Graphical abstract
Key Words: anatomy, cardiac electrophysiology, pacing
Abbreviations and Acronyms: HBP, His bundle pacing; LBB, left bundle branch; LBBB, left bundle branch block
Painful left bundle branch block (LBBB) syndrome is a disorder whereby rate-related ventricular conduction aberrancy can cause disabling symptoms with significant activity limitation. Its true prevalence in the general population is unknown due to coexisting coronary artery disease (CAD) and lack of widespread awareness of its existence, but it is overall considered a rare cause of chest pain (1,2).
Since first described in 1946, a variety of treatment strategies have been utilized with variable results, suggesting a complex underlying pathophysiology. Although incompletely understood, the proposed mechanisms are probably related to significant left ventricular dyssynchronous systolic septal motion due to early septal radial inward thickening followed by late posterior inward thickening.
Selective pacing of the atrioventricular conduction axis, usually known as His bundle pacing, is currently considered the most physiological methods for maintaining intrinsic atrioventricular conduction. His-bundle pacing has also been successfully employed to treat painful left bundle branch (LBB) syndrome by correction of the wide QRS complex and restoration of electrical dyssynchrony (3). Corrective His bundle pacing (HBP) is possible due to the proximal and focal nature of lesions causing intra- or infra-Hisian conduction abnormalities, resulting in complete conduction block or asynchronous conduction between longitudinally dissociated, pre-destined Purkinje fibers. The hypothesis for HBP to correct LBBB is that increased pacing output penetrates the LBBB region beyond the area of conduction block and hence normalizes LBBB by direct recruitment of the LBB (4). HBP represents an appealing strategy for the treatment of painful LBBB syndrome. However, HBP has some limitations, including knowledge of the precise location of the His bundle, which varies significantly between individual patients; the frequent occurrence of high and unstable pacing threshold; abnormal sensing; and permanent damage to the His bundle acutely or chronically. Because of these limitations, the QRS duration cannot be normalized in almost one-half of patients where HBP is achieved (5,6).
Conduction system pacing via the LBB (known as left bundle pacing [LBP]) has recently emerged as an alternative strategy for cardiac resynchronization therapy. LBP via intraseptal lead fixation can bypass conduction system pathology more distal to the left-sided His to produce near physiological activation of the heart with lower thresholds than corrective His bundle pacing. In this issue of JACC: Case Reports, Garg et al. (7) described the first 2 cases with painful LBBB syndrome successfully treated with LBP in combination with AV fusion, leading to complete alleviation of symptoms and resolution of the LBBB with remarkable narrowing of QRS.
Although this is one of the least frequent indications for LBP, the authors should be commended by their adequate patient selection, as evidenced by their great clinical outcome and by the beautifully descriptive iconography provided in their case report. In this “Da Vinci” corner, we sought to review the anatomical determinants necessary to understand LBB pacing and the normal anatomy (and its variants) of the left bundle branch in the human heart.
Location of the His Bundle as a Reference for LBB Pacing
In 2017, Huang et al. (8) reported LBP, which is a transvenous, transseptal implantation technique resulting in LBB capture, usually with low pacing capture thresholds. With the position of the His recording as a reference, the initial site for LBP was between 0 to 15 mm below the distal tricuspid valve annulus distal (ventricular aspect) and apical to the His position in right anterior oblique (RAO) (30°) fluoroscopic view. Thus, the fluoroscopic location of the His bundle is of critical importance as it helps identify an initial tentative position to deploy the LBP lead. Precise knowledge of the location of the atrial and penetrating components of the atrioventricular conduction axis and its relation with the His position as reference during LBP is of paramount importance for successful LBP.
In our anatomical studies, we have encountered marked interindividual differences, with significant variations in the site of transition from the atrioventricular node to the bundle of His relative to the tricuspid valve annulus and the atrioventricular membranous septum. As we have emphasized in a recent publication (9), we followed the precedent of Tawara (10) when taking the site of penetration into the insulating tissues of the central fibrous body as representing the transition from the node to the bundle of His. This penetration into the fibrous tissues of the central fibrous body occurred superiorly in 58.5% of specimens, with the transition found inferiorly within the triangle of Koch in the remaining 41.5% of the hearts. Consequently, we observed that the axis penetrated on the atrial side of the hinge-line in just over one-half of the hearts, whereas in the remainder, the transition was either at the hinge-line or on its ventricular aspect. This is in keeping with our findings regarding the location of the largest His bundle deflection relative to the septal leaflet and the angiographic vertex of the triangle of Koch. These anatomical variations are of importance for the interventional electrophysiologist when assessing for the best location for lead deployment into the LBB.
