Abstract
The guidance cue receptor DCC controls mesocortical dopamine development in adolescence. Repeated exposure to an amphetamine regimen of 4 mg/kg during early adolescence induces, in male mice, downregulation of DCC expression in dopamine neurons by recruiting the Dcc microRNA repressor, microRNA-218 (miR-218). This adolescent amphetamine regimen also disrupts mesocortical dopamine connectivity and behavioral control in adulthood. Whether low doses of amphetamine in adolescence induce similar molecular and developmental effects needs to be established. Here, we quantified plasma amphetamine concentrations in early adolescent mice following a 4 or 0.5 mg/kg dose and found peak levels corresponding to those seen in humans following recreational and therapeutic settings, respectively. In contrast to the high doses, the low amphetamine regimen does not alter Dcc mRNA or miR-218 expression; instead, it upregulates DCC protein levels. Furthermore, high, but not low, drug doses downregulate the expression of the DCC receptor ligand, Netrin-1, in the nucleus accumbens and prefrontal cortex. Exposure to the low-dose regimen did not alter the expanse of mesocortical dopamine axons or their number/density of presynaptic sites in adulthood. Strikingly, adolescent exposure to the low-dose drug regimen does not impair behavioral inhibition in adulthood; instead, it induces an overall increase in performance in a go/no-go task. These results show that developmental consequences of exposure to therapeutic- versus abused-like doses of amphetamine in adolescence have dissimilar molecular signatures and opposite behavioral effects. These findings have important clinical relevance since amphetamines are widely used for therapeutic purposes in youth.
Keywords: cognitive control, guidance cues, Netrin-1, Prefrontal cortex
1 ∣. INTRODUCTION
Adolescence represents a key neurodevelopmental stage when environmental factors can have a strong influence over ongoing structural, molecular, and neurochemical changes.1 In rodents, the adolescent growth of dopamine axons to the prefrontal cortex (PFC) is a particularly important event because it affects the structural and functional maturation of PFC circuitry and, in turn, behavioral flexibility and cognitive control in adulthood.2-5 We have shown that the guidance cue receptor DCC, which is highly expressed by mesolimbic dopamine axons,6 promotes targeting recognition events in the nucleus accumbens (NAcc) in adolescence, preventing them from continuing to grow ectopically to the PFC.4 Subtle changes in DCC levels in adolescence impact the extent of the dopamine innervation to the PFC, leading to substantial modifications in PFC circuitry connectivity and in cognitive processing in adulthood.4-9
Repeated noncontingent exposure to 4 mg/kg of amphetamine in early adolescence, but not in adulthood, downregulates Dcc mRNA and protein expression in the ventral tegmental area (VTA) in male mice.10,11 This effect is mediated by amphetamine-induced VTA upregulation of the microRNA-218 (miR-218), which is a potent repressor of DCC in both human and rodent neurons and appears to control DCC expression in dopamine neurons across postnatal life.10,12 Notably, exposure to 4 mg/kg of amphetamine in early adolescence also results in disruption to the development of mesocorticolimbic dopamine connectivity, leading to an increase in the expanse of the dopamine input to the PFC but to a significant reduction in the number of presynaptic sites of PFC dopamine axons in adulthood.5,9 These neuroanatomical alterations, which require downregulation of DCC receptors in dopamine neurons, are associated with deficits in PFC-dependent behaviors, including impaired behavioral inhibition.5,9,13-16
To date, we have explored the effects of amphetamine in adolescence on miR-218/Dcc expression, PFC dopamine development, and behavioral inhibition using a 4 mg/kg dose. In mice, approximately the same doses have been reported to achieve plasma amphetamine levels comparable with those reached by recreational doses used in humans, ranging from 500 to 2500 ng/mL.17-22 However, the doses of amphetamine, which are typically used for therapeutic purposes, have been shown to reach peak plasma levels ranging from 30 to 140 ng/mL.23-27 Whether exposure to equivalent low doses of amphetamine in mice downregulates miR-218/Dcc expression in the VTA, altering PFC dopamine development and cognitive control in adulthood, needs to be determined. Here, we quantified plasma amphetamine levels achieved by exposing early adolescent male mice to a 0.5 or 4 mg/kg dose and then determined the impact that the low-dose regimen has on the Dcc-dependent maturation of mesocortical dopamine connectivity and behavioral control.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Animals
All experiments and procedures were performed according with the guidelines of the Canadian Council of Animal Care and the McGill University/Douglas Mental Health University Institute Animal Care Committee. C57BL/6 wild-type male mice were obtained from Charles River Canada and maintained in the colony room of the Douglas Mental Health University Institute Neurophenotyping center on a 12-hour light–dark cycle (light on at 0800 h) with food and water available ad libitum. All the experiments were conducted during the light part of the cycle.
2.2 ∣. Drugs
d-Amphetamine sulfate (Sigma-Aldrich, Dorset, United Kingdom) was dissolved in 0.9% saline. All amphetamine injections were administered i.p. at a volume of 0.1 mL/10 g and doses of 0.5 or 4 mg/kg. Different routes of administration lead to significant differences in the bioavailability of different drugs, including methylphenidate and cocaine.28,29 In this study, we chose the i.p. route to be able to compare our findings with those reported by our lab and other groups.
2.3 ∣. Amphetamine plasma concentration
2.3.1 ∣. Amphetamine regimen and plasma collection
Male C57BL/6 mice received a single injection of amphetamine (0.5 or 4 mg/kg) at postnatal day (PND) 28, and trunk blood was collected after different time points (5, 15, 25, and 35 min) in tubes containing EDTA as anticoagulant. Blood plasma was obtained by centrifuging at 3000g for 10 minutes, at 4°C.
