Abstract
The overactivation of inflammatory pathways and/or a deficiency of neuroplasticity may result in the delayed recovery of neural function in traumatic brain injury (TBI). A promising approach to protecting the brain tissue in TBI is xenon (Xe) treatment. However, xenon’s mechanisms of action remain poorly clarified. In this study, the early-onset expression of 91 target genes was investigated in the damaged and in the contralateral brain areas (sensorimotor cortex region) 6 and 24 h after injury in a TBI rat model. The expression of genes involved in inflammation, oxidation, antioxidation, neurogenesis and neuroplasticity, apoptosis, DNA repair, autophagy, and mitophagy was assessed. The animals inhaled a gas mixture containing xenon and oxygen (ϕXe = 70%; ϕO2 25–30% 60 min) 15–30 min after TBI. The data showed that, in the contralateral area, xenon treatment induced the expression of stress genes (Irf1, Hmox1, S100A8, and S100A9). In the damaged area, a trend towards lower expression of the inflammatory gene Irf1 was observed. Thus, our results suggest that xenon exerts a mild stressor effect in healthy brain tissue and has a tendency to decrease the inflammation following damage, which might contribute to reducing the damage and activating the early compensatory processes in the brain post-TBI.
Keywords: TBI, xenon, neuroinflammation, Nanostring, rat, gene expression
1. Introduction
The search for effective methods for neuroprotection and neurorehabilitation after TBI remains a challenge for modern medicine. The use of medical gases represents an approach to overcoming or limiting brain damage post-TBI. Hydrogen, hydrogen sulfide, nitrogen monoxide, carbon dioxide, isoflurane, sevoflurane, oxygen (through hyperbaric oxygenation), and noble gases (helium, argon, and xenon) are currently being studied as neuroprotective gases [1]. Inert or noble gases are considered promising therapeutic molecules, of which xenon is the most extensively researched [2]. A distinctive feature of xenon compared to the other noble gases is its lipophilicity and ability to induce an anesthetic effect at a relatively low pressure (0.4–0.8 atm) [2,3]. Xenon has been employed in clinical medicine for inhalation anesthesia [4]. Several studies have demonstrated cytoprotective effects from xenon in various CNS injuries (trauma; ischemia, including severe neonatal asphyxia; subarachnoid hemorrhage) [5,6,7,8]. For instance, in newborns with severe asphyxia, 72 h-exposure to xenon led to better neurological outcomes [5]. However, little is known about changes in gene expression following xenon treatment after TBI. The potential mechanisms include a decrease in excitotoxicity through blocking NMDA receptors; an improvement in neuronal feeding, increasing the efficiency of metabolite supply; a decrease in inflammation; the alteration of the activity of microglial cells; diminishing apoptosis through BCL2 and TREK-1 proteins [9,10,11,12,13]. Xenon has previously been tested in combination with drugs (ketamine and memantine) for the treatment of neurodegenerative diseases [14,15]. The combined use of one of the drugs with xenon yielded the same results as monotherapy, but at smaller doses, which reduced the side effects of the drug. Some data on xenon’s mechanisms, however, appear to contradict each other. For instance, Breuer T. et al. (2015) noted a proinflammatory effect of xenon (a 3-fold increase in IL-6 and 3-fold decrease in IL-10, compared with sevoflurane) and the induction of oxidative stress in cardiac surgery patients [16]. On the other hand, there are data indicating anti-inflammatory responses to xenon [6]. To identify additional possible mechanisms of xenon treatment after TBI, we studied the dynamics of gene expression for signaling pathways associated with inflammation, antioxidation, autophagy, mitophagy, apoptosis, DNA repair, neurogenesis, and neuroplasticity, as well as the gene expression of potential xenon target proteins (NMDAR, TREK-1, and Clic4) in a TBI model in rats.
2. Materials and Methods
2.1. Animals
Male Wistar rats (200–300 g) were kept at 3–4 animals per cage, with a 12 h light/dark cycle; water and food were provided ad libitum. The animals (n = 24) were divided into 5 groups, group I (TBI-6 h, n = 5), group II (TBI-6 h + xenon, n = 5), group III (TBI-24 h, n = 5), group IV (TBI-24 h + xenon, n = 5), and group V (intact animals, n = 4). Gene expression analysis was carried out for 3 animals of each group. The animals were randomized into groups according to the subjects’ body weight.