The LBB System
The membranous septum is divided into atrioventricular and interventricular component at the base of the interleaflet triangle between the right and the noncoronary leaflets of the aortic valve. The length of the membranous septum is highly variable, with a mean value of 4.6 ± 1.5 mm, and a range from 1 to 9 mm. We observed that in almost three-fifths of the specimens, the interventricular component of the membranous septum was very small or nonexistent. In those cases, we found a rapid take-off of the fascicles of the LBB at the level of the hinge of the septal leaflet of the tricuspid valve.
Unlike HBP, where the target zone for effective pacing is very small, the LBB fibers, despite presenting large interindividual variations, are widely distributed as a subendocardial network, and it may be easier to reach some of the LBB areas during the intraseptal implantation technique. At the implantation of LBP, the pacing lead penetrates the ventricular septum to reach the left ventricular subendocardial trunk or ramifications of the left bundle subsequent to the branching of the axis. This part of the axis extends usually, but not always, from the inferior border of the membranous septum, sandwiched between the septum and the crest of the muscular ventricular septum. After penetrating the AV membranous septum, the conduction axis has a nonbranching component that, in 75% to 85% of cases, runs only for a short distance (1 to 3 mm) along the septal crest before giving rise to the fascicles of the left bundle (LBB) on the septal surface (Figure 1). The most anterior fibers of the LBB originate at the end of the branching portion located underneath the inferior edge of the interventricular membranous septum or—in a minority of cases—within the inferior margin of the membranous septum. Histological studies also demonstrated variations in some cases, where the His bundle distal to the central fibrous body traversed the left side of the muscular interventricular septum (IVS) between 2 and 3.5 mm below the membranous septum, or the His bundle anterior to the central fibrous body coursed to the right of the crest of the muscular IVS. As described by Massing et al. (11), in 5 hearts with “right-sided His bundles,” the right bundle branch (RBB) formed a direct continuation of the His bundle, whereas in the most common setting (27 cases), the His bundle–RBB junction formed a definite obtuse angle.
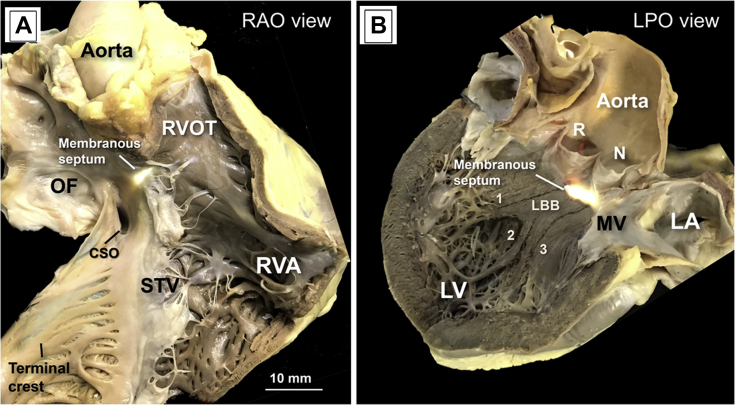
Figure 1.
Macroscopic View From a Human Postmortem Heart in Simulated RAO and LPO View
When viewed from the right atrial cavity (A), the fibrous membranous septum forms the apex of the triangle of Koch (transillumination). The hinge of the septal leaflet of the tricuspid valve (STV) provides the dividing line between the atrioventricular and interventricular components of the membranous septum. (B) The LPO view shows the transilluminated membranous septum located inferior to the interleaflet triangle between the right (R) and noncoronary (N) sinus of the aortic valve. Note that we have highlighted in dark color the limits of the endocardial position of the left bundle branch (LBB) of His and its 3 fascicles, the left anterior (1), the left septal or middle (2), and the left posterior (3). LPO = left posterior oblique; RAO = right anterior oblique.