2.3.2 ∣. Bioanalysis of d-amphetamine in plasma samples
d-Amphetamine was extracted from plasma samples (25 μL) by protein precipitation with methanol (125 μL) containing 0.5μM losartan (internal standard). Samples were vortexed and centrifuged (16 000g for 5 min) at 4°C, and supernatants were transferred to a 96-well plate. Separation of analytes was achieved on an Ultimate 3000 UPLC system (Thermo Fisher Scientific, Waltham, Massachusetts) with an Agilent Eclipse Plus C18 column (1.8 μm, 2.1 × 100 mm) (Agilent, Santa Clara, California) using a gradient run of 2.5:97.5 to 5:95 (water:acetonitrile) with 0.1% formic acid over 1.5 minutes and detected on a Thermo Q Exactive Focus orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, Massachusetts). The orbitrap was run in a positive ion product reaction monitoring (PRM) mode. The ions of 136.1121 and 423.1695 m/z are selected with a 1 m/z isolation window and fragmented with setting of 15 and 20 CE, respectively. Transitions of 91.0563 and 119.0867 (d-amphetamine) and 207.0915 and 377.1522 (losartan) were used and quantified for concentration determinations. Calibration curves over the range of 0.03 to 100 nmol/mL were constructed from the peak area ratio of the analyte to the internal standard using quadratic regression with a weighting factor of 1/(nominal concentration). Correlation coefficient greater than 0.99 was obtained in all analytical runs for the analytes. Noncompartmental analysis module in Phoenix WinNonlin version 7.0 (Certara USA, Inc., Princeton, New Jersey) was used to assess pharmacokinetic parameters. Peak plasma concentrations (Cmax) and time to Cmax (Tmax) were the observed values. Area under the curve (AUC) was calculated by log-linear trapezoidal rule to the end of sample collection (AUClast).
2.4 ∣. Amphetamine regimen
Male C57BL/6 early adolescent mice were treated with saline or amphetamine injections from PND 22 ± 1 to PND 31 ± 1. Consistent with our previous studies, different groups of mice received one injection of amphetamine (0.5 or 4 mg/kg, i.p.) or saline, every other day, for a total of 5 days. Locomotor activity was measured 15 minutes prior to and 90 minutes after each saline or amphetamine injection.
In concordance with our and other studies, we define early adolescence in mice as the period between the day of weaning and PND 32.3,4,8-11,30,31 While this range is not an absolute margin, but an age during which mice exhibit distinct neurobehavioral characteristics, this definition seems now to be a consensus in the rodent literature.1,3,32-34 Indeed, early adolescence is the critical period when exposure to a high-dose amphetamine regimen leads to impaired behavioral inhibition, aberrant PFC dopamine connectivity, and reduced PFC dopamine function in adulthood.5,9
2.5 ∣. Western blot analysis
One week after completing the saline or amphetamine treatment regimens, different cohorts of mice were rapidly decapitated, and their brains were flash-frozen in 2-methylbutane (Fisher Scientific, Hampton, New Hampshire) chilled with dry ice. Bilateral punches of the VTA, NAcc, and PFC were excised from 1-mm thick coronal slices starting from sections corresponding to plate 55 (−2.92 mm, anterior/posterior relative to Bregma) and 15 (1.94 mm, anterior/posterior relative to Bregma), respectively, of Paxinos and Franklin35 and processed for western blot as before.10,36 Briefly, protein samples (15 μg) were separated on a 10% SDS-PAGE and transferred to a nitrocellulose membrane that was incubated overnight at 4°C with antibodies against DCC (1:1000, Cat#554223, BD Pharmingen, Mississauga, ON, Canada), Netrin-1 (1:750 dilution, Cat#NB100-1605, Novus Biologicals, Littleton, Colorado) and β-actin (1:15000, Sigma-Aldrich, Oakville, ON, Canada).
2.6 ∣. RNA extraction and quantitative real-time PCR for mouse tissue
One week after the saline or amphetamine treatment, bilateral punches of the VTA were taken from coronal sections obtained as described above. Total RNA and microRNA fractions were isolated using the miRNeasy Micro Kit protocol (Qiagen, Toronto, ON, Canada) as previously.10 All RNA samples were determined to have 260/280 and 260/230 values greater than or equal to 1.8, using the Nanodrop 1000 system (Thermo Scientific, Toronto, ON, Canada). RNA integrity was further assessed using the denaturing gel electrophoresis method proposed by Aranda et al.37 The three well-defined 28S, 18S, and 5.8S/5S ribosomal bands were detected without any background. Reverse transcription for Dcc and Glyceraldehyde-3-phosphatedehydrogenase (Gapdh) mRNA was performed using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, California) according to manufacturer's instructions. Real-time polymerase chain reaction (PCR), using TaqMan assay (Applied Biosystems, Foster City, California) was carried out with an Applied Biosystems 7900HT RT PCR system. Data for Dcc mRNA expression were analyzed by using the relative quantification standard curve method and the level of these transcript was quantified relative to the expression of the reference gene Gapdh. Reverse transcription for miR-218 was performed using the TaqMan MicroRNA Reverse Transcription Kit together with the corresponding miRNA TaqMan probes (Applied Biosystems, Foster City, California). Expression levels were calculated using the relative quantification standard curve method. The small nucleolar RNA (snoRNA) RNU6B was used as endogenous control to normalize the expression of miR-218. In all cases, the real-time PCR was run in technical triplicates.
2.7 ∣. Neuroanatomical analysis
2.7.1 ∣. Perfusion
Adult mice received an intraperitoneal overdose of ketamine 50 mg/kg, xylazine 5 mg/kg, and acepromazine 1 mg/kg and were perfused intracardially with 50 mL of 0.9% saline followed by 75 mL of chilled fixative solution (4% paraformaldehyde in phosphate-buffered saline). Brains were dissected and placed in the fixative solution overnight at 4°C and were then transferred to phosphate-buffered saline and stored for a maximum of 2 days. Brains were sectioned using a vibratome (35-μm thick coronal slices).
2.7.2 ∣. Immunofluorescence
As we have done before, every second coronal section was processed (1:2 series).6,8,36 A rabbit polyclonal anti-TH antibody (1:500 dilution, catalog #AB152; Millipore Bioscience Research Reagents) and an Alexa Fluor 594-conjugated secondary antibody raised in goat (1:500 dilution, 1 h incubation, Invitrogen) were used.
2.7.3 ∣. Stereology
The TH antibody labels dopamine axons in the PFC with high specificity and rarely labels norepinephrine axons.6,8,38 As previously, and because of the lateralization of the dopamine system, we obtained counts only from the right hemisphere. To evaluate changes in mesocortical dopamine connectivity in animals exposed to 0.5 mg/kg of amphetamine during adolescence, we performed stereological quantification of the span of TH-positive fibers in the cingulate 1, prelimbic, and infralimbic subregions of the pregenual medial PFC. The total volume of TH-positive fiber innervation (in cubic micrometers) was assessed using the Cavalieri method using Stereoinvestigator (MicroBrightField).4,6,8,9 Counts were performed blind. The coefficient of error was below 0.1 for all regions of interest in all sampled brains.
