7.6 (4.2) | Referent |
| Comprehensive community cancer program | 582 (21.3) | 6.7 (4.5) | − 0.31 (− 1.36, 0.75) |
| Academic/research program | 863 (31.6) | 8.2 (5.1) | 0.63 (− 0.40, 1.65) |
| Integrated network cancer program | 181 (6.6) | 6.7 (4.1) | − 0.27 (− 1.47, 0.93) |
| Other or unknown | 1018 (37.3) | 7.2 (4.9) | Non-estimated |
No number; SD, standard deviation; CI confidence interval; and NOS not otherwise specified
Mean wait-time (weeks) from cervical cancer diagnosis to hysterectomy is shown
§Estimated parameters (beta) from generalized linear regression model. *P < 0.05, **P < 0.001. Due to the collinearity between age < 40, facility location and type unknown categories, betas were non-estimated for facility location and type unknown categories
Fig. 1.
Associations between hysterectomy wait-time and oncologic outcomes and all-cause mortality (adjusted model). A total of 2,732 women with clinical stage IA cervical cancer who had primary hysterectomy were examined. Adjusted-odds ratio for pathological stage T2b (A), LVSI (B), and nodal metastasis (C), and adjusted-hazard ratio for all-cause mortality (D) are shown by week of hysterectomy wait-time. Waiting time was coded using restricted cubic spline transformation with clinically relevant cut-points at 6, 12, and 18 weeks. The Y-axis represents the effect size (adjusted-odds ratio or adjusted-hazard ratio). The X-axis represents the wait-time (week) from cervical cancer diagnosis to surgical treatment with hysterectomy. Week 1 is set as the reference. The solid line represents the estimate as adjusted-effect size. The dashed lines are corresponding 95% confidence interval. Three dots represent the knots. P values indicate the overall associations. For the surgical-pathological factors, adjusting factors were age, year, race/ethnicity, insurance status, average neighborhood household income, average neighborhood education level, year of diagnosis, comorbidity score, urban/rural type, histology type, tumor differentiation, stage, and hospital factors (location and setting). For all-cause mortality, lympho-vascular space invasion, pathological parametrial tumor involvement, and lymph node metastasis were additionally included as covariates in the multivariable Cox proportional hazard regression model
The observed result with absence of association between hysterectomy wait-time and mortality risk is somehow reassuring. Notably, our results for micro-invasive cervical cancer differ from data for stage IB tumors in which longer wait times to hysterectomy are associated with increased mortality [5]. Our findings may be due, at least in part, to the favorable prognosis for micro-invasive cervical cancer. Important limitations in this study included missing information on underlying reason of hysterectomy delay, comorbidities, occult cancer diagnosis, and use of excisional biopsy prior to hysterectomy.
As there are few data to describe the survival effect of delay in hysterectomy in cervical cancer [4, 6], our analysis of stage IA tumors provides valuable information in the management of women with early-stage cervical cancer and suggests that recommendations by recent expert panels to postpone hysterectomy for 6–8 weeks among patients with early-stage cervical cancer in centers or regions with a high burden of COVID-19 disease are reasonable for stage IA disease and do not adversely impact survival [7].
Author contributions
KM: Conceptualization, funding acquisition, investigation, methodology, project administration, writing (original draft). YH: Investigation, data collection, analysis, validation, manuscript editing. SM: Investigation, interpretation, manuscript reviewing and editing. RR Deshpande: Investigation, interpretation, manuscript reviewing and editing. MK: Investigation, interpretation, manuscript reviewing and editing. LDR: Investigation, interpretation, manuscript reviewing and editing. JDW: Conceptualization, investigation, methodology, project administration, manuscript supervision, reviewing editing.
Funding
Ensign Endowment for Gynecologic Cancer Research (K.M.). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Availability
The data on which this study is based are publicly available upon request at https://www.facs.org/quality-programs/cancer/ncdb.
Declarations
Conflict of interest
The followings are outside this work: Consultant, Clovis Oncology, and research funding, Merck, royalties, UpToDate (J.D.W.); honorarium, Chugai, textbook editorial expense, Springer, investigator meeting attendance expense, VBL Therapeutics (K.M.); none for others.
Transparency
The manuscript’s corresponding author (J.D.W.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. National Cancer Database is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The program is the source of the de-identified data used; and the program has not verified and is not responsible for the statistical validity of the data analysis or the conclusions derived by the study team.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United States COVID-19 Cases and Deaths by State. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days (Accessed 7/2/2021).
- 2.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jazieh AR, Akbulut H, Curigliano G, et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020;6:1428–1438. doi: 10.1200/GO.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Huang Y, Matsuzaki S, Klar M, Wright JD. Effect of delay in surgical therapy for early-stage cervical cancer: an implication in the coronavirus pandemic. Eur J Cancer. 2020;139:173–176. doi: 10.1016/j.ejca.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo K, Novatt H, Matsuzaki S, et al. Wait-time for hysterectomy and survival of women with early-stage cervical cancer: a clinical implication during the coronavirus pandemic. Gynecol Oncol. 2020;158:37–43. doi: 10.1016/j.ygyno.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez PT, Chiva L, Eriksson AGZ, et al. COVID-19 global pandemic: options for management of gynecologic cancers. Int J Gynecol Cancer. 2020;30:561–563. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on which this study is based are publicly available upon request at https://www.facs.org/quality-programs/cancer/ncdb.