The most impressive feature of the LBB anatomy was its marked variability between individuals. The origin of the LBB is broad in some and narrow in others (ranging from <1 to 14 mm) and is significantly influenced by the anatomical relationship of the His bundle to the IV septum (9,11). As it coursed down the IVS from base toward apex, the LBB widens, in some hearts abruptly, and in others more gradually. The size, number, location, configuration, and distribution of LBB subdivisions is still a matter of active debate. Some researchers consider the LBB an exclusively bifascicular structure with an anterior and a posterior ramification (12). Early electrophysiological data from Durrer et al. (13), showed that 3 distinct endocardial areas in the left ventricle are synchronously activated during the first 5 ms of cardiac excitation. These 3 islands of initial excitation may reasonably be assumed to correspond to the terminal areas of the 3 main parts of the LBB system. Although some authors described the branching of the LBB as “unpredictable,” histopathological investigations confirmed the consistent presence of 3 rather than 2 main peripheral networks comprised of a thin and elongated anterior radiation, a wider posterior one and a third radiation designated to cover the midseptal surface (9,14, 15, 16). The septal branch emerged in most cases from the common left bundle, but in some cases from the anterior, from posterior radiations, or by a complex plexus of ramification given by both the anterior and posterior fascicles. The main LBB trunk (i.e., the target of the lead to be deployed) extends inferiorly 10 to 15 mm toward the apex; therefore, it is likely that in most cases, the pacing lead penetrates the midseptal area at this level of the conduction axis and is able to excite the LBB. However, based on the complexity and variability of the LBB system topography, the transseptal lead may often times reach the midseptal branch or even a wide posterior ramification (Figure 2).
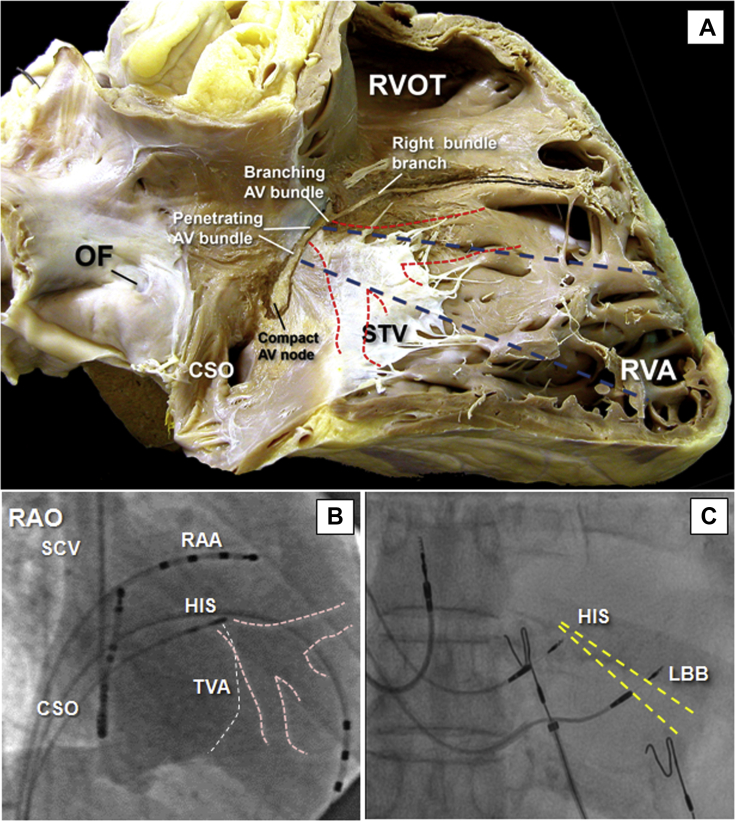
Figure 2.
Anatomy and Fluoroscopy of the Left Bundle Branch
(A) A gross dissection of the human heart to reveal in simulated right anterior oblique view (RAO) the location of the compact atrioventricular (AV) node and penetrating AV bundle relative to the septal leaflet of the tricuspid valve (STV) anteriorly. In this case, the transition from the compact AV node to the penetrating AV bundle occurs inferiorly, and the length of the AV bundle implies a large His bundle recording zone. A dashedblue line marks the position of the His bundle as a reference to identify the initial site for left bundle (LB) pacing about 0 to 15 mm below the STV tricuspid valve annulus distal (ventricular) and apical to the His position. Red dashed lines indicate the schematics of the left bundle branch (LBB) on the left side of the interventricular septum. (B) An RAO 50º fluoroscopic view of a right atrial angiography taken during an EP study to display the tricuspid valvar annulus (TVA) and the relationship between the site of recording of the largest His bundle potential (His) and the landmarks of the triangle of Koch. In this case, the His bundle was recorded superiorly at the vertex of the triangle. Pink dashed lines indicate the schematics of the LBB on the left side of the interventricular septum and the tricuspid valve annulus. (C) Fluoroscopy with minimal RAO view obtained during the implantation of LBP. Note the different fluoroscopic position of the leads when modifying the RAO angulation. The relationship with a His pacing lead is observed. The LBP lead is deployed ≈1.5 cm distally and ≈0.5 cm more inferiorly to achieve, via transseptal deployment, pacing of the LBB. Yellow dashed lines indicate the angulation and possible location of the LBB with respect to the His bundle.