The medial PFC subregions were delineated according to plates spanning 14 to 18 (1.98- to 1.54-mm anterior/posterior relative to Bregma) of the mouse brain atlas.35 A 5× magnification was used to trace the contours of the dense TH-positive innervation of the subregions using a Leica DM400B microscope. An unbiased counting frame (25 × 25 μm) was superimposed on each contour, and counts were made at regular predetermined intervals (x = 175 μm, y = 175 μm) from a random start point. Counting of varicosities was performed at 100× magnification within the rostrocaudal borders of our region of interest (plates 14-18; 1:4 series). A guard zone of 5 μm was used and the optical dissector height was set to 10 μm.
2.7.4 ∣. Number of DA neurons
The total number of dopamine neurons in the VTA was assessed using the optical fractionator probe of Stereoinvestigator as previously.4,7-9 The counting scheme used a 60 × 60 μm counting frame (x = 150 μm, y = 150 μm intervals) with a random start point. Counting was performed at 40× magnification in a 1:4 series. A 3-μm guard zone and a probe depth of 10 μm were used.
2.8 ∣. Behavioral evaluations
2.8.1 ∣. Go/no-go
We modified and optimized a go/no-go task for use with mice.4,5 Briefly, mice were food restricted for the duration of the behavioral testing to maintain a body weight of 85% of the initial weight. The task took place in operant behavioral boxes (Med Associates, Inc., St Albans, Vermont) equipped with a house light, two illuminated nose poke holes, an adjustable sonalert tone generator, and a pellet dispenser. We used chocolate-flavored dustless precision pellets (BioServ, Inc., Flemington, New Jersey) as the operant reinforcer. The experimental procedure consisted of three stages: conditioned reinforcement training, reaction time training, and the go/no-go task. Animals were subject to one training or testing session per day.
Conditioned reinforcement training
At the start of each conditioned reinforcement training session, the house light turns on and remains on throughout the 20-minute session. Each trial within this session consists of the presentation of an illuminated nose poke hole for 9 seconds. If the mouse does not respond by nose poking in the illuminated hole, the cue light is extinguished for a 10-second intertrial interval (ITI) before the next trial/cue presentation. Thus, the purpose of the house light in this session is to signal that there is a current ongoing session.
If the mouse responds to the cue light by nose poking in the illuminated hole, a chocolate food pellet is dispensed, and the trial is counted as a “reward” trial. The location of the active (cued) nose poke hole (either left or right) is counterbalanced within groups and stays consistent for each individual mouse for the duration of the session and throughout each stage of the experiment. Responses to the active nose poke hole when the cue light is off, as well as responses to the nonactive nose poke hole (where the cue light was never illuminated), do not result in a reward but are recorded and analyzed. Mice advanced to the next stage of training once they achieve a criterion of over 70% responses to cued trials. Mice received one 20-minute conditioned reinforcement training session per day.
Reaction time training
Once mice stably respond to the cued nose poke hole to receive the reinforcer, they are trained to (a) respond only following the illumination of the cue light and (b) to respond within 3 seconds of the cue illumination to receive the reinforcer. To train mice to respond only when the cue light is present, the structure of the session is changed to incorporate a pretrial period prior to each trial. In this pretrial period, the house light is illuminated for a variable amount of time (3, 6, or 9 s, distributed randomly) without the cue light. If the mouse nose pokes during this pretrial period, the house light is extinguished, and a 10-second ITI is initiated. This is recorded as a “premature response.” If the mouse does not respond during the pretrial period, the cue light is then illuminated for 3 seconds. Therefore, the purpose of the house light in this training phase is to signal the start of a pretrial period, and the purpose of this pretrial period is to train mice to respond specifically to the cue light. A response during the 3-second trial period results in the delivery of a reward pellet; if the mouse fails to respond within this window, the cue and house lights are extinguished, and a 10-second ITI began. In order to advance to the go/no-go task, mice have to end less than 25% of the pretrial periods with a premature response. Mice receive one 30-minute reaction time training session per day.
Go/no-go task
Following successful completion of both training stages, mice undergo 10 sessions of the go/no-go task. This task requires mice to respond to a lighted “go” cue, identical to the cue used in the training sessions, or inhibit their response to this cue when presented in tandem with an auditory “no-go” cue. In the go trials, mice had to respond to the illuminated nose poke hole in the 3-second timeframe during which the cue light is on in order to receive a reward. This is counted as a “hit” in our analysis. In the no-go trials, an 85-dB tone is paired with the 3-second cue light to signal that the mouse should withhold from responding. If mice respond during the 3-second no-go trial, an ITI is initiated and no reward is dispensed. This is counted as a “commission error” in our analysis. However, if mice withhold from responding for the 3-second duration of the tone/light no-go cue, a reward is dispensed. As in the reaction time training, a randomized, variable pretrial period of 3 to 9 seconds precedes each trial, and the number of premature responses was recorded. The purpose of this is to mimic the setting used during the reaction time training, where mice learn to withhold from nose poking prior to the presentation of the cue light. Within each session, the number of go and no-go trials is given in an approximately 1:1 ratio and presented in a randomized order. Each session lasts 30 minutes and consists of approximately 30 to 50 go and 30 to 50 no-go trials.
2.9 ∣. Statistical analysis
All values are represented as means ± SEM. A significance threshold of α = .05 was used in all the experiments. Statistical differences between two groups were analyzed with Student's t tests, and when required, the significance level used to evaluate the comparisons was adjusted using the Holm–Bonferroni sequentially rejective procedure.39 All data are normally distributed, and the variance is similar between groups. Statistical differences between more than two groups were analyzed with one or two-way ANOVAs. The sample size in all the experiment varied from four to nine animals per group.
