Abstract
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital defect and usually diagnosed within the first 2 months of life. Only 10% of patients survive to adulthood largely in part to the formation of extensive collaterals from the right to left coronary arteries. We present a case of ALCAPA diagnosed in an asymptomatic adult through a transthoracic echocardiogram (TTE). (Level of Difficulty: Beginner.)
Key Words: ALCAPA, coronary anomaly, dilated coronary artery, echocardiography
Abbreviations and Acronyms: ALCAPA, anomalous origin of the left coronary artery from the pulmonary artery; LCA, left coronary artery; RCA, right coronary artery
Graphical abstract

Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital defect.
History of Present Illness
A 37-year healthy man presented to establish care with a primary care physician. He had no specific complaints. His vitals were within normal limits with a heart rate of 74 beats/min, blood pressure of 124/60 mm Hg, respiratory rate of 16 breaths/min, and oxygen saturation of 98% on room air. His physical examination was notable for a grade 3 of 6 holosystolic murmur heard best at the apex that radiated to his back.
The patient had a history of measles when he was 2 years of age. Otherwise, his past medical history was unremarkable.
Learning Objectives
-
•
To highlight the importance of understanding the vascular anatomy of ALCAPA, clinical presentation in adults, and diagnosis.
-
•
To identify the echocardiographic features typical of ALCAPA and use in combination with clinical data to significantly reduce misdiagnosis or nondiagnosis, thereby improving survival.
-
•
To understand the post-operative management of patients with ALCAPA.
Differential Diagnosis
The differential diagnosis for an apical holosystolic murmur is mitral regurgitation owing to mitral valve dysfunction.
Investigations
Electrocardiography was notable for a left anterior fascicular block. His laboratory work-up was within normal limits. Transthoracic echocardiography revealed an ejection fraction of 45%, moderate posteriorly directed mitral regurgitation, mild hypokinesis of anteroseptum and anterior, anterolateral, and apical segments of the left ventricle. Color Doppler showed low-velocity, diastolic linear flow in the septum from inferoseptum to anteroseptum, consistent with coronary vessel flow. Low-velocity diastolic flow was also noted into the pulmonary artery, representing reversed flow from an anomalous left main emptying into the pulmonary artery (Videos 1, 2, 3, and 4). Overall findings were highly characteristic of an anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA). He underwent a stress echocardiogram in which he achieved target heart rate and exercised for 13 min without experiencing chest pain or discomfort. Stress electrocardiography was notable for 3-mm ST-segment depressions in the lateral leads (V4 to V6) and 1-mm ST-segment elevation in the aVR lead. Post-exercise echocardiography showed more prominent hypokinesis of the anterior wall and hypokinesis of anteroseptum. Mitral regurgitation remained unchanged from baseline. Cardiac catheterization confirmed the presence of an anomalous left coronary artery (LCA) originating from the pulmonary artery. Right coronary angiography showed a very large right coronary artery (RCA) that filled the entire LCA retrograde via collaterals with flow reversal in the LCA and contrast emptying into the pulmonary artery (Figures 1 and 2). Ascending aortography confirmed that there was no LCA arising from the ascending aorta (Figure 3). His filling pressures were normal, and there was no step-up in oxygen saturation present.
Online Video 1.
Transthoracic echocardiogram with color Doppler. Off-axis parasternal short-axis view at the aortic valve level, focusing on right ventricular outflow, showing retrograde flow into the pulmonary artery from the left coronary artery.
Online Video 2.
Transthoracic echocardiogram with color Doppler. Off-axis apical 4-chamber view with a focus on the right ventricle and interventricular septum, showing extensive collateral flow from the right coronary artery. Note that the Doppler flow is predominantly in diastole.
Online Video 3.
Transthoracic echocardiogram with color Doppler. Parasternal short-axis view focusing on the interventricular septum, showing diastolic flow in the interventricular septum indicative of collaterals from the right coronary artery.
Online Video 4.
Transthoracic echocardiogram with color Doppler. Apical 4-chamber view with color Doppler across the mitral valve, showing the eccentric jet of mitral regurgitation.