2.2. TBI
The rats were anesthetized with an intraperitoneal injection of 300 mg/kg chloral hydrate. Additionally, to ensure effective pain relief in the perioperative and postoperative periods, we used the repeated topical application of a long-acting local anesthetic, bupivacaine ointment. TBI was induced in accordance with the method of dosed contusion injury to the open brain as described previously [17]. The operator performing TBI was blinded to the treatment groups. The head skin in the operating area was shaved and treated with an antiseptic, chlorhexidine 0.05% (ROSBIO, St. Petersburg, Russia). The animal was placed in a stereotaxic frame, the head was fixed, and a skin incision was made along the sagittal suture. Trepanation drilling was performed in the parietal and frontal bones using a 5 mm cutter above the left hemisphere in the sensorimotor cortex area, along with the stereotaxic coordinates 2.5 mm laterally and 1.5 mm caudally, relative to the bregma. The trauma unit was placed so that the striker was above the dura mater. A 50-g mass pin was dropped to the striker from a height of 10 cm. The body temperatures of the rats during surgery were maintained at 37 ± 0.5 °C using a temperature-controlled heating blanket. After the exposure in the anesthesia induction chamber, the animals were warmed up to maintain a rectal body temperature of about 37.0 °C until they woke up (but for no more than 60 min). After waking up (or 1 h later at the end of the exposure), the animals were transferred to a cage.
2.3. Xenon Treatment
Within 15–30 min of the TBI, one or two rats were placed in a transparent chamber (5 L) of the Combi-Vet (Rothacher-Medical GmbH, Heitenried, Switzerland) anesthesia machine, and connected to a KNP-01 xenon anesthesia attachment with oxygen and air compressors. A semi-open breathing circuit was used. The passage of the fresh gas mixture through the chamber was ensured by periodic compression of the breathing bag located near the inlet to the chamber. A gas mixture entered the chamber (1 L/min)—achieved concentrations were ϕO2 25–30%/ϕXe 70–75% (KseMed®, Akela-N, Khimki, Russia) (groups II and IV) or air (ϕO2 21%) (groups I and III)—for 1 h. The oxygen and xenon concentrations, as well as the gas flow rate, were measured at the chamber inlet using a gas analyzer GKM-03-INSOVT (Insovt, St. Petersburg, Russia) and flowmeters. The animals breathed on their own during the procedure.
2.4. Limb-Placing Test
For assessing the neurological status of the animals from groups III and IV (24 h after TBI), a limb-placing test was conducted as described previously [18]. The test was carried out by an experimenter blinded to the treatment groups.
2.5. Sampling
The animals were anesthetized with sevoflurane (6 vol.%) and sacrificed. Brain tissue specimens (70–100 mg or 5 × 5 × 2 mm) were harvested from the TBI zone and from the healthy contralateral zone and placed into Eppendorf tubes (2 mL) with RNAlater solution (Thermo Fisher Scientific, Waltham, MA, USA) to stabilize the RNA molecules. According to the manufacturer’s protocol, the samples were kept overnight at +4 °C and then placed in a freezer to store them at −20 °C.
2.6. RNA Purification
Total RNA was isolated from brain tissue specimens using the RNeasy Mini kit (Qiagen, Hilden, Germany) and treated with DNase I in accordance with the manufacturers’ protocols. The purity of the isolated RNA was determined spectrophotometrically by using a NanoDrop™ OneC (Thermo Fisher Scientific, Waltham, MA, USA). An RNA Clean & Concentrator—5 kit (Zymo Research, Irvine, CA, USA) was used to remove contaminants from the RNA samples. The concentration of the RNA was assessed using a Qubit 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The RNA integrity number (RIN) was determined electrophoretically on an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) using an Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA); only samples with RIN > 5 were used for further analysis.
