Abstract
An 87-year-old woman had residual moderate to severe aortic insufficiency following transcatheter aortic valve replacement. A year later, she developed extensive Stanford type A aortic dissection originating at the supra-annular aortic edge of the transcatheter aortic valve replacement nitinol frame. Dissection repair, frozen elephant trunk with exclusion of prior insufficiency while preserving the transcatheter aortic valve replacement valve was performed. (Level of Difficulty: Beginner.)
Key Words: aortic valve, dissection, stenosis
Abbreviations and Acronyms: STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; TAAD, Stanford type A aortic dissection; TAVR, transcatheter aortic valve replacement; TEVAR, thoracic endovascular aortic repair
Graphical abstract
An 87-year-old woman had residual moderate to severe aortic insufficiency following transcatheter aortic valve replacement. A year later, she…
An 87-year-old woman presented to the emergency department with acute-onset mid and left periscapular back pain that awoke her from sleep. Blood pressure was 152/52 mm Hg and heart rate 64 beats/min. She reported no prior symptoms. On examination, she appeared to be in significant discomfort with a mild systolic ejection murmur II/VI at the left upper sternal border. She had tenderness to palpation over her spinous processes diffusely through the thoracic spine with notable kyphoscoliotic deformities. Rheumatic arthritis in the upper and lower extremities led to missing toes bilaterally.
Learning Objectives
-
•
To review broad indications for aortic stenosis and TAVR management.
-
•
To review considerations in Stanford Type A aortic dissection.
-
•
To present strategies for management.
Past Medical History
A remote history of chronic Stanford type B dissection was managed conservatively, with serial computed tomography angiogram surveillance demonstrating a well-remodeled descending thoracic aorta before development of severe aortic stenosis. Her previous medical history included atrial fibrillation on apixaban, osteoporosis, lumbar spinal stenosis, hypothyroidism, hypertension, and hyperlipidemia. Her rheumatoid arthritis was treated with prednisone, methotrexate, and etanercept.
The patient progressed to symptomatic severe nonrheumatic aortic stenosis. She had been followed up for several years with biannual echocardiograms, confirming progressive worsening moderate to severe aortic stenosis. The patient was referred for transcatheter aortic valve replacement (TAVR). Pre-operative coronary angiography did not demonstrate significant coronary artery disease. The patient had an Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) of 5.5% and a normal 6-min walk test result. Her frailty score was 2, and albumin was 4.0 g/dl before the operation. She underwent TAVR with a transfemoral 26-mm CoreValve Evolut PRO System (Medtronic Inc., Minneapolis, Minnesota). She had residual moderate to severe perivalvular aortic insufficiency post-TAVR along the left cusp annular plane. She was followed up closely with echocardiograms at regular intervals per Valve Academic Research Consortium 2 recommendations.
Differential Diagnosis
The differential diagnosis included aortic dissection, pulmonary embolism, spontaneous pneumothorax, spinal disc herniation, compression fracture, vertebral body fracture, or other musculoskeletal etiologies.
Investigations
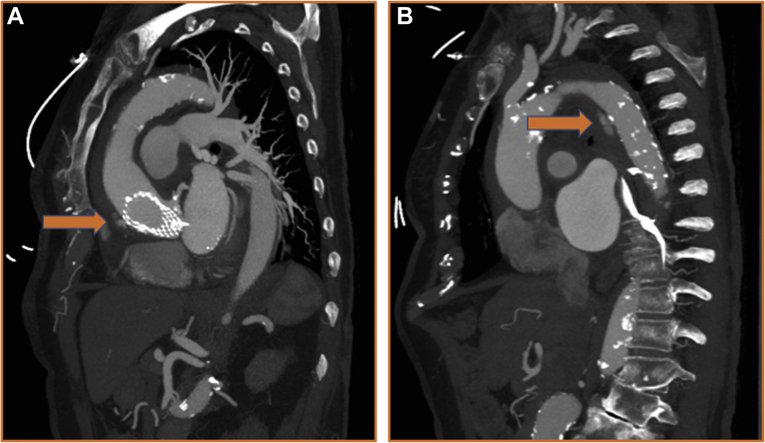
A gated computed tomography angiogram of the chest, abdomen, and pelvis was obtained and showed an acute Stanford type A aortic dissection (TAAD) with intramural hematoma originating from the supra-annular nitinol frame of the CoreValve, extending along the anterolateral ascending aorta through the arch to the perivisceral aorta (Figure 1A) and down to the root. The ascending aorta was 50 mm in diameter and was noted to be 42 mm on prior studies. There was pericardial effusion and concomitant Stanford type B dissection (Figure 1B). She was transferred with adequate dP/dt control and, after load reduction, maintaining systolic pressures at <110 mm Hg.
Figure 1.
Reconstructed CTA Images of Aortic Dissection
(A) Reconstructed sagittal view of gated computed tomography chest angiogram demonstrating type A aortic dissection with contrast extravasation. (B) Reconstructed sagittal view of gated computed tomography chest angiogram demonstrating descending aortic dissection with contrast extravasation.