Figure 1.
Dilated Tortuous Right Coronary Artery as Seen on Coronary Angiogram
Coronary angiogram (arrow) showing the dilated and tortuous right coronary artery with extensive collaterals to the left coronary artery.
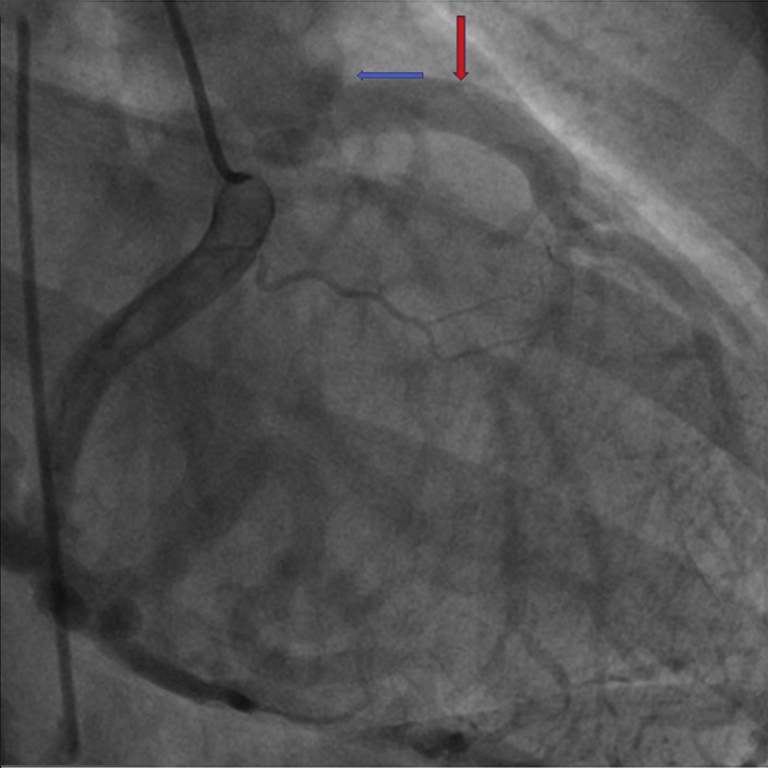
Figure 2.
Filling of Left Coronary Artery Through an Extensive Network of Collaterals From the Right System
Coronary angiogram showing filling of left coronary artery via collaterals (red arrow). The blue arrow points to the origin of the left coronary artery from the pulmonary artery.
Figure 3.
The Origin of the Left Coronary Artery From the Pulmonary Artery and Right Coronary From the Aorta
Aortogram shows origin of the left coronary artery from the pulmonary artery (blue arrow). The red arrow points to the origin of the right coronary artery from the right coronary sinus aorta.
Management
The patient then underwent successful reimplantation of the LCA with concurrent mitral valve repair (Figure 4, Figure 5, Figure 6). His post-operative course remained uneventful, and he was discharged on aspirin and warfarin, given the very robust competing collateral flow with a dilated RCA, which carries a risk of coronary thrombosis.
Figure 4.
Dilated Tortuous Epicardial Right Coronary Artery as Seen Intraoperatively
Intraoperative photo showing the tortuous and dilated right coronary artery (blue arrow) with extensive collaterals (red arrow).
Figure 5.
Intraoperative Image of Mitral Valve Repair
Intraoperative photo showing the pulmonary artery (blue arrow) being opened and the deep-seated origin of left coronary artery (red arrow).
Figure 6.
Intraoperative Image Showing Mitral Valve Repair
Follow-Up
On his 1-month post-operative follow-up, he reported doing well. He admitted to realizing that he may have had some chest discomfort while climbing hills but that this has completely resolved since his surgery. A murmur was no longer heard on a physical examination. He is compliant with his aspirin and coumadin without bleeding issues.
