Abstract
A woman with ischemic cardiomyopathy presented with recurrent syncope. Electrocardiogram showed complete heart block and torsade de pointes (TdP) secondary to amiodarone, recently started for paroxysmal atrial fibrillation. We describe a novel application of His bundle pacing that suppressed TdP and corrected the underlying left bundle branch block. (Level of Difficulty: Intermediate.)
Key Words: amiodarone, atrial fibrillation, cardiac pacemaker, cardiomyopathy, complete heart block, His bundle pacing
Abbreviations and Acronyms: CS, coronary sinus; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; TdP, torsade de pointes
Graphical abstract
A woman with ischemic cardiomyopathy presented with recurrent syncope…
A 78-year-old woman was admitted following recurrent syncope. Prior to this admission, she was just discharged 1 day prior after a 6-day admission for decompensated heart failure in the setting of atrial fibrillation with rapid ventricular response. A total of 2,250 mg of amiodarone was given during the previous hospitalization before she was discharged on oral amiodarone 200 mg twice a day. Her other medications were: atorvastatin 40 mg every night, apixaban 2.5 mg twice daily, furosemide 40 mg every morning, potassium chloride slow release 600 mg every morning, and omeprazole 20 mg every morning.
Learning Objectives
-
•
To make a differential diagnosis of causes of recurrent syncope episodes in a patient with paroxysmal atrial fibrillation started on amiodarone with background history of ischemic cardiomyopathy.
-
•
To recognize one of the subacute amiodarone side effects affecting the cardiovascular system, which include complete heart block, prolonged QTc, and recurrent TdP. In this setting, ventricular pacing from conventional sites was arrhythmogenic whereas pacing at the His bundle region suppressed TdP.
Upon arrival to emergency department, her blood pressure was 181/77 mm Hg and she had an irregular pulse of 56 beats/min. Physical examination revealed no signs of heart failure.
Medical History
The patient’s medical history included hypertension, ischemic cardiomyopathy with left ventricular ejection function (LVEF) of 38%, New York Heart Association functional class II, left bundle branch block with QRS complex duration of 155 ms and paroxysmal atrial fibrillation with CHA2DS2-VASc score of 6.
Differential Diagnosis
Differential diagnosis included significant post–atrial fibrillation conversion pause, ventricular tachycardia in the setting of ischemic cardiomyopathy, or TdP in the setting of long QTc secondary to amiodarone.
Investigations
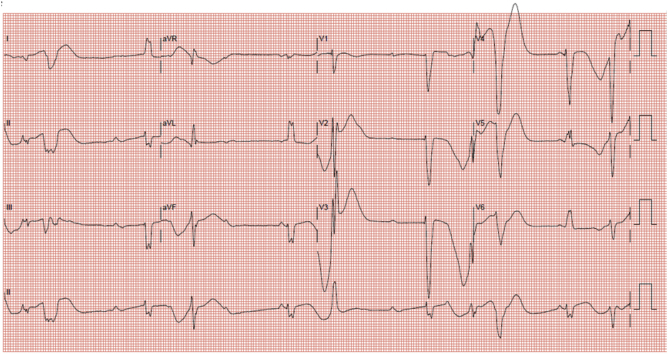
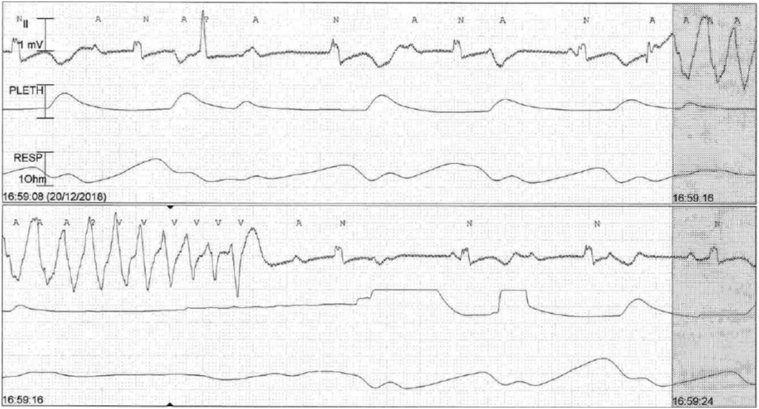
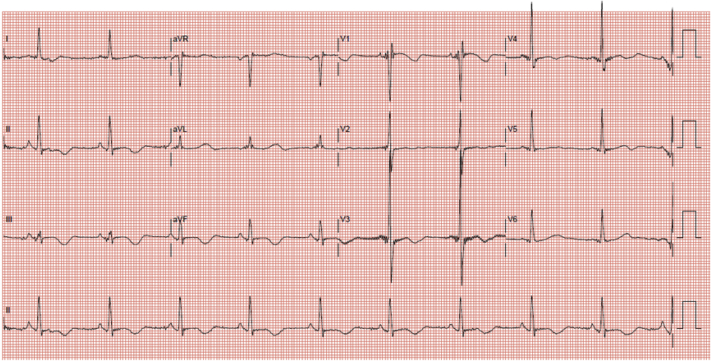
Her electrocardiogram (Figure 1) on this admission showed complete heart block with slow ventricular escape rhythm and long QT (QTc: 710 ms). Each ventricular escape beat was followed by late coupled premature ventricular ectopics of varying morphology. Serum potassium and magnesium were normal at 4.1 and 1.48 mmol/l, respectively. Liver and renal function tests were normal. Serial serum troponin level was not elevated. Telemetry showed multiple episodes of non-sustained TdP (associated with symptoms of pre-syncope) (Figure 2). Coronary angiogram did not show any significant obstructive lesion. Transthoracic echocardiography, specifically LVEF assessment, was unchanged from before.