3 ∣. RESULTS
3.1 ∣. A low amphetamine dose leads to plasma levels seen in therapeutic settings
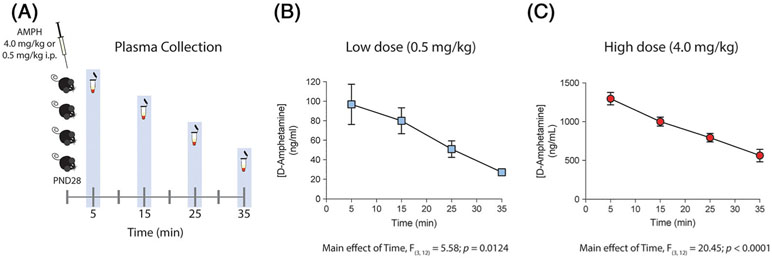
The concentration of d-amphetamine in plasma of early adolescent mice was measured at multiple time points post i.p. administration of 0.5 or 4 mg/kg (Figure 1A). d-Amphetamine showed quick absorption with a maximum concentration (Cmax) of 97 ± 21 ng/mL following administration of the 0.5 mg/kg dose (Figure 1B, 0.5 mg/kg: ANOVA with a main effect of time: F (3, 12) = 5.58, P = .0124). The 4 mg/kg dose, however, resulted in a concentration of 1300 ± 79 ng/mL observed 5 minutes post injection (Figure 1C, 4 mg/kg: ANOVA with a main effect of time: F (3, 12) = 20.45, P < .0001). The exposures based on area under the curve (AUClast) were 1928 ± 305 for 0.5 mg/kg and 26 349 ± 1922 for 4 mg/kg. An eightfold increase in the amphetamine dose resulted in almost a 14-fold increase in exposures. Thus, the increase in blood concentration of amphetamine was not simply proportional to the increase in the drug dose.
FIGURE 1.
Peak plasma concentrations achieved by an intraperitoneal injection of 0.5 or 4.0 mg/kg of d-amphetamine (AMPH) correspond to those measured in humans in therapeutic and recreational settings, respectively. A, Diagram describing AMPH regimen and the different time points for plasma collection. B, and C, Bioanalysis of d-amphetamine in plasma observed over time after a single intraperitoneal injection of 0.5 or 4 mg/kg dose (n = 4 per time point). B, Plasma concentration of AMPH showed a maximum concentration of 97 ± 21 ng/mL 5 minutes after low-dose injection. C, Plasma concentration of AMPH showed a maximum concentration of 1300 ± 79 ng/mL 5 minutes after high-dose injection
These results demonstrate that while the 0.5 mg/kg amphetamine dose reaches peak plasma concentrations within the range of those observed in therapeutic settings (ie, 30-140 ng/mL),23-27 peak plasma levels following the 4 mg/kg dose are within those seen in recreational use (i.e., 500-2500 ng/mL).17-22
3.2 ∣. Repeated exposure to a low dose of amphetamine in adolescence upregulates VTA DCC protein expression, without altering miR-218 or Dcc mRNA levels
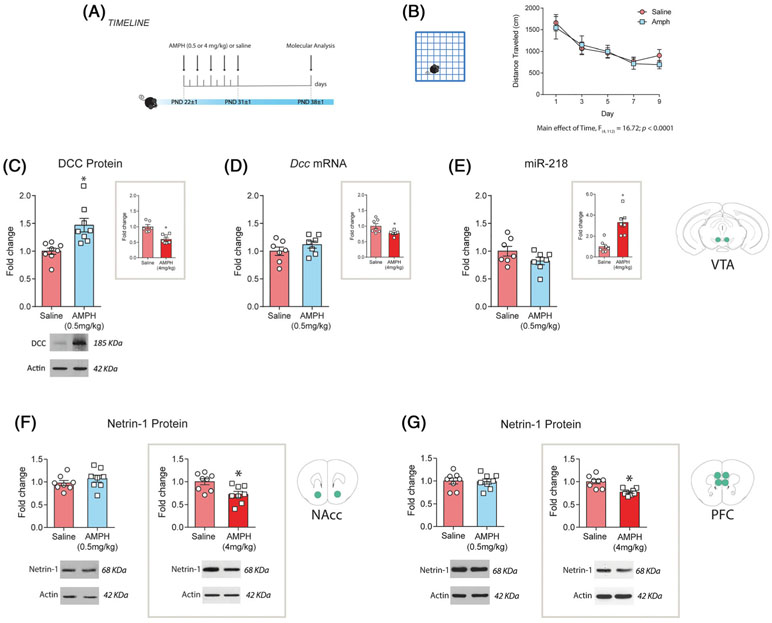
The expression levels of DCC protein, Dcc mRNA, and miR-218 in the VTA are regulated by noncontingent exposure to 4 mg/kg of amphetamine during early adolescence.10 To determine whether there is a threshold for these drug effects, we exposed early adolescent mice (PND 21 ± 1 to PND 31 ± 1; Figure 2A) to 0.5 mg/kg of amphetamine. Injections of 0.5 mg/kg of amphetamine do not alter locomotor activity in comparison to saline treatment (Figure 2B, two-way ANOVA for repeated measures: significant main effect of the day, F (4, 112) = 16.72, P < .0001; no significant effect of treatment, F (1, 28) = 0.09; P = .76; or treatment × day interaction, F (4, 112) = 0.49, P = .74). However, we found a significant increase in DCC protein expression in the VTA 1 week later in amphetamine-treated mice versus saline controls (Figure 2C; t(14) = 3.51, P = .0035). This contrasts the DCC downregulation we previously reported following exposure to a 4 mg/kg amphetamine treatment regimen (Figure 2C, inset; 10)). Furthermore, Dcc mRNA and miR-218 expression in the VTA did not differ between mice treated with 0.5 mg/kg of amphetamine or with saline in adolescence (Figure 2D Dcc mRNA: t(12) = 1.20, P = 0.25; Figure 2E miR-218: t(12) = 1.60, P = .14). These findings that are also opposite to the effects seen with the 4 mg/kg dose (Figure 2D,E, insets,10), indicate that the changes in DCC protein expression are posttranslational in nature.
FIGURE 2.
