Abstract
We describe a case of fulminant eosinophilic myocarditis as the first presentation of eosinophilic granulomatosis with polyangiitis, promptly managed with extracorporeal membrane oxygenation. This case highlights the multidisciplinary work involving all health care professionals in the acute management of these patients and discusses it from an educational point of view. (Level of Difficulty: Intermediate.)
Key Words: acute heart failure, autoimmune, cardiac assist devices, Churg-Strauss syndrome, heart team
Abbreviations and Acronyms: CMR, cardiac magnetic resonance; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; EGPA, eosinophilic granulomatosis with polyangiitis; EMB, endomyocardial biopsy; GDMT, guideline-directed medical therapy; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; TTE, transthoracic echocardiography
Graphical abstract

We describe a case of fulminant eosinophilic myocarditis as the first presentation of eosinophilic granulomatosis with polyangiitis, promptly managed…
History of Presentation and Past Medical History
A 22-year-old Caucasian man presented to the emergency department with mild chest discomfort and constitutional symptoms (i.e., nausea, vomiting, and anorexia) for the past 5 days. His history was notable for mild persistent asthma, allergic rhinitis, and previous smoking (5 pack-years).
Learning Objectives
-
•
To identify eosinophilic granulomatosis with polyangiitis, a rare systemic necrotizing vasculitis, as a potential cause of fulminant life-threatening eosinophilic myocarditis.
-
•
To recognize that mechanical circulatory support may be lifesaving in rapidly progressing eosinophilic myocarditis presenting as refractory cardiogenic shock.
-
•
To recognize endomyocardial biopsy as a valuable diagnostic tool to establish diagnosis and define treatment decisions in fulminant myocarditis.
The patient denied any past or current history of drug use. Other than sinus tachycardia at 126 beats/min, the physical examination was unremarkable. He was afebrile, with a normal breathing rate, blood pressure of 100/79 mm Hg, clear chest sounds, nontender abdomen, and no peripheral edema.
Investigations and Differential Diagnosis
On laboratory work-up there was a normal hemoglobin level (14.5 g/dl), leukocytosis (24.0 × 109/l) with marked eosinophilia (11.7 × 109/l; i.e., 48%), and heightened C-reactive protein (19.9 mg/dl) and erythrocyte sedimentation rate (28 mm/h). Liver enzymes, creatine kinase, and lactate dehydrogenase were elevated (aspartate aminotransferase: 119 IU/l; alanine aminotransferase: 50 IU/l; creatine kinase: 1042 IU/l; and lactate dehydrogenase: 986 IU/l), and mild acute kidney injury was present (serum creatinine, 1.45 mg/dl). Both high-sensitivity-cardiac troponin T and N-terminal pro–B-type natriuretic peptide were markedly increased (i.e., 2,500 ng/l and 18,795 pg/ml, respectively). A brief panel of autoantibodies was unremarkable. Electrocardiography (Figure 1A) was notable for inferolateral ST-segment depression and chest radiography (Figure 1B) showed bilateral interstitial lung infiltrates. Transthoracic echocardiography (TTE) (Figure 1C) revealed severe left ventricular systolic dysfunction (left ventricular ejection fraction [LVEF] <30%) secondary to global hypokinesis, a restrictive filling pattern, and pericardial thickening without effusion; right ventricular and valvular function was preserved. Given all the above, an eosinophil-associated disease with myocardial involvement was highly suspected (Table 1). On additional workup, chest and paranasal sinus computed tomography (CT) scans were performed and showed bilateral ground-glass lung infiltrates (Figure 2A) and maxillary and ethmoidal sinus opacifications, respectively.
Figure 1.
Main Findings on Initial Work-Up in the ED
(A) Electrocardiography. (B) Chest radiography. (C) Transthoracic echocardiography. (D) Re-evaluation transthoracic echocardiography showing very low cardiac output. Dopp = Doppler; ED = emergency department; Env Ti = envelope time; HR = heart rate; LVCO = left ventricular cardiac output; LVOT = left ventricular outflow tract; LVSV = left ventricular stroke volume; maxPG = maximum pressure gradient; meanPG = mean pressure gradient; Vmax = maximum velocity; Vmean = mean velocity; VTI = velocity time integral.
