Abstract
A 42-year-old man with a 6-month-long fever was found to have chronic active Epstein-Barr virus infection complicated by aneurysmal coronary arteries with other arteries. In adult patients with this infection, coronary aneurysms are rare but are a poor prognostic factor. (Level of Difficulty: Intermediate.)
Key Words: aortic artery aneurysm, chronic active Epstein-Barr virus infection, computed tomography, coronary artery aneurysm
Abbreviations and Acronyms: CAA, coronary artery aneurysm; CAEVB, chronic active Epstein-Barr virus infection; CIA, common iliac artery; CT, computed tomography; EBV, Epstein-Barr virus; Ig, immunoglobulin
Graphical abstract
A 42-year-old man with a 6-month-long fever was found to have chronic active Epstein-Barr virus infection complicated by aneurysms of coronary…
History of Presentation
A 42-year-old man was admitted to our hospital because of a fever lasting 6 months, anterior uveitis, hearing loss, systemic lymphadenopathy, splenomegaly, and abdominal aortic arteritis.
Learning Objectives
-
•
To consider the rare etiology of coronary and aortic artery aneurysm in patient with chronic active Epstein-Barr virus infection.
-
•
To identify the risk of cardiac complications such as coronary artery aneurysm in patient with chronic active Epstein-Barr virus infection.
-
•
To appreciate the importance of monitoring the progression of coronary artery aneurysm.
Medical History
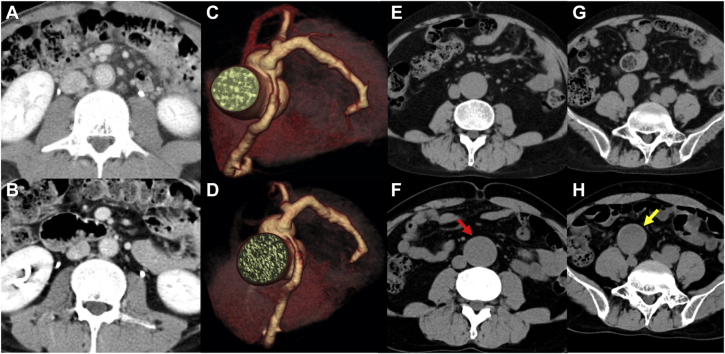
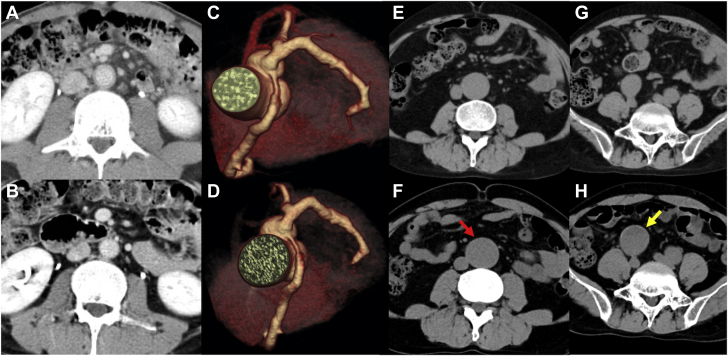
Epstein-Barr virus (EBV) antibody titers were as follows: viral capsid antigen immunoglobulin G (IgG), 1:1280; viral capsid antigen IgM, <1:10; early antigen IgG, 1:640; and EBV nuclear antigen, 1:80. The EBV-DNA load in peripheral blood was determined to be 3.7 × 103 copies/106 white blood cells. Monoclonal proliferation of EBV-infected cells was demonstrated in the peripheral blood with Southern blot analysis using EBV-terminal repeat. On the basis of these results, chronic active EBV infection (CAEBV) was diagnosed. Contrast-enhanced computed tomography (CT) showed that the coronary artery was diffusely dilated without aneurysm and that the wall of the abdominal aorta was also thickened, suggesting arteritis without aneurysm (Figure 1A). After CAEBV was diagnosed, prednisolone was administered at an initial dosage of 1 mg/kg/day and then slowly tapered depending on its effectiveness. The decreased wall thickness of the abdominal aorta was confirmed with CT (Figure 1B).