Exposure to a therapeutic-like dose of amphetamine in adolescence upregulates DCC protein expression without altering miR-218 or Dcc mRNA levels in the ventral tegmental area (VTA) and does not change Netrin-1 in dopamine terminal regions. A, Timeline of treatment and experimental procedures. B, Locomotor activity during the 90-minute test performed after each treatment injection. C, DCC expression is significantly increased in the VTA 1 week after the treatment with 0.5 mg/kg of amphetamine in adolescence. Inset: levels of DCC protein expression in the VTA in animals treated with 4 mg/kg of amphetamine, using the exact same schedule. In contrast to animals exposed to 4 mg/kg (D and E insets), animals treated with 0.5 mg/kg of amphetamine showed no changes in D, Dcc mRNA or E, miR-218 in the VTA compared with saline controls. F, and G, Netrin-1 expression in nucleus accumbens (NAcc) and prefrontal cortex (PFC) 1 week after termination of treatment with 0.5 or 4.0 mg/kg of amphetamine. No changes were observed in Netrin-1 expression levels in the F, NAcc or in the G, PFC in mice exposed to 0.5 mg/kg of amphetamines in comparison with saline-treated ones. Mice exposed to 4 mg/kg of amphetamine during adolescence showed a downregulation on Netrin-1 protein expression in the F, NAcc and the G, PFC compared with saline-treated controls. *Significantly different from saline group, P < .05. The data presented in the insets are reproduced from Cuesta et al.10 All data are shown as mean ± SEM
3.3 ∣. Dose-dependent effects of amphetamine in adolescence on Netrin-1 expression
The effects of DCC receptor on axonal targeting and synapse formation are mediated by its interaction with its ligand Netrin-1.40,41 Thus, we next determined the effect of high and low doses of amphetamine in adolescence on Netrin-1 expression in target regions of dopamine neurons (Figure 2F,G). Exposure to 4 mg/kg of amphetamine significantly downregulated Netrin-1 expression in the NAcc 1 week later, in comparison to saline groups (Figure 2F, right panel; t(14) = 0.89, P = .019). Thus, high doses of amphetamine in adolescence not only downregulate DCC expression in mesolimbic dopamine neurons but also reduce Netrin-1 levels in their target region. Intriguingly, this high-dose regimen also reduced Netrin-1 expression in the PFC (Figure 2G, right panel; t(14) = 4.24, P = .0008), an effect that may contribute to the reduced number of mesocortical dopamine varicosities in adulthood.5,9
In contrast, Netrin-1 expression in the NAcc and PFC did not differ between mice treated with the 0.5 mg/kg dose and saline (Figure 2F, left panel, NAcc: t(14) = 1.03, P = .32; Figure 2G, left panel, PFC: t(14) = 0.16, P = .88). The effects of amphetamine in adolescence on both DCC and Netrin-1 expression are therefore dose dependent.
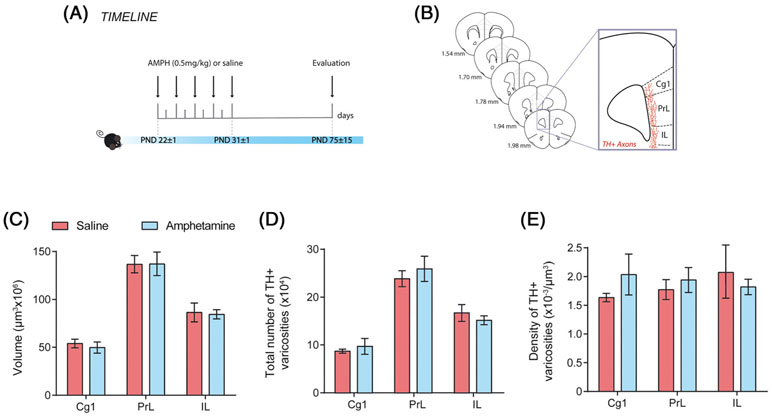
3.4 ∣. Exposure to low doses of amphetamine in adolescence does not alter mesocortical dopamine connectivity in adulthood
Next, we analyzed the extent of the mesocortical dopamine innervation to layers 5 and 6 of the medial PFC in adult mice exposed to amphetamine (0.5 mg/kg) or saline during adolescence (Figure 3A,B). We found no differences between groups in the span (ie, volume) of TH-positive fibers (Figure 3C: two-way ANOVA, no significant main effect of treatment, F (1, 8) = 0.036, P = .85; no significant treatment × medial PFC region interaction, F (2, 16) = 0.078, P = .93; significant main effect of medial PFC region, F (2, 16) = 111.2, P < .0001). In the medial PFC, nearly every dopamine varicosity forms a synapse with a dendritic spine or shaft42; therefore, we measured the total number and the density of TH-positive varicosities. There were no group differences in the total number and density of medial PFC TH-positive varicosities in any of the three subregions analyzed (total number: Figure 3D: two-way ANOVA, no significant main effect of treatment, F (1, 8) = 0.056, P = .81; no significant treatment × medial PFC region interaction, F (2, 16) = 1.01, P = .37; significant main effect of medial PFC region, F (2, 16) = 73.92, P < .0001; density: Figure 3E: two-way ANOVA, no significant main effect of treatment, F (1, 8) = 0.11, P = .75; no significant treatment × medial PFC region interaction, F (2, 16) = 1.59, P = .24; significant main effect of medial PFC region, F (2, 16) = 0.02, P = .82). These results contrast the ones we observed in the PFC of mice exposed to 4 mg/kg of amphetamine during adolescence, namely, an increase in the span of TH-positive innervation but a reduction in the total number and density of TH-positive varicosities.9
FIGURE 3.