Discussion
Bland-White-Garland syndrome, also known as ALCAPA, was first described in the literature in 1933 by Bland, White, and Garland (1). ALCAPA is a very rare congenital anomaly that occurs in 1 in 300,000 births, with a 90% mortality within the first year of life when left untreated (2). Although it most commonly presents in infancy, 10% of children with this congenital anomaly will survive to adulthood. Case reports on adults with ALCAPA show that their first presentation is on a spectrum of being asymptomatic to acute heart failure, myocardial ischemia, and sudden cardiac death (3,4). The degree of collateral formation between the RCA and LCA serves as a significant predictor for symptoms. Dilation of the RCA and the extensive collateralization from the RCA to the left system occurs as a compensation for the lower blood oxygen level in the LCA. Continuous blood from the RCA into the LCA and then into the low-pressure pulmonary artery results in coronary steal phenomenon. Papillary muscle and LV lateral wall dysfunction induced by chronic ischemia result in mitral regurgitation (5). Because the incidence of an adult type of ALCAPA is very low, often asymptomatic, and lacking ST-T changes on electrocardiography, patients with this congenital anomaly go through life either undiagnosed or misdiagnosed. Echocardiography is usually the initial diagnostic tool. Echocardiographic features including color Doppler mapping can show a continuous shunt retrograde into the pulmonary artery lumen at the abnormal origin of the LCA. Abundant collateral blood flow may be observed in the myocardium, especially in the interventricular septum. Other secondary features include left ventricular dilation and dysfunction, papillary muscle fibrosis, mitral valve prolapse or regurgitation, abnormal wall motion, and dilatated RCA (5). Although magnetic resonance angiography and computed tomography angiography have been increasingly used for the diagnosis of ALCAPA, these modalities are unable to determine intravascular blood flow conditions and require administration of contrast dye. Echocardiography is an indispensable diagnostic tool because it is noninvasive, is low cost, and has the ability to show the origin of the coronary artery. An obvious drawback is when the echocardiographer does not have comprehensive knowledge of ALCAPA. Of note, the diagnosis may be difficult in the setting of pulmonary hypertension, as the blood flow in the LCA might be forward, and the “stealing blood” sign of the coronary artery is not present.
Conclusions
ALCAPA is a rare congenital heart disease that carries a high mortality rate and may lead to sudden death if left untreated. An understanding of the typical echocardiographic features of ALCAPA is critical in order to obtain the often elusive diagnosis. Relatively young patients noted to have depressed left ventricular systolic function and concomitant mitral regurgitation without underlying coronary artery disease should raise suspicion for anomalous origin of a coronary artery. Ultrasound imaging with transthoracic echocardiography serves as an essential tool for the early diagnosis of this congenital anomaly. An operator’s comprehensive knowledge, diagnostic awareness, and echocardiographic skills are critical for the accurate diagnosis of ALCAPA.
Acknowledgment
The authors would like to thank Robin Brides, RDCS, for performing the transthoracic electrocardiography and obtaining great images.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Bland E.F., White P.D., Garland J. Congenital anomalies of the coronary arteries: report of an unusual case associated with cardiac hypertrophy. Am Heart J. 1933;8:787–801. [Google Scholar]
- 2.Yau J.M., Singh R., Halpern E.J., Fischman D. Anomalous origin of the left coronary artery from the pulmonary artery in adults: a comprehensive review of 151 adult cases and a new diagnosis in a 53-year-old woman. Clin Cardiol. 2011;34:204–210. doi: 10.1002/clc.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fierens C., Budts W., Denef B. A 72-year-old woman with ALCAPA. Heart. 2000;83:e2. doi: 10.1136/heart.83.1.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S., Xie M., Lv Q. Misdiagnosis of anomalous origin of the left coronary artery from the pulmonary artery by echocardiography: single-center experience from China. Echocardiography. 2020;37:104–113. doi: 10.1111/echo.14578. [DOI] [PubMed] [Google Scholar]
- 5.Ghaffari S., Ranjibar A., Hajizadeh R. Association of significant mitral regurgitation and left ventricular dysfunction with ALCAPA syndrome in a young patient. Crescent J Med Biol Sci. 2018;5:67–68. [Google Scholar]