Management
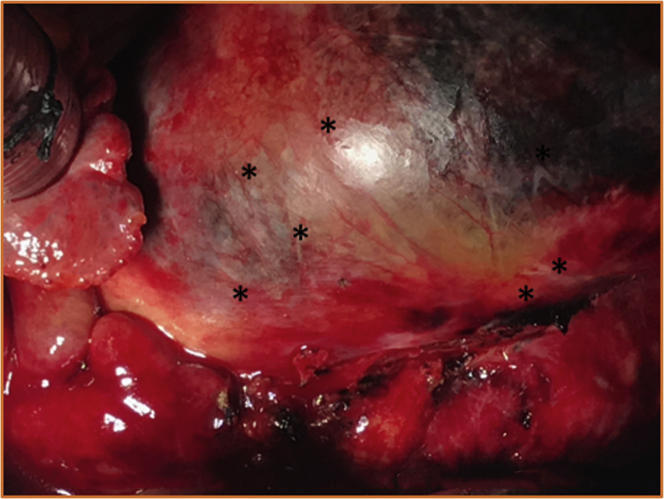
The patient was taken to the operating room, and she underwent ascending aortic replacement, dissection, and perivalvular leak repair along with frozen elephant trunk (Figure 2). The aortic insufficiency between the left and noncoronary sinus was repaired from just below the paravalvular skirt of the CoreValve Evolut PRO valve and the native aortic annulus (Figures 3A to 3C). No aortic insufficiency or residual flaps in the arch were noted on post–cardiopulmonary bypass transesophageal echocardiography. Post-operatively, the patient recovered as expected, and she was eventually transferred to the ward, with planned disposition to physiatry and rehabilitation facilities. Given that the patient was referred from a center offering cardiovascular surgical services, we coordinated local follow-up.
Figure 2.
Intraoperative Image of Type A Aortic Dissection
Intraoperative image following cannulation on cardiopulmonary bypass of the CoreValve imprinted onto the ascending aorta (∗) imminently pending rupture.
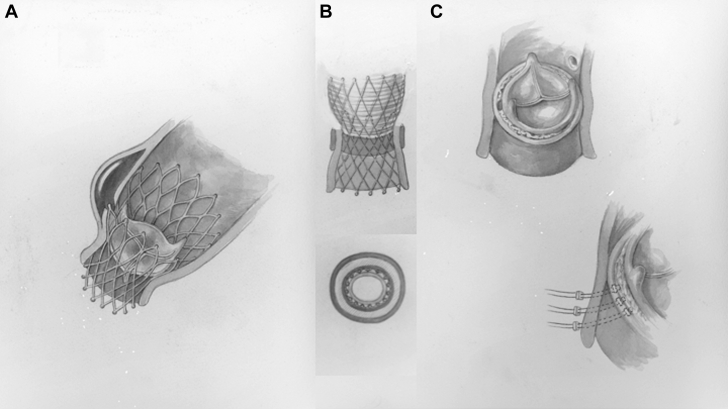
Figure 3.
Illustration of Repair Technique
(A) Schematic of dissection location. (B) Repair strategy of aortic dissection, preserving the CoreValve. (C) Repair strategy of aortic insufficiency under the CoreValve skirt and the native aortic valve annular plane.
Discussion
We discuss a patient almost 90 years old with a pre-TAVR STS-PROM of 5.5% who developed a late TAAD originating at the supra-annular nitinol frame of the CoreValve 1 year after TAVR. We postulate that treatment with steroids and immunologics for rheumatoid disease weakened the aortic wall and enabled the aortic dissection by the metallic frame around the transcatheter valve. More broadly, hypertension and malpositioning of the TAVR valve also contributed to aortic dissection. It is unclear which of these confounders were major factors, but given late discovery following TAVR, without known acute precipitating factors, an insidious (e.g., biological) process is likely. The patient underwent complex open reconstruction of the ascending aorta, perivalvular leak repair, and frozen elephant trunk. With TAVR procedures having increased 33-fold (from 445 in 2007 to 14,946 in 2011) and being provided now to an estimated 35,000 patients, an increased prevalence of associated complications such as TAAD may be anticipated. Each episode of care in such patients may have to be evaluated independent of prior decisions and care plans.
TAAD historically has carried an untreated mortality rate of 1%/h up to 48 h, where up to 90% of patients die within 30 days when not treated (1). It is typically spontaneous; however, it could be iatrogenic. TAAD has an incidence between 0.6% and 1.9% following the TAVR procedure (2). There are limited data on dissection rates in balloon-expanding devices versus self-expanding devices. TAAD is a rare but known complication of TAVR. There have been few cases describing acute TAAD within the post-operative course of the TAVR procedure (3,4); however, there have been no reports of late TAAD associated with TAVR. Patients with TAAD managed surgically have a significantly lower mortality rate than those treated with guideline-directed medical management. The incidence of TAAD following TAVR could rise as experience broadens, and hence, carefully tailored multidisciplinary care of these patients is critical.