Low amphetamine doses in adolescence do not alter mesocortical dopamine connectivity in adulthood. A, Timeline of treatment and experimental procedures. B, Schematic representation of the regions of interest in the medial prefrontal cortex outlined according to the Mouse Brain Atlas (Paxinos and Franklin, 2008). The cingulate (Cg1), prelimbic (PrL), and infralimbic (IL) subregions of the medial prefrontal cortex were analyzed. C, Volume, D, total number, and E, density of the TH-positive fiber innervation to the inner layers of the medial prefrontal cortex. Mice treated with 0.5 mg/kg of AMPH in adolescence do not show significant differences to their saline counterparts. All data are shown as mean ± SEM (n = 4-6 per group)
It is important to note that the number of TH-positive neurons in the VTA is similar between the amphetamine- and saline-treated groups (Dopamine neuron number: mean ± SEM: Saline = 7204 ± 1135, AMPH = 5744 ± 1005, t(8) = 0.95, P = .37; data not shown), similar to our findings using the 4 mg/kg dose.9
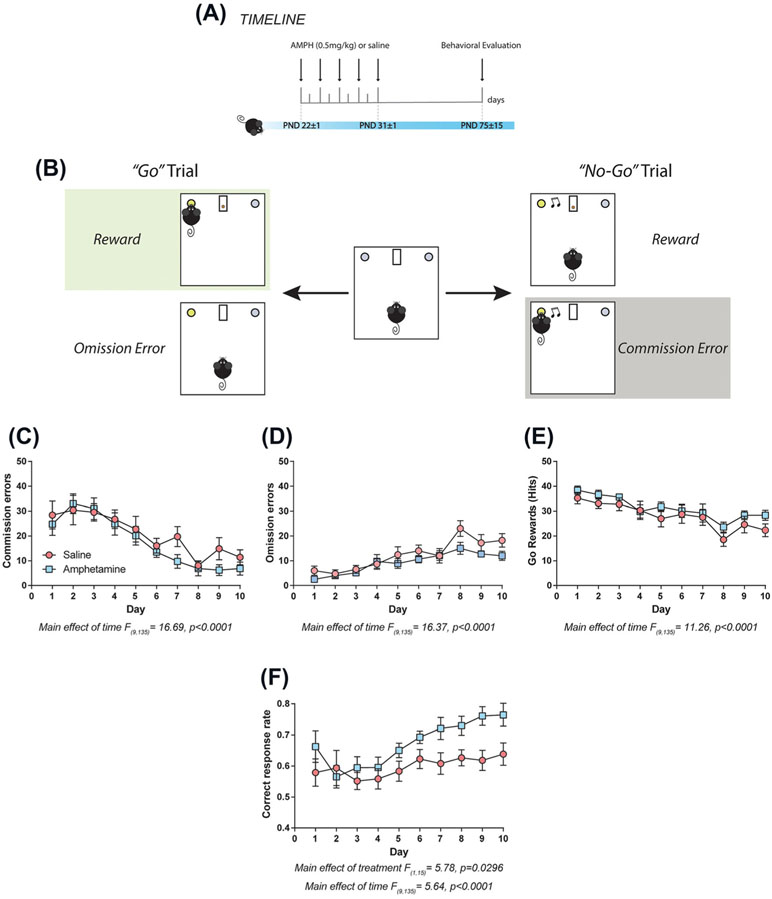
3.5 ∣. Exposure to low doses of amphetamine in adolescence does not induce behavioral inhibition deficits in adulthood
We then assessed the effects of 0.5 mg/kg of amphetamine in adolescence on adult using the go/no-go task4,5 (Figure 4A,B). There were no differences in the number of commission errors, “hits,” or omission errors between amphetamine and saline groups (Figure 4C, commission errors: two-way repeated measures ANOVA, no significant effect of treatment, F (1, 15) = 0.76, P = .396 or time × treatment interaction, F (9, 135) = 0.79, P = .625, significant main effect of time, F (9, 135) = 16.69, P < .0001; Figure 4D, hits: two-way repeated measures ANOVA, no significant effect of treatment, F (1, 15) = 1.42, P = .251 or time × treatment interaction, F (9, 135) = 0.47, P = .895, significant main effect of time, F (9, 135) = 11.26, P < .0001; Figure 4E, omission errors: two-way repeated measures ANOVA, no significant effect of treatment, F (1,15) = 1.875, P = .1911 or time × treatment interaction F (9,135) = 1.351, P = .2167, significant main effect of time, F (9,135) = 16.37, P < .0001).
FIGURE 4.
Low doses of amphetamine in adolescence improve overall cognitive performance in adulthood. A, Timeline of treatment and experimental procedures. B, Diagram of the go/no-go task adapted for mice. (C-E) There are no differences in the number of C, commission errors, D, omission errors, or E, “hits” between animals treated with 0.5 mg/kg or saline during adolescence. However, amphetamine exposure during adolescence induce a significant increase in the correct response rate across the test, when compared with saline-treated ones F. All data are shown as mean ± SEM (n = 8-9 per group)
We also calculated the total number of correct responses across the go/no-go task using the following formula modified from Gubner et al.43:
Interestingly, the amphetamine-treated group had a higher number of correct responses in comparison to the saline group (Figure 4F: two-way repeated measures ANOVA, significant effect of treatment, F (1, 15) = 5.78, P = .0296; no significant time × treatment interaction, F (9, 135) = 1.50, P = .155, significant main effect of time, F (9, 135) = 5.64, P < .0001). These results indicate that exposure to low doses of amphetamine in adolescence do not lead to deficits in cognitive control in adulthood but in fact improve performance across the overall task.
4 ∣. DISCUSSION
In this study, we show that there is a dose effect of amphetamine in early adolescent male mice on miR-218/DCC/Netrin-1 expression, mesocortical dopamine development, and cognitive control. The two doses used in the present study reached plasma levels within the range of those observed in humans using the drug in therapeutic and recreational settings. For adolescent and adult mice, plasma levels are a good proxy for drug brain levels44; thus, it is expected that the doses used for the present studies achieved brain levels of the drug that can have relevant molecular changes.
First, while exposure to the high amphetamine dose regimen recruits miR-218 to downregulate DCC in the VTA,10,11 the low drug dose increases DCC protein without altering Dcc mRNA or mir-218 levels. Second, only the high-dose amphetamine regimen reduces Netrin-1 expression in the NAcc and PFC. Third, while the high dose regimen leads to a reduction in adult mesocortical dopamine connectivity and function5,9 inducing in turn a reduction in inhibitory control,5 the low-dose regimen does not disrupt behavioral inhibition and, in fact, leads to an overall improvement in adult cognitive performance, as measured by an increase in the total number of correct responses in the go and no-go trials.
Exposure to 0.5 mg/kg of amphetamine increases DCC protein expression in the VTA, without altering Dcc mRNA levels or miR-218 expression in this region. This is in contrast to the downregulation of Dcc mRNA and protein expression induced by exposure to the 4 mg/kg dose regimen, which is actually mediated by drug-induced upregulation of miR-218 in the VTA.10 Thus, DCC protein upregulation by the therapeutic-like treatment might be mediated by posttranslational mechanisms. The SIAH (seven in absentia homolog) protein family has been shown to ubiquitinate and regulate DCC protein levels in different organs, including rat brain.45,46 In future studies, we will assess whether SIAH protein expression and/or activity is altered by amphetamine administration in adolescence.
