Abstract
A 52-year-old man following surgery for Ebstein’s anomaly after repair developed acute hemodynamically significant left ventricular outflow tract obstruction with systolic anterior motion of the mitral valve and severe mitral regurgitation. Fluid resuscitation and weaning of inotropes were unsuccessful. Left ventricular outflow tract obstruction and mitral regurgitation resolved by using esmolol. (Level of Difficulty: Intermediate.)
Key Words: Ebstein’s anomaly, tricuspid valve, left ventricle, postoperative
Abbreviations and Acronyms: ASD, atrial septal defect; LV, left ventricular; LVOT, left ventricular outflow tract; MRI, magnetic resonance imaging; RV, right ventricular; TEE, transesophageal echocardiogram; SAM, systolic anterior motion
Graphical abstract
A 52-year-old man following surgery for Ebstein anomaly after repair developed acute hemodynamically significant left ventricular outflow tract…
History of Presentation
A 52-year-old male was diagnosed with severe Ebstein malformation of the tricuspid valve (Carpentier type D) and a small secundum atrial septal defect (ASD) when he presented acutely with breathlessness, mild hypoxia, and progressively decreasing exercise tolerance. Echocardiogram and magnetic resonance imaging (MRI) demonstrated anatomy consistent with Ebstein’s anomaly, with moderate to severe tricuspid regurgitation, a dilated right atrium and right ventricular (RV) outflow tract, and small left ventricle. From his past medical history, he had known Ebstein’s anomaly.
Learning Objectives
-
•
To understand the anatomy and pathophysiology of Ebstein’s anomaly.
-
•
To understand postoperative management and hemodynamics of an Ebstein patient, in the intensive care unit setting
Differential Diagnosis
Dilated cardiomyopathy, arrhythmogenic right ventricle cardiomyopathy, could have been considered as a differential diagnosis. In this case, the crucial aspect was making the right diagnosis and management of the case postoperatively, as the patient became progressively hypotensive, oliguric, and acidotic despite fluid resuscitation and inotropic support.
Investigations
Preoperative echocardiogram and cardiac MRI demonstrated moderate-to-severe tricuspid regurgitation, dilated right atrium (right atrial area of 35 cm2), dilated RV outflow tract, small left ventricular (LV) volumes and a structurally normal mitral valve with no regurgitation (Figure 1). The echocardiogram and an MRI scan helped us to establish our diagnosis. Postoperative transesophageal echocardiogram (TEE) played a key role in making the diagnosis as the patients was progressively deterioratiNG.
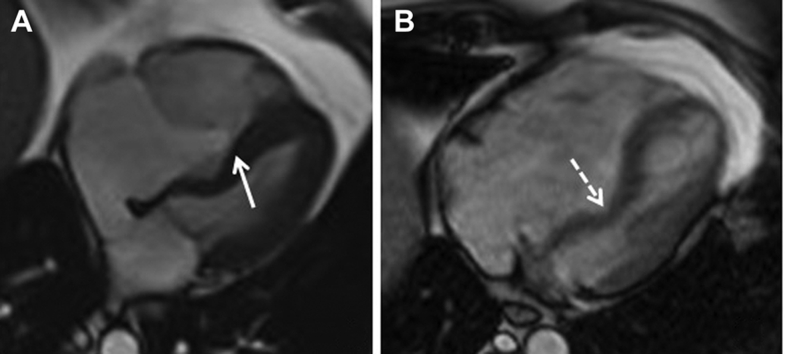
Figure 1.
Preoperative Cardiac MRI
Apical 4-chamber magnetic resonance images taken (A) in end-diastole and (B) systole. (A) The apical displacement of the point of coaptation of the tricuspid valve leaflets (solid arrow) is seen, allowing a large “atrialized” portion of the inlet of the right ventricle. (B) There is significant deviation of the atrialized septum (dashed arrow) into the small-sized left ventricular cavity.