Figure 1.
12-Lead Electrocardiogram Showed Complete Heart Block With Slow Ventricular Escape Rhythm and Long QT (QTc: 710 ms)
Each ventricular escape rhythm is followed by premature ventricular ectopic of varying morphology.
Figure 2.
Telemetry Showed Complete Heart Block, Slow Ventricular Escape Rhythm With Long QT Followed by Spontaneous Initiation and Termination of Nonsustained Torsade de Pointes
Telemetry electrocardiogram showed 2 continuous tracings and each tracing (from top to bottom) showed electrocardiogram lead II, plethysmography waveform, and respiratory waveform.
The most likely explanation for her recurrent syncope was nonsustained TdP in the setting of long QTc secondary to amiodarone.
Management
As the patient had a background of broad left bundle branch block with significant cardiomyopathy, as well as an urgent need for pacing for recurrent episodes of TdP, a decision was made to implant a cardiac resynchronization therapy defibrillator (device: Compia MRI CRT-D Sure Scan; right atrial lead: CapSure Fix Novus 5076/52 cm; right ventricular [RV] lead: Sprint SC DF4 6935M/62 cm; all Medtronic, Minneapolis, Minnesota).
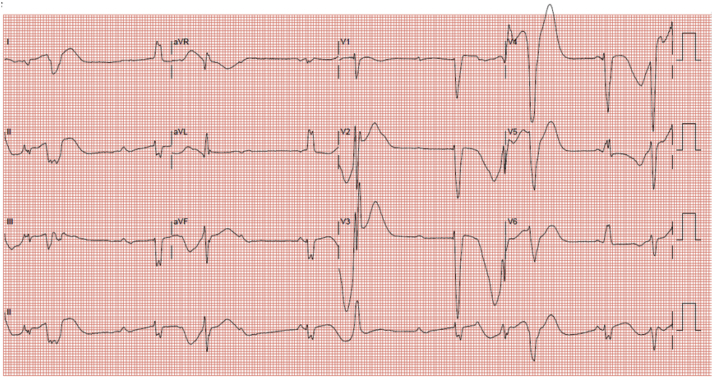
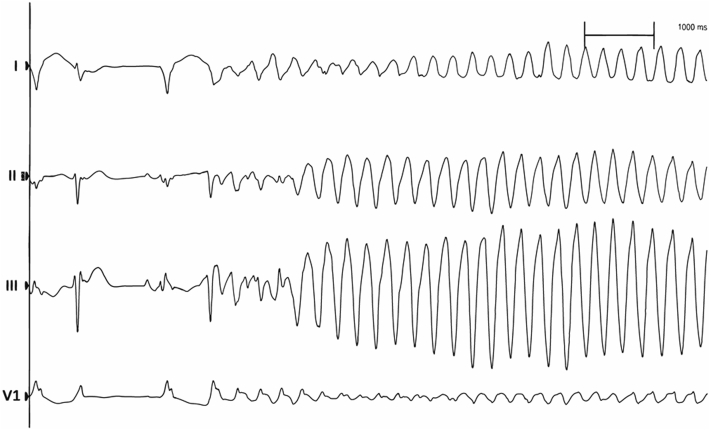
During the procedure, pacing from the RV apical lead and left ventricular (LV) lead (Medtronic Attain Performa Advanced Quadripolar lead 4298/88 cm) positioned at the posterolateral coronary sinus (CS) branch invariably induced nonsustained TdP (Figure 3). Pacing-induced nonsustained TdP also occurred at other sites of CS branches. Thus, the LV lead was not placed. In view of this, we proceeded to perform His bundle pacing by placing a lumenless bipolar pace/sense lead (Medtronic 3830/69 cm Select Secure) using C315 His sheath (Medtronic). Nonselective pacing at the His bundle region did not induce TdP (Figure 4) with a satisfactory pacing threshold (1.0 V at 1.0 ms). The QTc (calculated using Bazett formula) at baseline (on admission) was 710 ms compared with QTc during RV pacing of 560 ms as well as QTc during nonselective His bundle pacing of 529 ms. QTc during LV pacing was not measured, as pacing at LV invariably induced TdP.
Figure 3.