Figure 1.
Contrast-Enhanced Computed Tomography of the Coronary Artery, Abdominal Aorta, and Iliac Artery
Cross-sectional view of the contrast-enhanced computed tomography (A) at first admission and (B) after prednisolone was administered. (C) Three-dimensional reconstruction of the coronary computed tomographic image 1 month after the allogenic bone marrow transplantation. (D) The coronary artery aneurysm had further expanded by 4 years after it had first been confirmed. The (E) abdominal aortic aneurysm and (G) the common iliac artery aneurysm 2 years after the diagnosis of CAEBV. Four years later, both the (F) abdominal aorta (red arrow) and (H) right common iliac artery aneurysm (yellow arrow) had expanded.
Differential Diagnosis
Differential diagnosis included coronary artery aneurysms associated with inflammatory disorders such as Kawasaki disease, Takayasu disease and Churg-Strauss syndrome, or connective tissue disorders such as Marfan syndrome and Ehlers-Danlos syndrome.
Investigations
Two years after the diagnosis of CAEBV, an abdominal aortic aneurysm and bilateral common iliac artery (CIA) aneurysms were discovered with CT just before allogeneic bone marrow transplantation was performed as a curative treatment for CAEBV. The maximum diameter of the aortic abdominal aorta was 34 mm (Figure 1E), and the maximum diameter of the right CIA was 26 mm (Figure 1G). Owing to prolonged fever, CT examination was performed 1 month later and demonstrated a coronary artery aneurysm from the left main trunk through the left anterior descending and right coronary arteries, with a maximum diameter of 7 mm in the left main trunk and 6 mm in the right coronary artery (Figure 1C). Regarding treatment, the decision was made for close monitoring without intervention at that time. The EBV-DNA level in peripheral blood finally decreased to beneath the detection limit. Follow-up CT 6 years after diagnosis of CAEBV showed fusiform dilatation of the aneurysms, with a maximum diameter of 11 mm in the left main trunk, 7 mm in the right coronary artery (Figure 1D), 40 mm in the abdominal aorta (Figure 1F), and 40 mm in the right CIA (Figure 1H, Table 1). The patient has been followed on aspirin with close monitoring until the present time.
Table 1.
Timeline
| Time | Event |
|---|---|
| 2 months before the diagnosis (first admission to our institution) | Coronary artery was diffusely dilated without aneurysm and that the wall of an abdominal aorta was thickened without aneurysm. |
| Diagnosis of CAEBV | After prednisolone was administered, the wall thickness of an abdominal aorta was decreased. |
| 2 yrs after the diagnosis (allogenic bone marrow transplantation) | An abdominal aortic aneurysm and CIA aneurysm with maximum diameter of 34 mm in the abdominal aorta, and that of 26 mm in the right CIA. |
| 1 month after allogenic bone marrow transplantation | A coronary artery aneurysm from LMT through left anterior descending and RCA with maximum diameter of 7 mm in the LMT, and that of 6 mm in the RCA. |
| 5 yrs after the diagnosis | The EBV-DNA level in peripheral blood decreased to beneath the detection limit. |
| 6 yrs after the diagnosis | Dilation of the aneurysms, with a maximum diameter of 11 mm in the LMT, that of 7 mm in the RCA, and that of 40 mm in the right CIA. |
CAEBV = chronic active EBV; CIA = common iliac artery; EBV = Epstein-Barr virus; LMT = left main trunk; RCA = right coronary artery.
Discussion
Coronary artery aneurysm (CAA) is classically defined as a focal dilatation of more than 1.5 times the diameter of an adjacent artery (1). The prevalence of CAA was reported to be 0.2% to 4.9% of patients undergoing coronary angiogram (2,3). Patients with CAA can be completely asymptomatic, as in our patient, but can present with a variety of symptoms, including chest pain, congestive heart failure, acute coronary syndrome, and sudden cardiac death (1). The Coronary Artery Aneurysm Registry, the largest multicenter registry, found in adult patients a mortality rate of 15.3% and a major adverse cardiac events rate of 31% (4). However, no reports have been published with respect to specific prognosis of patients with CAEVB complicated by CAA.