Management
At the start of the operation, TEE confirmed the earlier findings, with no left ventricular outflow tract (LVOT) obstruction or mitral regurgitation. Because repair of the tricuspid valve was not feasible on inspection of the valve, he underwent elective tricuspid valve replacement (Carpentier-Edwards 33 mm), along with plication of the atrialized right ventricle and right atrium, “reinforcement” of RV inlet and direct ASD closure. The patient was successfully weaned off cardiopulmonary bypass and immediate TEE demonstrated good tricuspid valve function with trace mitral regurgitation. On return to the intensive care unit, the patient became progressively hypotensive, oliguric, and acidotic, despite adequate fluid resuscitation, requiring escalating doses of noradrenaline. On auscultation, there was a new grade 2/6 ejection systolic murmur over his left lower sternal edge and a grade 3/6 pan-systolic murmur over his apex.
Emergency TEE demonstrated significant turbulence within the LVOT, with a peak velocity above 4 m/s. There was significant systolic anterior motion (SAM) of the anterior leaflet of the mitral valve with resulting incoaptation of leaflets and severe mitral regurgitation (Figures 2A to 2D). Furthermore, there was reduced opening of the aortic valve because of the narrow LVOT jet directing blood flow through the posterior and left coronary cusps (Figures 2E to 2F). Despite further fluid resuscitation and weaning of the vasopressors and inotropes, there was little improvement in his hemodynamic status, and the decision was made to commence esmolol infusion. Within a few minutes of starting esmolol (starting dose 25 μg/kg/min), the LVOT obstruction significantly improved and there was no significant SAM of the mitral valve, with mild mitral regurgitation (Figures 3A to 3B). Over the next 12 h, his hemodynamic and metabolic status improved (Figures 4A to 4B), and he was extubated the following day. Esmolol was gradually weaned, and after 48 h he was discharged from the intensive care unit and continued to recover uneventfully.
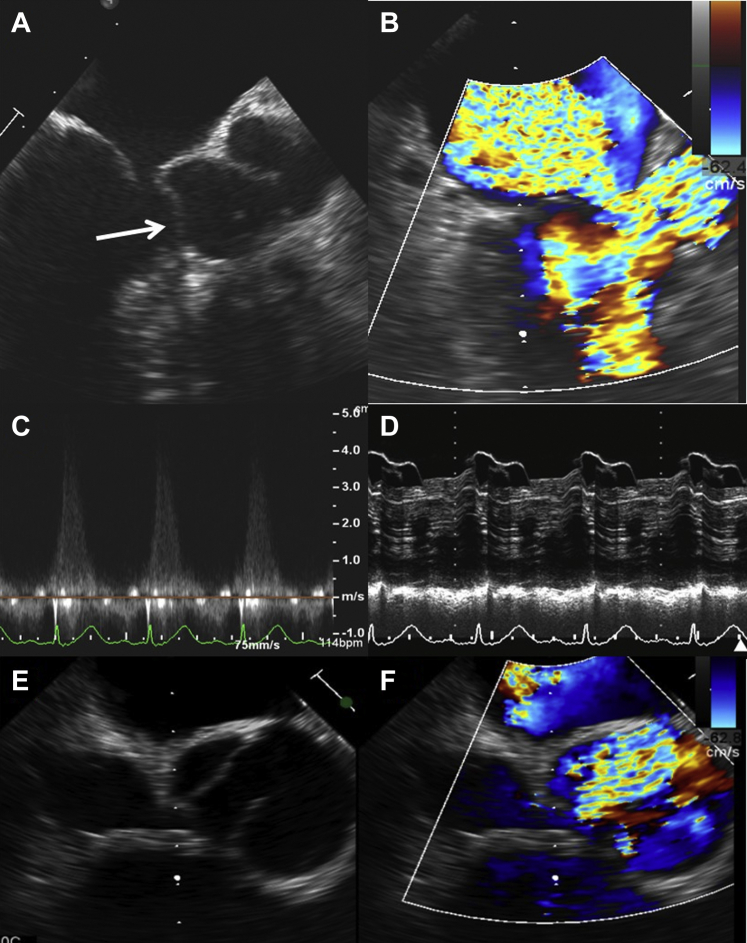
Figure 2.