The Risk of LBB Injury and Cardiac Perforation
During LBP implantation, it is important to place the pacing lead perpendicular to the ventricular septum to avoid an excessively apical position, where the IVS becomes thicker. Following this recommendation improves the ability to rapidly advance the pacing lead helix into the left side of the IVS. Observing a unique electrocardiographic transition and an LBB potential is useful to guide the lead advancement inside the IVS while pacing. During intraseptal lead fixation, the paced electrocardiogram morphology, impedance trends, and characteristic findings of LBB capture are assessed using different pacing outputs. The presence of LBB current of injury may suggest that the tip of the lead is in close contact with the left-sided conduction system with possible penetration into the fibrous insulation of the LBB (17).
As the LBB runs subendocardially on the left ventricular septum, septal perforation is possible during LBP using this technique. Anatomic and histological information provide guidance with regard to the depth of lead penetration to avoid septal perforation into the left ventricle. There are a number of determinants that may influence the development of LBB injury during the procedure. The thickness of the IVS is not just composed of working myocardium, but one needs to consider the: 1) variable thickness of the right ventricular endocardium; 2) fatty infiltration in the elderly; 3) connective tissue surrounding the LBB; and 4) variable amount of fibrous tissue that may be linked to a patient’s conduction disorder. Moreover, the LBB thickness can be extremely variable and involve from 1 to 25 cell layers of specialized smooth myocardium conducting tissue, which is surrounded by a varying but considerable thickness of left subendocardial collagen and smooth muscle. This is illustrated in Figure 3. It is rather obvious that multiple lead deployments in this region can result in permanent RBB injury, cardiac perforation and/or damage to a septal artery.
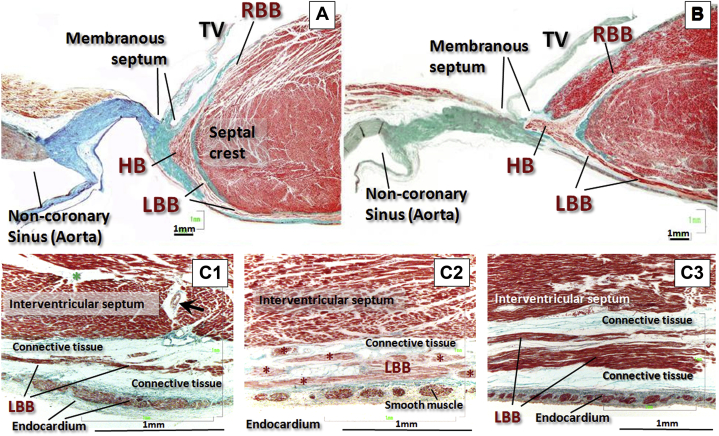
Figure 3.
Histological Findings of the His Bundle Area and the LBB Area
(A and B) Histological sections (stained with Masson's trichrome) through the division of the bundle of His and its right and left branches at the level of the interventricular component of the membranous septum, being attached inferiorly to the crest of the muscular interventricular septum. Note the relationship of the left branch (always subendocardial) with the nadir of the noncoronary leaflet of the aortic valve. (B) The right branch is running intramyocardially 1 or 2 mm from the endocardium of the right ventricle. (C1, C2, C3) Histological sections of 3 specimens stained with Masson's trichrome of the left branch 12 to 20 mm distal from its origin. The left branch is located between the muscular septum and the endocardium of the left ventricle. The thickness of the muscle fibers of the left branch is very variable, small in C1 and abundant in C3; the endocardium has smooth muscle cells in its thickness; and surrounding the left branch there is connective tissue of variable thickness. C1: Green star indicates fibrous tissue; black arrow indicates septal artery. ∗Left bundle branch myocytes separated by adipose/connective tissue. HB = His bundle; LBB = left bundle branch; RBB = right bundle branch; TV = tricuspid valve.