2.7. Multiplex Gene Expression Analysis
To explore the expression of 91 genes (for the full list of target genes, see Table S1) in the brain tissue specimens, the nCounter FLEX Analysis System and the CodeSet reagent kit (Nanostring Technology, Inc., Seattle, WA, USA) were employed. This technology allows the direct assay of gene transcriptional activity in a multiplex manner. The method is based on the labeling of targets with unique color barcodes attached to target-specific probes and their subsequent detection. Analysis was performed according to the manufacturer’s protocol.
2.8. Statistics
Analysis of variance (One-way ANOVA, the Holm–Sidak method) was performed using SigmaStat 3.1 (Systat Software Inc., San Jose, CA, USA). Two-fold differences in expression vs. control were considered significant at p < 0.00055 and a tendency at 0.00055 < p < 0.05 for target genes, and were considered significant at p < 0.05 for HKGs. The control values for housekeeping genes were calculated as described in the Results (3.1). The variability was assessed using the web page https://ncalculators.com/statistics/coefficient-of-variance-calculator.htm (accessed on April–May 2021).
3. Results
3.1. Housekeeping Gene Selection
There are various approaches to the assessment of housekeeping genes (HKGs) [19]. However, what the optimal method is remains controversial. Based on literature screening, we selected 5 HKGs, B2m, Gapdh, Hmbs, Ppia, and Ywhaz, employed in various studies as proven HKGs [20,21,22]. We did not find any data on the optimal HKG for TBI in rats receiving xenon treatment. Therefore, the selected HKGs were analyzed using the coefficient of variation (CV) and comparing the means of the changes within the groups.
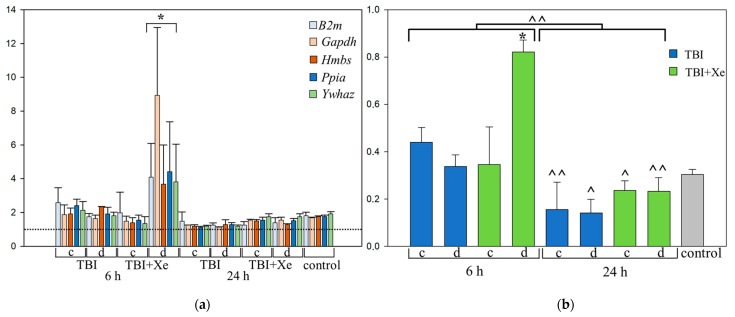
The analysis revealed that the CV of B2m gene expression was the highest (40%) (Table 1). We analyzed the frequencies of changes in the expression of the HKGs in each group. The greatest changes (at least two-fold, p < 0.00001) were observed in the 6h-TBI-xenon group in the injury zone (Figure 1b), while the Gapdh gene showed the greatest variation (p > 0.05) (Figure 1a).
Table 1.
Expression stability of candidate reference genes in sensorimotor region in TBI rats treated with xenon, evaluated using coefficient of variation (CV).
| HKG | CV% | SD | Rank |
|---|---|---|---|
| Gapdh | 28.6 | 20.2 | 1 |
| Ppia | 28.8 | 22.1 | 2 |
| Ywhaz | 29.8 | 18.5 | 3 |
| Hmbs | 30.1 | 18.2 | 4 |
| B2m | 40.0 | 19.1 | 5 |
Figure 1.
Housekeeping gene variations. (a) Mean of changes in the expression of HKGs in each group. (b) Coefficient of variation of HKG expression in brain tissue after TBI. * p < 0.05 to each group; 24 h vs. 6 h: ^ p < 0.05, ^^ p < 0.0001. One-way ANOVA, Holm–Sidak method; c—contralateral area, d—damaged area. www.BioRender.com.
We analyzed whether there were changes in the CVs of the HKGs in the TBI rats compared to those in the intact animals. After 6 h, there was a 1.28-fold increase in variability (p = 0.0555); after 24 h, there was a more than 2-fold decrease (p = 0.00124). The animals who inhaled xenon after TBI showed a more pronounced increase in the CVs of the HKGs than the intact animals at 6 h (1.93-fold, p = 0.0092), while the CVs were close to those for the intact animals at 24 h after the injury (p = 0.497).