Table 1.
Differential Diagnosis of Eosinophil-Associated Diseases With Myocardial Involvement
| Hypersensitivity |
| Antibiotics (mainly minocycline and beta-lactam antibiotics) |
| Central nervous system acting agents (mainly clozapine followed by carbamazepine) |
| Vaccines (e.g., tetanus toxoid, smallpox, and diphtheria/pertussis/tetanus) |
| Antitubercular agents (e.g., isoniazid) |
| NSAIDs, ACE inhibitors, diuretics, digoxin, among others |
| Autoimmune diseases |
| Eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome) |
| Giant cell arteritis |
| Sarcoidosis |
| Allergic bronchopulmonary aspergillosis |
| Polyarteritis nodosa |
| Heart transplantation allograft acute rejection |
| Primary hypereosinophilic syndrome (HES) |
| Chronic eosinophilic leukemia |
| PDGFRα-associated HES |
| FIP1-negative variant |
| Infection |
| Viral infections (e.g., HIV) |
| Parasitic infections (e.g., Toxocara canis, Ascaris, Strongyloides, Schistosoma) |
| Infections by protozoa (e.g., Toxoplasma gondii) |
| Malignant disease |
| Lymphoma (particularly Hodgkin, T- and B-cell lymphomas) |
| Leukemia (acute myelogenous leukemias most commonly, B-cell ALL) |
| Primary immunodeficiency diseases (hyper-IgE syndrome, Omenn syndrome) |
| Idiopathic/undefined |
ACE = angiotensin-converting enzyme; ALL = acute lymphoblastic leukemia; IgE = immunoglobulin E; HIV = human immunodeficiency virus; NSAID = nonsteroidal anti-inflammatory drug; PDGFR = platelet-derived growth factor receptor.
Figure 2.
Comparison of Admission and Re-Evaluation Chest CT
(A) Bilateral ground-glass lung infiltrates. (B) Resolution of lung infiltrates. CT = computed tomography.
Management
Despite clinical stability at presentation, the patient showed rapid clinical deterioration within the first hours, noted by acute heart failure, type 1 respiratory failure, and Acute Kidney Injury Network stage 3 oliguric acute kidney injury, and he was admitted to the cardiac intensive care unit on an emergency basis. Sedation, invasive ventilation, and intravenous (IV) inotropes were started. Repeat TTE revealed near cardiac standstill (Figure 1D, Video 1). Despite these measures, clinical deterioration resumed with refractory cardiogenic shock, ultimately requiring venoarterial extracorporeal membrane oxygenation (ECMO) at 14 h after emergency department admission. Coronary angiography was not performed immediately because acute coronary syndrome was highly unlikely and emergent hemodynamic support was needed.
Online Video 1.
Pre-Extracorporeal Membrane Oxygenation Transthoracic Echocardiography
Near Cardiac Standstill
Given the fulminant presentation, it was decided to perform an endomyocardial biopsy (EMB) for diagnostic work-up. After viral replication was excluded on cardiac biopsy, 1 mg/kg IV methylprednisolone and broad-spectrum antibiotics were started. Over the next few days, TTE revealed progressive improvement in cardiac function, and ECMO was discontinued on day 7, while the patient was weaned from both the ventilator and inotropes. During this period, no arrhythmic events were documented.
On hospital day 10, the patient was transferred to the cardiology ward, where further work-up was performed. Results of autoimmune screening, including antinuclear antibodies and cytoplasmic and perinuclear antineutrophil cytoplasmic antibodies, were negative. EMB later revealed severe myocardial necrosis with myocyte damage and a diffuse eosinophilic inflammatory infiltrate (Figures 3A and 3B). Accordingly, eosinophilic granulomatosis with polyangiitis (EGPA) was confirmed, with 4 out of 6 criteria (1) present: 1) asthma; 2) eosinophilia >10%; 3) extravascular eosinophils on EMB; and 4) paranasal sinusitis (Table 2). Re-evaluation chest CT was performed (Figure 2B), revealing resolution of lung infiltrates and further confirming the diagnosis (i.e., nonfixed fleeting lung infiltrates—5 out of 6 criteria). Furthermore, cardiac magnetic resonance (CMR) was remarkable for left ventricular widespread subendocardial late gadolinium enhancement (LGE) (Figures 4A and 4B). CT angiography, performed once clinical stability was achieved, revealed no epicardial coronary artery disease.