Our previous work shows that DCC expression in mesolimbic dopamine axons determines where and when they recognize their final target and, accordingly, reduced DCC signaling in adolescence results in targeting errors and ectopic growth of mesolimbic dopamine axons from the NAcc to the PFC.3,4,9 The fact that the high amphetamine regimen downregulates both DCC in dopamine neurons and Netrin-1 in the NAcc suggests that this experience potentiates the ectopic growth of mesolimbic dopamine axons to the PFC, causing disorganization of dopamine connectivity in this region. This in turn may lead to alterations in executive function,47 negatively impacting performance in tasks which require inhibition of a learned or ongoing behavior.5,14,15
Recently, Netrin-1 has been demonstrated to promote synapse formation in the PFC41 and to potentiate excitatory synaptic transmission via the insertion of GluA1 AMPA receptors in the hippocampus of adult mice.48 Thus, the downregulation of Netrin-1 in the PFC induced by the high-dose regimen may contribute to the reduction in the number and density of mesocortical dopamine synaptic sites observed in adulthood.5,9 Interestingly, a similar amphetamine regimen in adolescence has been shown to induce a reduction in the expression of D1 receptors in the PFC of adult male rats49 suggesting also alterations in the electrophysiological properties at excitatory and/or inhibitory synapses in this region. In line with this idea, it has been reported that high, but not low, doses of the stimulant drug methylphenidate in adolescence reduces PFC long-term potentiation in adulthood.50 These findings suggest that high doses of stimulant drugs in adolescence lead to functional reorganization of PFC synaptic circuitry and that local alterations in Netrin-1 signaling may contribute to this effect.
We have reported that both improvements and deficits in behavioral inhibition during the No Go task are correlated with alterations in dopamine connectivity in the adult PFC. Specifically, we have observed improved inhibitory control in mice that have increased PFC dopamine synaptic connectivity.4 In contrast, adult mice that have reduced PFC dopamine connectivity and turnover show deficits in inhibitory control.5 In this study, however, we do not find changes in the span (ie, volume) of the dopamine input or in the number/density of dopamine varicosities in the PFC of mice treated with low amphetamine doses in adolescence. We are currently setting new methodologies to assess directly dopamine neurotransmission in the PFC as well as to investigate changes in connectivity in other dopamine terminal regions or in other neurotransmitter systems.
Whether exposure to amphetamine in adolescence leads to changes in dopamine connectivity in the NAcc in adulthood remains to be addressed. The stereological methods used in this study are not sensitive enough to capture subtle changes in dopamine varicosity density and/or number in the NAcc.6,8,9 Therefore, we are planning to use more sensitive methods (ie,4) in future studies to address this issue.
To date, we have conducted all our studies in male mice. However, there is evidence of sex differences in the enduring behavioral effects of amphetamine exposure during adolescence,51-53 emphasizing the importance of addressing this issue in our studies. We are now beginning to assess whether the effects of amphetamine (high and low doses) in adolescence on DCC receptor signaling, PFC dopamine maturation, and behavioral control are sexually dimorphic. In addition, we also plan to examine whether adolescent exposure to the other typically prescribed psychostimulant, methylphenidate, also increases DCC protein expression 1 week later and leads to improved cognitive performance in adulthood. Indeed, methylphenidate administration can alter DCC expression in the VTA in adulthood.54
To our knowledge, this is the first study to compare the effects of amphetamine doses equivalent with those used by humans for recreational versus therapeutic purposes on the expression of developmental genes coordinating the adolescent maturation of PFC dopamine circuitry. Although it is important to keep in mind that the limitation of noncontingent drug administration models and that the duration of therapeutic treatment in humans may vary and sometimes last until adulthood, these and our previous findings9,10 show that therapeutic versus abused doses of amphetamine in adolescence have very different long-term consequences: while abused-like doses disrupt miR-218/DCC/Netrin-1-dependent dopamine development and behavioral control, therapeutic-like doses actually increase DCC expression and improve cognitive performance in adulthood. Our findings provide insight into the critical question of whether therapeutic exposure to stimulant drugs in adolescence induces detrimental effects on ongoing neurodevelopmental events.
ACKNOWLEDGEMENTS
We thank Dr Philip Vassilev for providing comments on drafts of this manuscript and Dominique Nouel for providing technical help.
FUNDING INFORMATION
This work was supported by the National Institute on Drug Abuse (R01DA037911 to C.F; F31DA041188 to LMR), the Canadian Institutes of Health Research (MOP-74709 to C.F.), the Natural Sciences and Engineering Research Council of Canada (2982226 to C.F.). C.F. is a research scholar of the Fonds de Recherche du Québec–Santé. S.C. received support from a Conrad F. Harrington postdoctoral fellowship and JMRL from the Healthy Brains for Healthy Lives initiative.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. [DOI] [PubMed] [Google Scholar]
- 2.Hoops D, Flores C. Making dopamine connections in adolescence. Trends Neurosci. 2017;40(12):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoops D, Reynolds LM, Restrepo-Lozano JM, Flores C. Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence. eNeuro. 2018;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds LM, Pokinko M, Torres-Berrio A, et al. DCC receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence. Biol Psychiatry. 2018a;83(2):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds LM, Yetnikoff L, Pokinko M, et al. Early adolescence is a critical period for the maturation of inhibitory behavior. Cereb Cortex. 2018b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manitt C, Mimee A, Eng C, et al. The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci. 2011;31(23):8381–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant A, Hoops D, Labelle-Dumais C, et al. Netrin-1 receptor-deficient mice show enhanced mesocortical dopamine transmission and blunted behavioural responses to amphetamine. Eur J Neurosci. 2007;26(11): 3215–3228. [DOI] [PubMed] [Google Scholar]