Thus, xenon significantly increased the variability of all 5 HKGs in the damaged area at 6 h, especially Gapdh. Based on the data obtained on the variability of the HKGs’ expression, three HKGs were chosen: Hmbs, Ppia, and Ywhaz. Gene expression of the target genes was normalized to the chosen HKGs in each experimental group and time-point.
3.2. Xenon and Targeted Genes
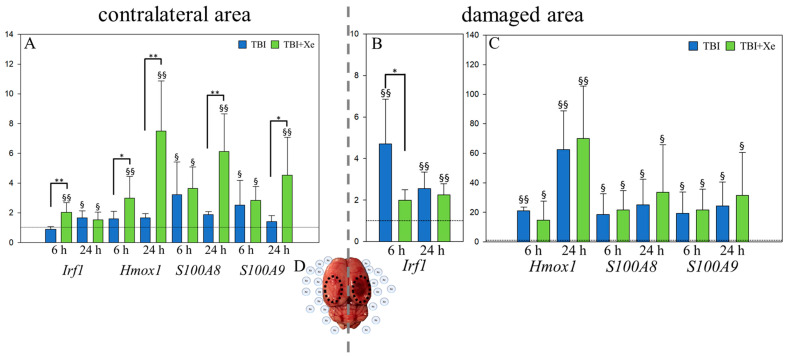
The most notable changes in gene expression after xenon treatment were found in 4 of the 91 target genes: the proinflammatory Irf1 gene; the gene of the main antioxidant enzyme in the brain, Hmox1; genes for the S100 family proteins associated with neuroinflammation, neuroprotection and neuritogenesis—S100A8 and S100A9 [23,24,25].
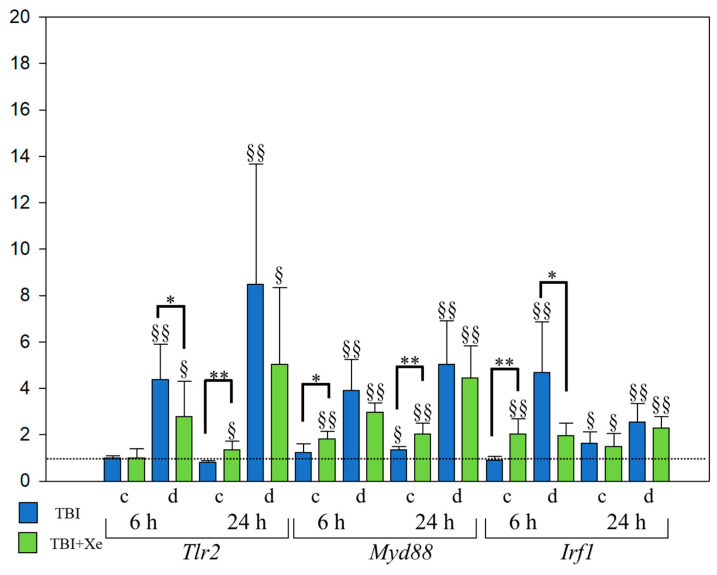
In the contralateral area, in the TBI + Xe group vs. the TBI group, the expression of Irf1 was increased 2.3-fold at 6 h (p < 0.00055), while Hmox1 was increased 4.5-fold (p < 0.00055) and S100A8 3.3-fold (p < 0.00055) at 24 h (Figure 2a). The expression of Tlr2 was increased 1.8-fold (p < 0.00055) and Myd88 1.6-fold (Figure 3) (p < 0.00055) at 24 h. There was a tendency toward increased expression of the Hmox1 gene (1.9-fold) at 6 h, and that of the S100A9 gene was increased 3.3-fold at 24 h after TBI.
Figure 2.
Gene expression analysis. (A) Gene expression in the contralateral region. (B) Irf1 gene expression in the damaged area. (C) Hmox1, S100A8, and S100A9 gene expression in the damaged area. TBI vs. TBI + Xe: * p < 0.05; ** p < 0.00055. § p < 0.05 and §§ p < 0.00055, compared to intact animals. (D) The state of the rat brain 6 h after TBI with selected contralateral (left) and damaged (right) areas. One-way ANOVA, Holm-Sidak method.
Figure 3.