Open repair of TAAD has been the gold standard of treatment in suitable candidates. Within the last few years, thoracic endovascular aortic repair (TEVAR) has become a potential treatment option in select high-risk patients. Akin to TAVR, TEVAR requires satisfactory access for device delivery with adequate proximal and distal landing zones, including the important need to cover the entry tear. TEVAR could help address TAAD pathology distal to the sinotubular junction and proximal to the head vessels. Nascent criteria recommend at least 2 cm of proximal and distal landing zone with sinotubular junction diameter <38 mm (5). New endovascular devices and advanced image-guided procedures are evolving and may prove to be a viable treatment option in patients not eligible for open repair (ARISE trial is currently enrolling). To this end, an important consideration in the management of patients with TAAD includes goal-directed medical therapy with aggressive impulse control, noninterventional/surgical treatment, observation without further care, comfort/palliative care, limited intervention/surgery, endovascular therapy, and open surgical therapy as potential strategies. The ideal approach is one that is symbiotic between center capability and patient goals.
Based on PARTNER (Placement of AoRTic TraNscathetER Valve) trial data (6, 7, 8), TAVR is becoming an equivalent strategy in patients with STS-PROM ≥4%, increasing the application of this technology to a larger subset of patients who could also have an increased incidence of TAAD. That said, low-risk patients treated with TAVR may require unique and independent consideration for surgical procedures such as TAAD repair when faced with such a decision. In the OCEAN (Optimized Catheter Valvular Intervention) study, in which TAVR outcomes were analyzed, age was not an independent predictive factor of increased midterm mortality risk.
Considering age in TAAD, treatment options for octogenarians and older patients who experience Stanford Type A dissection have evolved. In 2 different Japanese studies of octogenarians who underwent open repair versus conservative treatment in TAAD, there was equipoise on the ideal treatment strategy for patients (9,10). As TAVR becomes more mainstream, being offered at academic and nonacademic centers alike, where risk tolerance may be significantly different based on resources and expertise, subsequent treatments should be decoupled from the original assessment for TAVR therapy. Age is often argued as an important risk predictor in outcome when evaluating for cardiac surgery. In fact, age independently informs the EuroSCORE and STS-PROM calculators significantly. However, given the outcome in our patient, we challenge the current paradigm in the aging population shifting to the right, and suggest that individualized treatment options be considered for patients.
Follow-Up
The patient underwent the operation without neurological, renal, visceral, or peripheral malperfusion sequelae. She was discharged to the ward, convalescing expectantly with planned rehabilitation.
Conclusions
TAVR adoption is increasing, and outcomes continue to influence treatment of aortic stenosis. Procedural and selection bias could potentially overshadow other clinical decisions needed for patient care while potentially contributing to an uptick in complications. Open surgery is the current standard for treating TAAD (that might be related to TAVR) and should be considered a viable treatment option in risk-stratified patients.
Footnotes
Dr. Song is a consultant for Medtronic. The spouse of Dr. Fuss is a shareholder in ViewRay. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Bonser R.S., Ranasinghe A.M., Loubani M. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455–2474. doi: 10.1016/j.jacc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 2.Plichta R.P., Hughes G.C. Thoracic endovascular aortic repair for the ascending aorta: experience and pitfalls. J Vis Surg. 2018;4:92. doi: 10.21037/jovs.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berfield K.K., Sweet M.P., McCabe J.M. Endovascular repair for type A aortic dissection after transcatheter aortic valve replacement with a Medtronic CoreValve. Ann Thorac Surg. 2015;100:1444–1446. doi: 10.1016/j.athoracsur.2014.11.077. [DOI] [PubMed] [Google Scholar]
- 4.Ong S.H., Mueller R., Gerckens U. Iatrogenic dissection of the ascending aorta during TAVI sealed with the CoreValve revalving prosthesis. Catheter Cardiovasc Interv. 2011;77:910–914. doi: 10.1002/ccd.22900. [DOI] [PubMed] [Google Scholar]
- 5.Mangla A., Gupta S. Vascular complications post-transcatheter aortic valve procedures. Indian Heart J. 2016;68:724–731. doi: 10.1016/j.ihj.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack M.J., Leon M.B., Thourani V.H. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 7.Popma J.J., Deeb G.M., Yakubov S.J. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 8.Waksman R., Rogers T., Torguson R. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol. 2018;72:2095–2105. doi: 10.1016/j.jacc.2018.08.1033. [DOI] [PubMed] [Google Scholar]
- 9.Shiono M., Hata M., Sezai A., Iida M., Yagi S., Negishi N. Emergency surgery for acute type A aortic dissection in octogenarians. Ann Thorac Surg. 2006;82:554–559. doi: 10.1016/j.athoracsur.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 10.Hata M., Sezai A., Niino T. Should emergency surgical intervention be performed for an octogenarian with type A acute aortic dissection? J Thorac Cardiovasc Surg. 2008;135:1042–1046. doi: 10.1016/j.jtcvs.2007.08.078. [DOI] [PubMed] [Google Scholar]