Figure 3.
Eosinophilic Myocarditis on EMB (H&E, 400×)
(A) Severe myocardial necrosis (asterisk) with destruction of myocytes and adjacent normal myocardium (arrow). (B and C) Perivascular spaces with mixed inflammatory infiltrate consisting almost entirely of eosinophils (arrows). (C) Interstitial spaces with edema and eosinophils (arrows). EMB = endomyocardial biopsy; H&E = hematoxylin and eosin.
Table 2.
EGPA Diagnostic Criteria
| 1. History of asthma |
| 2. Eosinophilia (>10% of leukocytes on differential white cell count) |
| 3. Mononeuropathy (including multiplex) or polyneuropathy |
| 4. Migratory or transient pulmonary infiltrates |
| 5. Paranasal sinus abnormality |
| 6. Biopsy showing a blood vessel with extravascular eosinophils |
Reprinted with permission from Masi et al. (1).
EGPA = eosinophilic granulomatosis with polyangiitis.
Figure 4.
CMR Findings
(A and B) Widespread subendocardial late gadolinium enhancement (arrows) on cardiac magnetic resonance (CMR).
During the hospital stay, management was adjusted to EGPA-directed treatment with oral corticosteroid and twice monthly IV cyclophosphamide (2), in addition to up-titration of guideline-directed medical therapy (GDMT) for heart failure with reduced ejection fraction (HFrEF) and cardiac rehabilitation. Despite these measures, LVEF did not fully recover and plateaued at 34% on TTE serial evaluation. Given these findings and the widespread LGE on CMR, the heart team decided to insert an implantable cardioverter-defibrillator after 35 days of GDMT. On hospital day 45, the patient was doing well and was discharged on 40 mg oral prednisolone and twice monthly IV cyclophosphamide pulse for 6 months in addition to HFrEF GDMT.
Discussion
Formerly known as Churg-Strauss syndrome, EGPA is a rare systemic necrotizing vasculitis involving small to medium-sized vessels and is associated with asthma and with blood and tissue eosinophilia. According to the American College of Rheumatology, the presence of 4 or more criteria (Table 2) establishes the diagnosis with a sensitivity of 85% and a specificity of 99.7% (1).
EGPA is one of the most common systemic vasculitides to involve the heart, which is typically associated with eosinophilia and a negative antineutrophil cytoplasmic antibody work-up. It often leads to myocarditis and acute heart failure, coronary vasculitis, and myocardial infarction, as well as ventricular arrhythmias and sudden cardiac death. However, a significant proportion of patients will be asymptomatic or have an insidious, rather than fulminant, presentation (1). Cardiac imaging plays a pivotal role in the detection of myocardial, pericardial, or valvular involvement, although findings are often nonspecific. TTE should be promptly performed, whereas CMR may further refine the diagnosis and determine the extent of myocardial necrosis. However, definitive diagnosis is exclusively established by EMB (3,4). Invasive coronary angiography (or CT angiography in selected patients) should be performed to rule out coronary disease (5). In this case, and although arguable, the heart team decided not to perform coronary angiography immediately because an alternative diagnosis seemed very likely (i.e., myocarditis).