Inflammatory gene expression in the rat brain tissue with TBI. TBI vs. TBI + Xe: * p < 0.05, ** p < 0.00055; § p < 0.05, §§ p < 0.00055, compared to intact animals. One-way ANOVA, Holm-Sidak method.
The Irf1 gene expression of the TBI + Xe group was 2.4-fold lower than that of the TBI group in the damaged area (p = 0.0021) (Figure 2B,C and Figure 3).
3.3. Limb-Placing Test
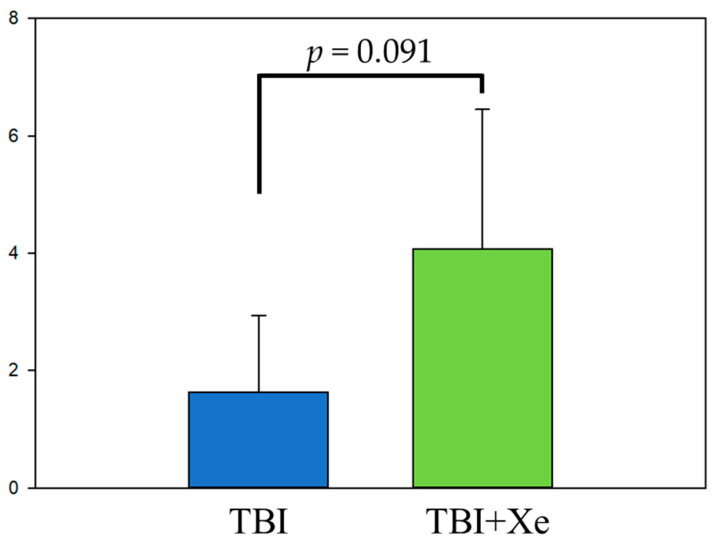
The neurological status was assessed in the rats from the TBI group (n = 5) and the TBI + xenon group (n = 5) at 24 h after TBI by the limb-placing test. The animals that inhaled xenon exhibited 2.5-fold-higher test scores than the TBI group (p = 0.091) (Figure 4), indicating a trend toward less motor weakness due to xenon.
Figure 4.
Limb-placing test in 24 h after TBI. Ordinate—the number of test scores.
4. Discussion
Noble gases, due to their chemical inertness, are attractive targets for use as neuroprotectants. Various noble gases are being tested, but xenon is the most common for clinical studies [2]. Despite many data indicating xenon’s clinical efficacy [5,6,7], the effect of xenon on the expression of genes involved in neuroprotection and their transient expression after TBI remain poorly understood. In this study, we observed a high variability in the expression of HKGs, within the damaged area in the 6h-TBI-Xe group, which could be a potential confounder. Three HKGs with less-variable expression (Ppia, Ywhaz, and Hmbs) were chosen as internal controls for the gene expression study. An additional search for the most stable HKGs at different time points may be required to prove the effects of xenon in a TBI rat model.
An explanation of these possible effects of xenon, is its ability to stabilize the membranes of brain cells [26], binding at the glycine site of the NMDA receptor (GluN1 subunit) [10,27]. When xenon is removed, a “withdrawal syndrome” occurs, triggering lipid peroxidation (LPO), with an adequate response by brain cells observed [26]. The xenon therapy regimen used in newborns with severe asphyxia by Amer A.R. (2018) appears to be optimal in terms of the anti-inflammatory effect and protection against LPO afterward: it entails long-term xenon treatment (up to 72 h) in the asphyxia acute period in combination with therapeutic hypothermia [5]. A gradual increase in a baby’s body temperature for 4 h or more after the procedure [28] will probably contribute to a slow increase in or a blockade of LPO when xenon is eliminated. However, there are no data on the consequences of mild post-xenon LPO. Apparently, in response to xenon, both inflammatory and antioxidative genes are activated in intact brain tissue, which was observed in the current study: the expression of an inflammatory gene (Irf1) the main gene of the antioxidant system (Hmox1), and genes associated with neurogenesis and neuroplasticity (S100A8 and S100A9) increased. Notably, Hmox1’s expression was elevated for longer and more strongly, for up to 24 h, than Irf1’s. Early increases in the contralateral area expression of the antioxidant Hmox1 gene and S100A8/S100A9 genes encoding the dimer calgranulin contribute to neuroplasticity [25], and might protect normal neurons from secondary damage (pro-oxidant stress signaling products from the damaged area including circulating DNA and other DAMP molecules). That xenon-induced feature in normal brain tissue might be crucial for developing both early and long-term neuroprotection post-TBI. Indeed, xenon-induced long-term (20 months) protection was associated with reduced white matter loss and neuronal loss in the corpus callosum and hippocampal CA1 and dentate in a contralateral area in mice post-TBI [6]. Whether this protective effect of xenon is true for mice of different ages is still unknown, since aging may diminish xenon’s cytoprotection because of alterations of microcirculation in aging brain tissue causing less accumulation of the gas molecules [29].