Postoperative TEE Demonstrating LVOT Obstruction and Significant MR
Postoperative transesophageal images demonstrating in (A) incoaptation of the mitral valve leaflets with significant SAM of the anterior mitral leaflet (white arrow). In the same view in B, color Doppler demonstrates significant turbulence within the LVOT and severe mitral regurgitation. (C) The peak velocity obtained across the LVOT was over 4 m/s despite perfect alignment of the Doppler beam, suggesting severe obstruction. (D) M-mode demonstrating significant SAM of the anterior mitral leaflet. (E) Reduced opening of the aortic valve in systole because of the jet generated by the LVOT obstruction, seen in F. LVOT = left ventricular outflow tract; MR = mitral regurgitation; SAM = systolic anterior motion; TEE = transesophageal echocardiogram.
Figure 3.
TEE Images After Interruption of Inotropes and Start of Beta-Blocker
(A) After interruption of inotropes and administration of esmolol, there is mild turbulence in the LVOT and the SAM is mild, with no significant incoaptation of the mitral valve leaflets and mild MR. (B) The peak gradient across the LVOT is near-normal. Abbreviations as in Figure 2.
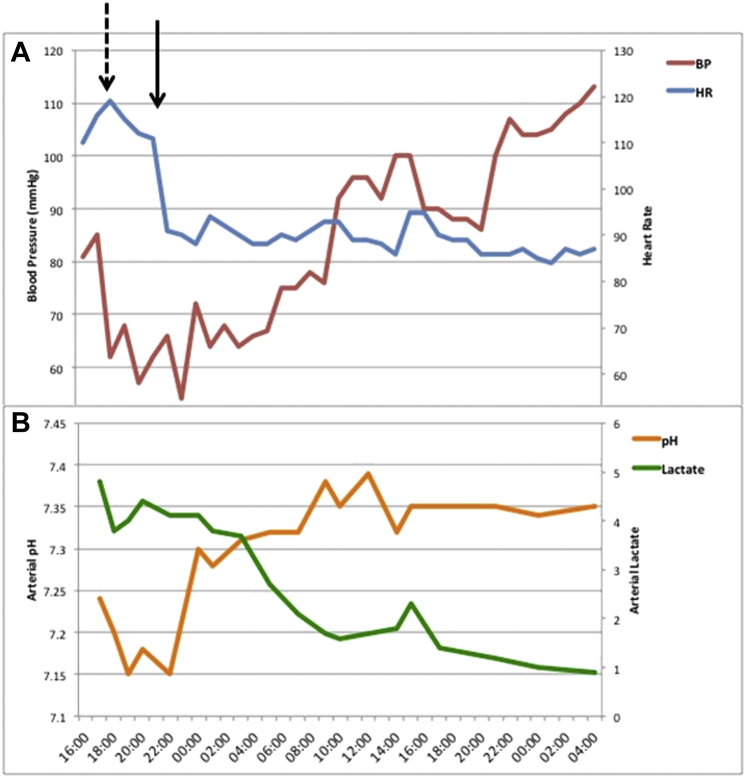
Figure 4.
Change in Postoperative Hemodynamics and Biochemistry
Changes (A) in heart rate (HR) and blood pressure (BP) and (B) arterial pH and serum lactate postoperatively. Dashed arrow indicates the time when diagnosis of LVOT obstruction was made and inotropes were weaned unsuccessfully; the solid arrow represents commencement of esmolol infusion.
Discussion
Ebstein’s anomaly accounts for 1% of congenital heart disease cases, with an incidence of approximately 1 per 200,000 live births (1). The malformation affects the tricuspid valve and right ventricle in a variable manner and is characterized by adherence of the valve leaflets to the underlying myocardium, apical displacement of the functional annulus, dilatation of the “atrialized” portion of the right ventricle, redundancy, fenestrations and tethering of the anterior leaflet and dilatation of the right atrioventricular junction (2, 3, 4). It can be associated with other congenital lesions such as ASDs, ventricular septal defects, left ventricular noncompaction and abnormalities of the mitral valve (5). Even in the absence of associated lesions, patients with Ebstein’s anomaly can develop left ventricular dysfunction, which may influence perioperative risk.