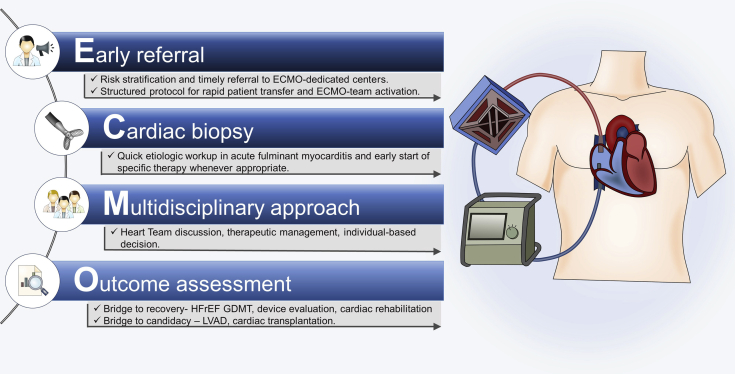
This patient presented with rapidly progressive fulminant myocarditis with a need for emergent hemodynamic support. ECMO, an extracorporeal life support technique able to provide cardiopulmonary bypass, may be lifesaving in refractory cardiogenic shock. Venoarterial peripheral ECMO is of utmost value in supporting a compromised heart until a definitive diagnosis is made and cause-specific treatment is initiated, whenever possible. The optimal timing for implementation is not uniformly established and must rely on an informed individualized heart team decision (6). Standard operating procedure must include early contact with the ECMO tertiary referral center with expertise in the management of the critically ill patient (Figure 5). Indeed, this case of EGPA-related fulminant eosinophilic myocarditis emphasizes the crucial role of such timing and the successful role of ECMO as a bridge-to-recovery strategy.
Figure 5.
ECMO Management Algorithm
ECMO = extracorporeal membrane oxygenation; GDMT = guideline-directed medical therapy; HFrEF = heart failure with reduced ejection fraction; LVAD = left ventricular assist device.
EMB is recommended early in the work-up of unexplained new-onset or recent (<2 weeks) heart failure and hemodynamic deterioration (Class I, Level of Evidence: B) because it may lead to treatment decisions based on histopathological findings that may substantially improve prognosis (3,4). Indeed, cardiac involvement in EGPA further worsens the prognosis and is the cause of death in roughly one-half of the patients. However, survival significantly improves with appropriate immunosuppression. It is recommended to use a combination of glucocorticoids and either cyclophosphamide or rituximab for remission induction in patients with organ or life-threatening disease (2). The Five Factor Score (FFS) (7) is the most commonly used prognostic tool, and it is helpful for selecting patients who may benefit from immunosuppressive agents (i.e., cyclophosphamide) (FFS ≥1).
Finally, in this particular case we decided to insert a subcutaneous implantable cardioverter-defibrillator before discharge, despite the general recommendation to wait until 3 months of HFrEF GDMT are completed. The rationale for this decision included the absence of patients with acute myocarditis in the clinical trials that support this recommendation and the extensive LGE that made reverse remodeling unlikely. A wearable defibrillator vest could have been a valid alternative, but unfortunately it is not available at our center.
Follow-Up
At 1-year follow-up, the patient is doing well, in New York Heart Association functional class I. He has resumed his work as a barber, and no vasculitis relapse has been documented. He is currently taking prednisolone 10 mg and HFrEF GDMT (bisoprolol: 10 mg daily; ramipril: 10 mg daily; and spironolactone: 25 mg daily). Nonetheless, his LVEF remained severely depressed (34%) on TTE re-evaluation. Thus far, he has had no heart failure hospitalizations or implantable cardioverter-defibrillator-related events.
Conclusions
We report a rare case of rapidly progressing vasculitis in a patient who presented with fulminant myocardial involvement. Multidisciplinary teams are paramount to improve the prognosis of such rare yet life-threatening conditions. Early ECMO implementation and EMB were key strategic decisions for a successful outcome.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For a supplemental video, please see the online version of this paper.
References
- 1.Masi A.T., Hunder G.G., Lie J.T. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 2.Yates M., Watts R.A., Bajema I.M. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 3.Cooper L.T., Baughman K.L., Feldman A.M. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a–d. [DOI] [PubMed] [Google Scholar]
- 5.Caforio A.L.P., Adler Y., Agostini C. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J. 2017;38:2649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
- 6.Rao P., Khalpey Z., Smith R., Burkhoff D., Kociol R.D. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.004905. [DOI] [PubMed] [Google Scholar]
- 7.Guillevin L., Lhote F., Gayraud M. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 1996;75:17–28. doi: 10.1097/00005792-199601000-00003. [DOI] [PubMed] [Google Scholar]