There was a decrease (tendency) in the expression of the inflammatory genes Irf1 and Tlr2 at 6 h in the damaged area. This might be associated with the stabilization of the cytoplasmic and organoid membranes of activated immune cells of the nervous tissue and blood by xenon and its demonstrated anti-inflammatory effect.
Lastly, the rats’ neurological status 24 h after the injury and xenon treatment was evaluated. Xenon treatment (postconditioning) resulted in a better neurological outcome (tendency), as observed by Campos-Pires R. (2020) in a rat TBI model [8].
The study has some limitations. The animals from TBI and TBI + Xe received different concentrations of oxygen in the induction chamber (Δ ϕO2 = 5–9%). A high concentration of inhaling oxygen in rats leads to the alteration of inflammatory and antioxidative genes [30,31,32]. However, the concentration of oxygen is much higher (ϕO2 more than 80%) than in our study. Therefore, the difference in O2 in the inhaling air between the TBI and TBI + Xe groups was neglected.
Thus, xenon treatment or postconditioning in TBI has the potential to reduce inflammation in the brain-damaged area, and at the same time, it represents a mild stressor agent for intact brain tissue, which probably contributes to the activation of compensatory mechanisms in the brain, improving neurological outcomes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci11070889/s1, Table S1: The panel of genes for multiplex gene expression analysis.
Author Contributions
A.D.F. substantially contributed to the design of the study, team management, performance of the experiments, gene expression analysis and data interpretation, and was involved in the preparation of the manuscript and its publication. D.N.S. substantially contributed to the performance of the experiments, and took part in the revision of the manuscript and preparation for publication. I.A.R. substantially contributed to planning the study and performing the xenon treatment as an anesthesiologist. K.N.L. substantially contributed to the study design and performance of the experiments as a veterinarian. A.S.B. substantially contributed to assessing the organ states post-mortem as a pathologist. O.A.G. substantially contributed to team management and manuscript revision. V.M.P. substantially contributed to the design of the study and the major revision of the manuscript, and gave final approval for this version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Ministry of Science and Higher Education of the Russian Federation (No AAAA-A19-119032090049-0 and AAAA-A19-119100990120-3) and RFBR Grant No. 19-34-90072.