Rare case reports describe an association between Ebstein’s anomaly and LVOT obstruction, usually in the context of hypertrophic obstructive cardiomyopathy (6,7). We present the case of a patient who developed hemodynamically significant LVOT obstruction following surgical repair for Ebstein’s anomaly, in the absence of hypertrophic cardiomyopathy or mitral valve abnormalities. Below, we discuss the potential pathophysiological mechanisms involved, management options, and review the literature for the association between Ebstein’s anomaly and LVOT obstruction.
We searched Medline and EMBASE for any literature on LVOT obstruction and SAM of the mitral valve in patients with Ebstein’s anomaly. We included global publications in all ages and languages as far back as the databases would go. After screening through references for additional papers, we found 6 case reports of patients with Ebstein’s anomaly who had evidence of LVOT obstruction (Table 1).
Table 1.
Case Reports Reporting the Presence of LVOTO in Patients With Ebstein’s Anomaly
| First Author (Ref. #) | Year | Pre- or Post-Operative | Associated Lesions | Cause of LVOT |
|---|---|---|---|---|
| Isobe (8) | 1996 | Pre-operative | ASD and accessory mitral valve | Parachute-shaped accessory mitral tissue |
| de Agustin (7) | 2008 | Pre-operative | HOCM | HOCM |
| Ulus (9) | 2011 | Pre-operative | Enlarged, redundant mitral valve leaflets and chordae | Anterior leaflet mitral valve |
| Lee (6) | 2016 | Pre-operative | HOCM | HOCM |
| Waterhouse (10) | 2016 | Pre-operative | Nil | Bowing of atrialized RV septum into LVOT |
| Hirata (11) | 2016 | Pre-operative | SAM and severe MR | Bowing of atrialized RV septum into LVOT |
ASD = atrial septal defect; HOCM = hypertrophic obstructive cardiomyopathy; LVOT = left ventricular outflow tract; MR = mitral regurgitation; RV = right ventricular; SAM = systolic anterior motion.
Mechanisms of LVOT obstruction in Ebstein’s anomaly
A spectrum of left heart abnormalities can coexist with Ebstein’s anomaly (5). In a cohort of 106 consecutive Ebstein patients undergoing echocardiographic evaluation at the Mayo Clinic, 39% were found to have an abnormality of the left-sided myocardium or valves (5). Mechanisms responsible for LVOT obstruction in Ebstein’s anomaly include accessory mitral valve tissue (8), enlargement and redundancy of mitral valve leaflets (9) and, more commonly, coexisting features of hypertrophic cardiomyopathy (6,7). However, LVOT obstruction has also been noted in Ebstein patients with no associated left heart abnormalities. Severe atrialization of the ventricular septum with severe tricuspid regurgitation and a regurgitant jet directed toward the atrialized septum can result in significant deviation of the basal septum into the LVOT, causing obstruction at rest, with or without SAM of the mitral valve (10,11). Our patient had no demonstrable LVOT obstruction, neither on echocardiogram or MRI preoperatively. We submit that hemodynamically new, significant LVOT obstruction can develop postoperatively in Ebstein patients because of the underlying anatomical substrate (e.g., reduced left ventricular end-diastolic dimensions, dyskinetic basal ventricular septum, small LV volumes), hypovolemia, and a hyperdynamic state exacerbated by inotropes. Indeed, adequate fluid resuscitation and interruption of inotropes was not sufficient to correct the LVOT obstruction, and beta blockade was required to achieve hemodynamic improvement.
