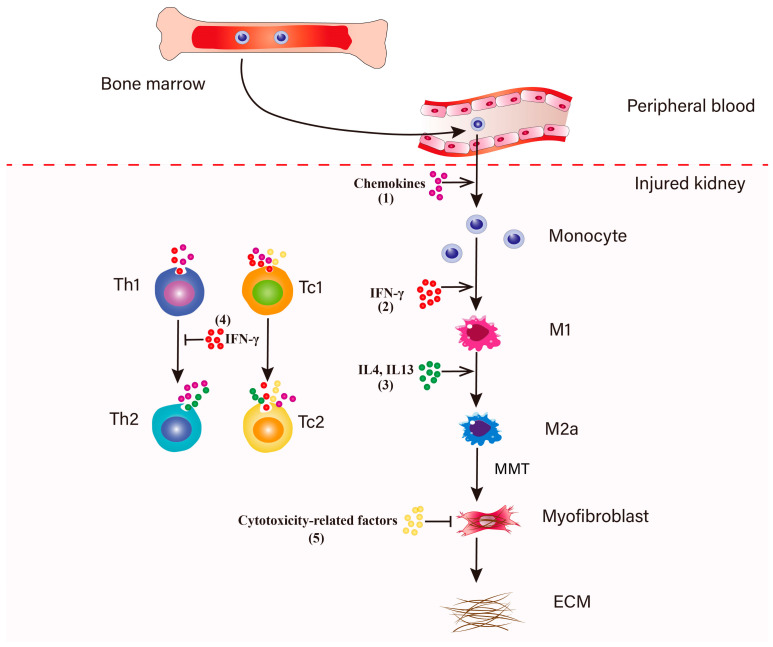
Figure 2.
Regulation of macrophage-to-myofibroblast transition. (1) In response to kidney injury, the bone marrow increases the production and release of monocytes into the peripheral blood. Circulating monocytes are recruited to the injured kidney, a process that is dependent on the chemokines partly secreted by resident T lymphocytes and macrocytes [118,119]. Recruited monocytes can proliferate in the kidney to amplify the inflammatory response. (2) IFN-γ produced by Th1 and CD8+ T lymphocytes promote macrophages polarization towards a pro-inflammatory M1 phenotype [85,90]. (3) The infiltrated macrophages adopt an anti-inflammatory M2 phenotype in response to IL4 and IL13 which are expressed by Th2 and Tc2 cells [82]. The ongoing activation of TGF-β triggers macrophage transition from a M2a phenotype to myofibroblasts within the injured kidney (MMT) [38,39,66,67,68,69]. These MMT-derived myofibroblasts induce the accumulation and deposition of ECM and subsequently promote the development of renal fibrosis [38,39,43]. (4) During this process, two subsets of CD8+ T cells, Tc1 and Tc2 cells secrete IFN-γ to inhibit the differentiation of Th2 cells, which can prevent the Th2 cells-induced excessive polarization of M2a cells [34]. (5) Tc1 and Tc2 cells could secrete cytotoxicity-related factors to induce fibroblasts apoptosis, subsequently suppressing the expansion of myofibroblasts.