Institutional Review Board Statement
The experiments were carried out in accordance with the requirements of Directive 2010/63/EU of the European Parliament and the Council of the European Community on the protection of animals used for scientific purposes. The study was approved by the ethical committee of the Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitology (protocol No. 5/20/1, dated 23 December 2020).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Y.Z., Li T.T., Cao H.L., Yang W.C. Recent advances in the neuroprotective effects of medical gases. Med. Gas Res. 2019;9:80–87. doi: 10.4103/2045-9912.260649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson R., Franks N.P. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit. Care. 2010;14:229. doi: 10.1186/cc9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence J.H., Loomis W.F., Tobias C.A., Turpin F.H. Preliminary observations on the narcotic effect of xenon with a review of values for solubilities of gases in water and oils. J. Physiol. 1946;105:197–204. doi: 10.1113/jphysiol.1946.sp004164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Z., Piazza O., Ma D., Scarpati G., De Robertis E. Xenon anesthesia and beyond: Pros and cons. Minerva Anestesiol. 2019;85:83–89. doi: 10.23736/S0375-9393.18.12909-9. [DOI] [PubMed] [Google Scholar]
- 5.Amer A.R., Oorschot D.E. Xenon Combined with Hypothermia in Perinatal Hypoxic-Ischemic Encephalopathy: A Noble Gas, a Noble Mission. Pediatr. Neurol. 2018;84:5–10. doi: 10.1016/j.pediatrneurol.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Campos-Pires R., Hirnet T., Valeo F., Ong B.E., Radyushkin K., Aldhoun J., Saville J., Edge C.J., Franks N.P., Thal S.C., et al. Xenon improves long-term cognitive function, reduces neuronal loss and chronic neuroinflammation, and improves survival after traumatic brain injury in mice. Br. J. Anaesth. 2019;123:60–73. doi: 10.1016/j.bja.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldeman M., Coburn M., Rossaint R., Clusmann H., Nolte K., Kremer B., Höllig A. Xenon Reduces Neuronal Hippocampal Damage and Alters the Pattern of Microglial Activation after Experimental Subarachnoid Hemorrhage: A Randomized Controlled Animal Trial. Front. Neurol. 2017;8:511. doi: 10.3389/fneur.2017.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos-Pires R., Onggradito H., Ujvari E., Karimi S., Valeo F., Aldhoun J., Edge C.J., Franks N.P., Dickinson R. Xenon treatment after severe traumatic brain injury improves locomotor outcome, reduces acute neuronal loss and enhances early beneficial neuroinflammation: A randomized, blinded, controlled animal study. Crit. Care. 2020;24:667. doi: 10.1186/s13054-020-03373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franks N.P., Dickinson R., de Sousa S.L., Hall A.C., Lieb W.R. How does xenon produce anaesthesia? Nature. 1998;396:324. doi: 10.1038/24525. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson R., Peterson B.K., Banks P., Simillis C., Martin J.C., Valenzuela C.A., Maze M., Franks N.P. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: Evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 11.Gruss M., Bushell T.J., Bright D.P., Lieb W.R., Mathie A., Franks N.P. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol. Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 12.Bantel C., Maze M., Trapp S. Noble gas xenon is a novel adenosine triphosphate-sensitive potassium channel opener. Anesthesiology. 2010;112:623–630. doi: 10.1097/ALN.0b013e3181cf894a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahlenkamp A.V., Coburn M., Haase H., Kipp M., Ryang Y.M., Rossaint R., Beyer C. Xenon enhances LPS-induced IL-1β expression in microglia via the extracellular signal-regulated kinase 1/2 pathway. J. Mol. Neurosci. 2011;45:48–59. doi: 10.1007/s12031-010-9432-z. [DOI] [PubMed] [Google Scholar]
- 14.Lavaur J., Le Nogue D., Lemaire M., Pype J., Farjot G., Hirsch E.C., Michel P.P. The noble gas xenon provides protection and trophic stimulation to midbrain dopamine neurons. J. Neurochem. 2017;142:14–28. doi: 10.1111/jnc.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L., Zhao H., Weng H., Ma D. Lasting effects of general anesthetics on the brain in the young and elderly: “mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. J. Anesth. 2019;33:321–335. doi: 10.1007/s00540-019-02623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breuer T., Emontzpohl C., Coburn M., Benstoem C., Rossaint R., Marx G., Schälte G., Bernhagen J., Bruells C.S., Goetzenich A., et al. Xenon triggers pro-inflammatory effects and suppresses the anti-inflammatory response compared to sevoflurane in patients undergoing cardiac surgery. Crit. Care. 2015;19:365. doi: 10.1186/s13054-015-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feeney D.M., Boyeson M.G., Linn R.T., Murray H.M., Dail W.G. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 18.Jolkkonen J., Puurunen K., Rantakömi S., Härkönen A., Haapalinna A., Sivenius J. Behavioral effects of the α2-adrenoceptor antagonist, atipamezole, after focal cerebral ischemia in rats. Eur. J. Pharmacol. 2000;400:211–219. doi: 10.1016/S0014-2999(00)00409-X. [DOI] [PubMed] [Google Scholar]