Cases of CAEBV have various symptoms, including fever, lymphadenopathy, hepatosplenomegaly, and cytopenia, which can lead to such cardiovascular complications as CAA. According to previous studies, of patients with CAEBV, a CAA develops in approximately 9% (5). Pathological studies have revealed that lymphoid infiltration is present in CAA and that these aneurysms result from lymphoid vasculitis (6).
In the present case, an abdominal aortic aneurysm and a CIA aneurysm had already been detected in addition to a CAA at the time of bone marrow transplantation, 2.5 years after disease onset. Furthermore, the aneurysms had gradually enlarged despite successful eradication of EBV-infected cells. Because coronary artery lesions, including dilation, stenosis, and calcification, have been reported to regress within several years after cord blood transplantation, allogeneic hematopoietic stem cell transplantation being performed soon after CAEBV is diagnosed might prevent aneurysms from developing (7). Although the relationships of the size of aneurysms with disease activity or prognosis remain uncertain, high EBV load, fever, and cytopenia are reported to be involved in the cardiovascular complications (7). The present case was considered to be of high risk of development of cardiac complications because of the presence of high EBV load and fever.
With respect to medical treatment for CAAs, the decision was made to administer aspirin alone to prevent coronary artery disease, because the main etiology of CAA is atherosclerosis (1). In addition, the Coronary Artery Aneurysm Registry has reported that aspirin is the preventative agent most often prescribed (4). Moreover, although a previous report has shown the efficacy of anticoagulation therapy in a specific patient with the complication of thrombus (8), the present patient had no evidence of thrombus of the coronary artery.
Follow-Up
The current practice guideline does not recommend a specific approach to monitoring CAA (9). In the present case, greater attention should have been paid to monitor the size of the CAA by performing serial contrast-enhanced CT.
Conclusions
Despite the scarce evidence of CAAs in cases of CAEBV, carefully investigating cardiovascular complications, including CAAs, is important because they are poor prognostic factors.
Acknowledgment
The authors are grateful to Prof. Masao Okazaki at the Jikei University School of Medicine for his editing contribution.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Pham V., Hemptinne Q., Grinda J.M., Duboc D., Varenne O., Picard F. Giant coronary aneurysms, from diagnosis to treatment: a literature review. Arch Cardiovasc Dis. 2020;1113:59–69. doi: 10.1016/j.acvd.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Swaye P.S., Fisher L.D., Litwin P. Aneurysmal coronary artery disease. Circulation. 1983;67:134–138. doi: 10.1161/01.cir.67.1.134. [DOI] [PubMed] [Google Scholar]
- 3.Tunick P.A., Slater J., Kronzon I., Glassman E. Discrete atherosclerotic coronary artery aneurysms: a study of 20 patients. J Am Coll Cardiol. 1990;15:279–282. doi: 10.1016/s0735-1097(10)80049-x. [DOI] [PubMed] [Google Scholar]
- 4.Nunez-Gil I.J., Cerrato E., Bollati M. Coronary artery aneurysms, insights from the international coronary artery aneurysm registry (CAAR) Int J Cardiol. 2020;299:49–55. doi: 10.1016/j.ijcard.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H., Morishima T., Kanegane H. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. 2003;187:527–533. doi: 10.1086/367988. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa A., Ito M., Iwaki T., Yatabe Y., Asai J., Hayashi K. Chronic active Epstein-Barr virus infection with giant coronary aneurysms. Am J Clin Pathol. 1996;105:733–736. doi: 10.1093/ajcp/105.6.733. [DOI] [PubMed] [Google Scholar]
- 7.Muneuchi J., Ohga S., Ishimura M. Cardiovascular complications associated with chronic active Epstein-Barr virus infection. Pediatr Cardiol. 2009;30:274–281. doi: 10.1007/s00246-008-9343-8. [DOI] [PubMed] [Google Scholar]
- 8.Doi T., Kataoka Y., Noguchi T. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2017;37:2350–2355. doi: 10.1161/ATVBAHA.117.309683. [DOI] [PubMed] [Google Scholar]
- 9.Fihn S.D., Gardin J.M., Abrams J. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]