In cases of severe Ebstein malformation, the apical displacement of the tricuspid annulus results in the “atrialization” of the basal ventricular septum, which may be responsible for the predisposition to LVOT obstruction. This refers to the portion of the septum lying between the true and functional annulus and, in Ebstein’s anomaly, is typically thin and dyskinetic, devoid of any muscle tissue (12). Elevated right atrial pressures resulting from the tricuspid regurgitation and reduced compliance of the small (functional) right ventricle result in fixed deviation of the atrialized septum toward the LV, reducing the effective LV diameter and outflow tract (10,11). Both pathology (13) and echocardiographic (14) studies confirm the presence of altered LV geometry in Ebstein’s anomaly. On echocardiography (14), patients with Ebstein’s anomaly had higher LV eccentricity index scores compared with morphologically normal hearts (1.35 ± 0.23 vs. 1.02 ± 0.05) and a higher ratio of RV-to-LV cavity size (1.7 ± 0.44 vs. 0.65 ± 0.30). LV eccentricity correlated well with markers of Ebstein severity, such as the area of the functional right atrium and the degree of tricuspid valve displacement. All Ebstein patients had “paradoxical” motion of their atrialized septum and these alterations in geometry were associated with impaired LV function, as determined by radionuclide angiography (14). This can act as an anatomical substrate for LVOT obstruction.
Minimizing the risk of LVOT obstruction in Ebstein’s anomaly
We would advocate caution in escalating inotropes when faced with postoperative hypotension and hemodynamic instability in Ebstein patients. TEE is instrumental in differentiating between LVOT obstruction and other causes of hemodynamic compromise (e.g., ventricular dysfunction, tamponade). In the case reported here, further inotropic support would have proven detrimental, promoting further dynamic LVOT obstruction and mitral regurgitation. Given acceptable biventricular function, interruption of inotropes and initiation of a short-acting beta-blocker resulted in prompt resolution of both the LVOT obstruction and mitral regurgitation, with an obvious hemodynamic improvement.
Surgery for Ebstein’s anomaly aims at repairing the tricuspid valve, and resorts to a valve replacement only when repair is not achievable. The cone procedure is nowadays commonly employed and is applicable to almost all anatomical types of Ebstein’s anomaly (15). This uses valve tissue shaped in a cone rather than as a monocusp valve, which appears to improve durability of the valve repair. Da Silva recommended performing this repair early in life, around 5 years of age, to avoid the development of long-term complications. In fact, by performing an early childhood repair, one can hope for restoration of some muscle in the previously atrialized portion of the ventricular septum, avoiding protrusion into the LVOT and reducing the risk of dynamic obstruction (16).
Follow-Up
Six months after discharge, our patient has recovered fully, and remains asymptomatic with a significantly improved exercise tolerance. Transthoracic echocardiography 6 months after his operation demonstrated a normal sized LV cavity with no evidence of LVOT obstruction, trivial mitral regurgitation, and a well-functioning tricuspid valve prosthesis.
Conclusions