- 19.Sundaram V.K., Sampathkumar N.K., Massaad C., Grenier J. Optimal use of statistical methods to validate reference gene stability in longitudinal studies. PLoS ONE. 2019;14:e0219440. doi: 10.1371/journal.pone.0219440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubern C., Hurtado O., Rodríguez R., Morales J.R., Romera V.G., Moro M.A., Lizasoain I., Serena J., Mallolas J. Validation of housekeeping genes for quantitative real-time PCR in in-vivo and in-vitro models of cerebral ischaemia. BMC Mol. Biol. 2009;10:57. doi: 10.1186/1471-2199-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swijsen A., Nelissen K., Janssen D., Rigo J.M., Hoogland G. Validation of reference genes for quantitative real-time PCR studies in the dentate gyrus after experimental febrile seizures. BMC Res. Notes. 2012;5:685. doi: 10.1186/1756-0500-5-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholami K., Loh S.Y., Salleh N., Lam S.K., Hoe S.Z. Selection of suitable endogenous reference genes for qPCR in kidney and hypothalamus of rats under testosterone influence. PLoS ONE. 2017;12:e0176368. doi: 10.1371/journal.pone.0176368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C., Iashchishyn I.A., Kara J., Foderà V., Vetri V., Sancataldo G., Marklund N., Morozova-Roche L.A. Proinflammatory and amyloidogenic S100A9 induced by traumatic brain injury in mouse model. Neurosci. Lett. 2019;699:199–205. doi: 10.1016/j.neulet.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Qiu X., Ping S., Kyle M., Longo J., Chin L., Zhao L.R. S100 Calcium-Binding Protein A9 Knockout Contributes to Neuroprotection and Functional Improvement after Traumatic Brain Injury. J. Neurotrauma. 2020;37:950–965. doi: 10.1089/neu.2018.6170. [DOI] [PubMed] [Google Scholar]
- 25.Sun J., Pan X., Christiansen L.I., Yuan X.L., Skovgaard K., Chatterton D.E.W., Kaalund S.S., Gao F., Sangild P.T., Pankratova S. Necrotizing enterocolitis is associated with acute brain responses in preterm pigs. J. Neuroinflamm. 2018;15:180. doi: 10.1186/s12974-018-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khlusov I.A., Naumov S.A., Vovk S.M., Kornetov N.A., Shpisman M.N., Lukinov A.V., Naumov A.V. Vliianie ksenona na kletki i retseptory [Xenon effects on cells and receptors] Vestn. Ross. Akad. Med. Nauk. 2003;9:32–37. (In Russian) [PubMed] [Google Scholar]
- 27.Armstrong S.P., Banks P.J., McKitrick T.J., Geldart C.H., Edge C.J., Babla R., Simillis C., Franks N.P., Dickinson R. Identification of two mutations (F758W and F758Y) in the N-methyl-D-aspartate receptor glycine-binding site that selectively prevent competitive inhibition by xenon without affecting glycine binding. Anesthesiology. 2012;117:38–47. doi: 10.1097/ALN.0b013e31825ada2e. [DOI] [PubMed] [Google Scholar]
- 28.Wyllie J., Bruinenberg J., Roehr C.C., Rüdiger M., Trevisanuto D., Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249–263. doi: 10.1016/j.resuscitation.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Amano T., Meyer J.S., Okabe T., Shaw T., Mortel K.F. Stable xenon CT cerebral blood flow measurements computed by a single compartment--double integration model in normal aging and dementia. J. Comput. Assist. Tomogr. 1982;6:923–932. doi: 10.1097/00004728-198210000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Gerstner B. Interaction of inflammation and hyperoxia in a rat model of neonatal white matter damage. PLoS ONE. 2012;7:e49023. doi: 10.1371/journal.pone.0049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright C.J., Dennery P.A. Manipulation of gene expression by oxygen: A primer from bedside to bench. Pediatr. Res. 2009;66:3–10. doi: 10.1203/PDR.0b013e3181a2c184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greco F., Wiegert S., Baumann P., Wellmann S., Pellegrini G., Cannizzaro V. Hyperoxia-induced lung structure-function relation, vessel rarefaction, and cardiac hypertrophy in an infant rat model. J. Transl. Med. 2019;17:91. doi: 10.1186/s12967-019-1843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.