Electrocardiogram Strip Showed Left Ventricular Pacing-Induced Torsade de Pointes
Electrocardiogram leads configuration from top to bottom: I, II, III, and V1.
Figure 4.
12-Lead Electrocardiogram Showed Nonselective His Bundle Pacing
QTc of 529 ms.
Discussion
Amiodarone is a class III antiarrhythmic agent with well-documented long-term side effects involving multiple systems, including but not limited to pulmonary toxicity, thyroid dysfunction, and liver injury. Subacute amiodarone syndrome, however, is rare and affects mainly the liver (1, 2, 3, 4, 5) rather than heart. Sequeira et al. (6) were the first to describe the association of intravenous amiodarone-induced third-degree atrioventricular block and extreme QT interval prolongation generating TdP in a patient with paroxysmal atrial fibrillation (6). In our case, amiodarone was initially administered intravenously and the patient was already on oral amiodarone when she presented with TdP. This was in contrast with the case of Sequeira et al. (6), in which TdP happened during administration of intravenous amiodarone. Another interesting observation in our case was that pacing from both ventricles induced non-sustained TdP whereas non-selective His bundle pacing was not arrhythmogenic.
In general, amiodarone has low proarrhythmic effect both in normal and heart failure patients despite QT prolongation due to its fast phase III repolarization, a low incidence on dispersion of repolarization, a lower potential to induce early after depolarization, and a weak effect on reverse frequency dependence (7,8). However, the proarrhythmic effect of amiodarone has been well described (7,8). In our case, the proarrhythmic effect of amiodarone was likely exacerbated by low repolarization reserve in this patient. However, the patient declined genetic testing for long QT syndrome.
Conventional pacing at the RV apex and epicardial LV through the CS vasculature triggered TdP (Figure 3). This would have precluded the use of temporary RV pacing, which may have been attempted in most centers without access to urgent electrophysiological service. Pacing at the His bundle region, however, managed to suppress the premature ventricular complexes and TdP. A plausible explanation is a lowering of the dispersion of repolarization by pacing at the His bundle region and relative shortening of QT interval.
Follow-Up
Subsequent follow-up (up to 12 months) showed no ventricular arrhythmia event, stable nonselective His bundle pacing threshold, as well as RV lead parameters. There was no recurrent hospitalization. Her effort tolerance improved markedly to New York Heart Association functional class I. Repeat transthoracic echocardiography showed LVEF had improved from 38% to 45%.
Conclusions
We describe a case of subacute amiodarone side effect resulting in complete heart block, prolonged QTc, and recurrent TdP. In this setting, ventricular pacing from conventional sites (RV apex and coronary sinus) was arrhythmogenic, whereas pacing at the His bundle region suppressed TdP. To the best of our knowledge, this is the first case reporting on the novel application of His bundle pacing in the context of amiodarone-induced TdP.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Rhodes A., Eastwood J.B., Smith S.A. Early acute hepatitis with parenteral amiodarone: a toxic effect of the vehicle? Gut. 1993;34:565–566. doi: 10.1136/gut.34.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng X.R., Wee L.Y., Chadachan V. Acute amiodarone syndrome after a single intravenous amiodarone bolus. Singapore Med J. 2012;53:e225–e227. [PubMed] [Google Scholar]
- 3.Pye M., Northcote R.J., Cobbe S.M. Acute hepatitis after parenteral amiodarone administration. Br Heart J. 1988;59:690–691. doi: 10.1136/hrt.59.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupon-Roses J., Simo-Canonge R., Lu-Cortez L., Permanyer-Miralda G., Allende-Monclus H. Probable early acute hepatitis with parenteral amiodarone. Clin Cardiol. 1986;9:223–225. doi: 10.1002/clc.4960090512. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson R.N., Nayani T.H., Davies J.R. Acute hepatic dysfunction following parenteral amiodarone administration. Postgrad Med J. 1989;65:707–708. doi: 10.1136/pgmj.65.767.707-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sequeira O.R., Aquino N.J., Gomez N.B. Amiodarone-induced third degree atrioventricular block and extreme qt prolongation generating torsade des pointes in paroxysmal atrial fibrillation. J Atr Fibrillation. 2016;9:1502. doi: 10.4022/jafib.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milberg P., Ramtin S., Monnig G. Comparison of the in vitro electrophysiologic and proarrhythmic effects of amiodarone and sotalol in a rabbit model of acute atrioventricular block. J Cardiovasc Pharmacol. 2004;44:278–286. doi: 10.1097/01.fjc.0000129581.81508.78. [DOI] [PubMed] [Google Scholar]
- 8.Meierhenrich R., Helguera M.E., Kidwell G.A., Tebbe U. Influence of amiodarone on QT dispersion in patients with life-threatening ventricular arrhythmias and clinical outcome. Int J Cardiol. 1997;60:289–294. doi: 10.1016/s0167-5273(97)00073-9. [DOI] [PubMed] [Google Scholar]