In conclusion, in this Da Vinci Corner editorial, we have described the most common location of the His bundle to help guide the location of the LBB. In addition, we have summarized the branching patterns of the LBB and the determinants of LBB injury during LBP. We hope this editorial will be useful for the implanting physicians that seek to perform anatomy-guided LBP in the safest and most effective way possible.
Acknowledgment
The authors would like to recognize with this paper the huge effort made by the global population, and especially the health care workers, in the fight against COVID-19.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Eichert H. Transient bundle branch block associated with tachycardia. Am Heart J. 1946;31:511–518. doi: 10.1016/0002-8703(46)90436-x. [DOI] [PubMed] [Google Scholar]
- 2.Heinsimer J.A., Skelton T.N., Califf R.M. Rate-related left bundle branch block with chest pain and normal coronary arteriograms treated by exercise training. Am J Med Sci. 1986;292:317–319. doi: 10.1097/00000441-198611000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Shvilkin A., Ellis E.R., Gervino E.V., Litvak A.D., Buxton A.E., Josephson M.E. Painful left bundle branch block syndrome: Clinical and electrocardiographic features and further directions for evaluation and treatment. Heart Rhythm. 2016;13:226–232. doi: 10.1016/j.hrthm.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S., Zhou X., Gold M.R. Left bundle branch pacing: JACC review topic of the week. J Am Coll Cardiol. 2019;74:3039–3049. doi: 10.1016/j.jacc.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Viles-Gonzalez J.F., Mahata I., Anter E., d'Avila A. Painful left bundle branch block syndrome treated with his bundle pacing. J Electrocardiol. 2018;51:1019–1022. doi: 10.1016/j.jelectrocard.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Upadhyay G.A., Vijayaraman P., Nayak H.M. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol. 2019;74:157–159. doi: 10.1016/j.jacc.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Garg A., Master V., Ellenbogen K.A., Padala S.K. Painful left bundle branch block syndrome successfully treated with left bundle branch area pacing. J Am Coll Cardiol Case Rep. 2020;2:568–571. doi: 10.1016/j.jaccas.2019.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W., Su L., Wu S. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33 doi: 10.1016/j.cjca.2017.09.013. 1736 e1–3. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera J.-Á., Anderson R.H., Macías Y. Variable arrangement of the atrioventricular conduction axis within the triangle of Koch: implications for permanent His bundle pacing. J Am Coll Cardiol EP. 2020;6:362–377. doi: 10.1016/j.jacep.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Tawara S. Fischer; 1906. Das reizleitungssystem des Säugetierherzens. [Google Scholar]
- 11.Massing G.K., James T.N. Anatomical configuration of the His bundle and bundle branches in the human heart. Circulation. 1976;53:609–621. doi: 10.1161/01.cir.53.4.609. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum M.B., Elizari M.V., Lazzari J.O. Paidos; Buenos Aires: 1968. Los hemibloqueos: por Mauricio B. Rosenbaum, Marcelo V. Elizari y Julio O. Lazzari. [Google Scholar]
- 13.Durrer D., Van Dam R.T., Freud G., Janse M., Meijler F., Arzbaecher R. Total excitation of the isolated human heart. Circulation. 1970;41:899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- 14.Uhley H.N., Rivkin L.M. Visualization of the left branch of the human atrioventricular bundle. Circulation. 1959;20:419–421. doi: 10.1161/01.cir.20.3.419. [DOI] [PubMed] [Google Scholar]
- 15.Rossi L. Sistema di conduzione trifascicolare ed emiblocchi di branca sinistra. Considerazioni anatomiche ed istopatologiche. G Ital Cardiol. 1971;1:55–62. [PubMed] [Google Scholar]
- 16.Demoulin J.C., Kulbertus H.E. Histopathological examination of concept of left hemiblock. Br Heart J. 1972;34:807–814. doi: 10.1136/hrt.34.8.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L., Xu T., Cai M. Electrophysiological characteristics and clinical values of left bundle branch current of injury in left bundle branch pacing. J Cardiovasc Electrophysiol. 2020;31:834–842. doi: 10.1111/jce.14377. [DOI] [PubMed] [Google Scholar]