In patients with Ebstein’s anomaly, LVOT obstruction can result from abnormalities of the mitral valve or underlying myocardium, or can occur in the absence of any associated left-sided abnormalities. Deviation of the thin atrialized basal ventricular septum can alter the structure and geometry of the left ventricle and lead to dynamic LVOT obstruction, exacerbated by changes in filling status and inotropy in the perioperative period. Physicians caring for postoperative Ebstein patients should be aware of this phenomenon and, once confirmed by echocardiography, should avoid a vicious cycle of escalating inotropic support and worsening LVOT obstruction. Increasing doses of a beta-blocker may prove helpful in relieving this dynamic obstruction and achieve hemodynamic stability.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Dearani J.A., Danielson G.K. Congenital heart surgery nomenclature and database project: Ebstein’s anomaly and tricuspid valve disease. Ann Thorac Surg. 2000;69:S106–S117. doi: 10.1016/s0003-4975(99)01265-5. [DOI] [PubMed] [Google Scholar]
- 2.Paranon S., Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult. Heart. 2008;94:237–243. doi: 10.1136/hrt.2006.105262. [DOI] [PubMed] [Google Scholar]
- 3.Anderson K.R., Zuberbuhler J.R., Anderson R.H., Becker A.E., Lie J.T. Morphologic spectrum of Ebstein’s anomaly of the heart: a review. Mayo Clin Proc. 1979;54:174–180. [PubMed] [Google Scholar]
- 4.Zuberbuhler J.R., Allwork S.P., Anderson R.H. The spectrum of Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1979;77:202–211. [PubMed] [Google Scholar]
- 5.Attenhofer Jost C.H., Connolly H.M., O’Leary P.W., Warnes C.A., Tajik A.J., Seward J.B. Left heart lesions in patients with Ebstein anomaly. Mayo Clin Proc. 2005;80:361–368. doi: 10.4065/80.3.361. [DOI] [PubMed] [Google Scholar]
- 6.Lee W.-C., Fu M., Fang H.-Y. Unusual combination: Ebstein’s anomaly and hypertrophic obstructive cardiomyopathy. J Echocardiogr. 2016;14:42–44. doi: 10.1007/s12574-016-0273-9. [DOI] [PubMed] [Google Scholar]
- 7.de Agustin J.A., Perez de Isla L., Zamorano J.L. Ebstein anomaly and hypertrophic cardiomyopathy. Eur Heart J. 2008;29:2525. doi: 10.1093/eurheartj/ehn186. [DOI] [PubMed] [Google Scholar]
- 8.Isobe M., Tanaka M., Sekiguchi M. Subaortic stenosis due to accessory tissue of the mitral valve associated with Ebstein’s anomaly in an adult. Int J Cardiol. 1996;57:286–288. doi: 10.1016/s0167-5273(96)02825-2. [DOI] [PubMed] [Google Scholar]
- 9.Ulus T., Nadir A., Birdane A., Ata N. Native mitral valve causing left ventricular outflow tract obstruction in an adult with Ebstein’s anomaly. Anadolu Kardiyol Dergisi/The Anatol J Cardiol. 2011;11:651–652. doi: 10.5152/akd.2011.173. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse D.F., Murphy T.M., McCreery C.J., O’Hanlon R. An unusual cause of dynamic left ventricular outflow obstruction. Int J Cardiol. 2015;197:282–283. doi: 10.1016/j.ijcard.2015.06.084. [DOI] [PubMed] [Google Scholar]
- 11.Hirata K., Yagi N., Kubota S., Wake M., Tengan T. Case of Ebstein anomaly complicated by left ventricular outflow tract obstruction secondary to deformed basal septum attributable to atrialized right ventricle. Circulation. 2016;133:e33–e37. doi: 10.1161/CIRCULATIONAHA.115.016208. [DOI] [PubMed] [Google Scholar]
- 12.Anderson K.R., Lie J.T. Pathologic anatomy of Ebstein’s anomaly of the heart revisited. Am J Cardiol. 1978;41:739–745. doi: 10.1016/0002-9149(78)90826-3. [DOI] [PubMed] [Google Scholar]
- 13.Edwards W.D., Mierop L., Van Kutsche L. Embryology and pathologic features of Ebstein’s anomaly. Prog Pediatr Cardiol. 1993;2:5–15. [Google Scholar]
- 14.Benson L.N., Child J.S., Schwaiger M., Perloff J.K., Schelbert H.R. Left ventricular geometry and function in adults with Ebstein’s anomaly of the tricuspid valve. Circulation. 1987;75:353–359. doi: 10.1161/01.cir.75.2.353. [DOI] [PubMed] [Google Scholar]
- 15.da Silva J.P., Baumgratz J.F., da Fonseca L. The cone reconstruction of the tricuspid valve in Ebstein’s anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg. 2007;133:215–223. doi: 10.1016/j.jtcvs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Lange R., Burri M., Eschenbach L.K. Da Silva’s cone repair for Ebstein’s anomaly: effect on right ventricular size and function. Eur J Cardio-Thoracic Surg. 2015;48:316–321. doi: 10.1093/ejcts/ezu472. [DOI] [PubMed] [Google Scholar]