- 8.Manitt C, Eng C, Pokinko M, et al. DCC orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl Psychiatry. 2013;3:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology. 2015;40(5):1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuesta S, Restrepo-Lozano JM, Silvestrin S, et al. Non-contingent exposure to amphetamine in adolescence recruits miR-218 to regulate DCC expression in the VTA. Neuropsychopharmacology. 2018;43(4):900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yetnikoff L, Almey A, Arvanitogiannis A, Flores C. Abolition of the behavioral phenotype of adult netrin-1 receptor deficient mice by exposure to amphetamine during the juvenile period. Psychopharmacology (Berl). 2011;217(4):505–514. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Berrío A, Lopez JP, Bagot RC, et al. DCC confers susceptibility to depression-like behaviors in humans and mice and is regulated by miR-218. Biol Psychiatry. 2017;81(4):306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boomhower SR, Newland MC. Effects of adolescent exposure to methylmercury and d-amphetamine on reversal learning and an extradimensional shift in male mice. Exp Clin Psychopharmacol. 2017;25(2):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerslag LR, Waldman AJ, Gulley JM. Effects of amphetamine exposure in adolescence or young adulthood on inhibitory control in adult male and female rats. Behav Brain Res. 2014;263:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Änggård E, Gunne L-M, Jönsson L-E, Niklasson F. Pharmacokinetic and clinical studies on amphetamine dependent subjects. Eur J Clin Pharmacol. 1970;3(1):3–11. [Google Scholar]
- 18.Cravey RH, Jain NC. Testing for amphetamines: medico-legal hazards. Trauma. 1973;15(49). [Google Scholar]
- 19.Gustavsen I, Morland J, Bramness JG. Impairment related to blood amphetamine and/or methamphetamine concentrations in suspected drugged drivers. Accid Anal Prev. 2006;38(3):490–495. [DOI] [PubMed] [Google Scholar]
- 20.Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse. JAMA. 1967;201:305–309. [DOI] [PubMed] [Google Scholar]
- 21.Riffee WH, Ludden TM, Wilcox RE, Gerald MC. Brain and plasma concentrations of amphetamine isomers in mice. J Pharmacol Exp Ther. 1978;206(3):586–594. [PubMed] [Google Scholar]
- 22.Van Swearingen AE, Walker QD, Kuhn CM. Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology (Berl). 2013;225(3):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borcherding BG, Keysor CS, Cooper TB, Rapoport JL. Differential effects of methylphenidate and dextroamphetamine on the motor activity level of hyperactive children. Neuropsychopharmacology. 1989;2(4):255–263. [DOI] [PubMed] [Google Scholar]
- 24.Brown GL, Hunt RD, Ebert MH, Bunney WE Jr, Kopin IJ. Plasma levels of d-amphetamine in hyperactive children. Serial behavior and motor responses. Psychopharmacology (Berl). 1979;62(2):133–140. [DOI] [PubMed] [Google Scholar]
- 25.Greenhill LL, Swanson JM, Steinhoff K, et al. A pharmacokinetic/pharmacodynamic study comparing a single morning dose of adderall to twice-daily dosing in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1234–1241. [DOI] [PubMed] [Google Scholar]
- 26.Kramer WG, Read SC, Tran B, Zhang Y, Tulloch SJ. Pharmacokinetics of mixed amphetamine salts extended release in adolescents with ADHD. CNS Spectr. 2005;10(S15):6–13. [PubMed] [Google Scholar]
- 27.McGough JJ, Biederman J, Greenhill LL, et al. Pharmacokinetics of SLI381 (ADDERALL XR), an extended-release formulation of Adderall. J Am Acad Child Adolesc Psychiatry. 2003;42(6):684–691. [DOI] [PubMed] [Google Scholar]
- 28.Gerasimov MR, Franceschi M, Volkow ND, et al. Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295(1): 51–57. [PubMed] [Google Scholar]
- 29.Pan WJ, Hedaya MA. An animal model for simultaneous pharmacokinetic/pharmacodynamic investigations: application to cocaine. J Pharmacol Toxicol Methods. 1998;39(1):1–8. [DOI] [PubMed] [Google Scholar]
- 30.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. [DOI] [PubMed] [Google Scholar]
- 31.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176(2):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1-2):19–31. [DOI] [PubMed] [Google Scholar]
- 33.Schneider M Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013;354(1):99–106. [DOI] [PubMed] [Google Scholar]
- 34.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27(1-2):163–178. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates, Compact 2nd edn. Amsterdam; Boston: Elsevier Academic Press; 2004. [Google Scholar]
- 36.Manitt C, Labelle-Dumais C, Eng C, et al. Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS ONE. 2010;5(7):e11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aranda PS, LaJoie DM, Jorcyk CL. Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis. 2012;33(2):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466(4):478–494. [DOI] [PubMed] [Google Scholar]
- 39.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;1:65–70. [Google Scholar]
- 40.Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19(3):575–589. [DOI] [PubMed] [Google Scholar]
- 41.Goldman JS, Ashour MA, Magdesian MH, et al. Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J Neurosci. 2013;33(44):17278–17289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seguela P, Watkins KC, Descarries L. Ultrastructural features of dopamine axon terminals in the anteromedial and the suprarhinal cortex of adult rat. Brain Res. 1988;442(1):11–22. [DOI] [PubMed] [Google Scholar]
- 43.Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin Exp Res. 2010;34(8):1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl). 2008;201(4):589–599. [DOI] [PubMed] [Google Scholar]
- 45.Hu G, Zhang S, Vidal M, La Baer J, Xu T, Fearon ER. Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway. Genes Dev. 1997;11(20):2701–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rott R, Szargel R, Haskin J, et al. Monoubiquitylation of α-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J Biol Chem. 2008;283(6):3316–3328. [DOI] [PubMed] [Google Scholar]
- 47.Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1463–1470. discussion 1470-1461 [DOI] [PubMed] [Google Scholar]
- 48.Glasgow SD, Labrecque S, Beamish IV, et al. Activity-dependent Netrin-1 secretion drives synaptic insertion of GluA1-containing AMPA receptors in the hippocampus. Cell Rep. 2018;25(1):168–182 e166. [DOI] [PubMed] [Google Scholar]
- 49.Kang S, Wu MM, Galvez R, Gulley JM. Timing of amphetamine exposure in relation to puberty onset determines its effects on anhedonia, exploratory behavior, and dopamine D1 receptor expression in young adulthood. Neuroscience. 2016;339:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgos H, Cofre C, Hernandez A, et al. Methylphenidate has long-lasting metaplastic effects in the prefrontal cortex of adolescent rats. Behav Brain Res. 2015;291:112–117. [DOI] [PubMed] [Google Scholar]
- 51.Cirulli F, Laviola G. Paradoxical effects of D-amphetamine in infant and adolescent mice: role of gender and environmental risk factors. Neurosci Biobehav Rev. 2000;24(1):73–84. [DOI] [PubMed] [Google Scholar]
- 52.Hankosky ER, Gulley JM. Adolescent Exposure to Amphetamines and Vulnerability to Addiction. In Neuropathology of Drug Addictions and Substance Misuse. Academic Press; 2016;292–299. [Google Scholar]
- 53.Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age-and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl). 2008;196(1):71–81. [DOI] [PubMed] [Google Scholar]
- 54.Argento JK, Arvanitogiannis A, Flores C. Juvenile exposure to methylphenidate reduces cocaine reward and alters netrin-1 receptor expression in adulthood. Behav Brain Res. 2012;229(1):202–207. [DOI] [PubMed] [Google Scholar]