Abstract
Pericardial decompression syndrome, defined as paradoxical hypotension and pulmonary edema after pericardiocentesis, is a rare complication of pericardiocentesis. Stress cardiomyopathy, caused by excess catecholamine response resulting in left ventricular dysfunction and elevated cardiac enzymes, can overlap with pericardial decompression syndrome, and both might belong to the same spectrum of disease. (Level of Difficulty: Intermediate.)
Key Words: cardiomyopathy, pericardial decompression syndrome, pericardial effusion, stress cardiomyopathy, systolic dysfunction
Abbreviations and Acronyms: LV, left ventricular; LVEF, left ventricular ejection fraction; PDS, pericardial decompression syndrome; SCM, stress cardiomyopathy; TTE, transthoracic echocardiography
Graphical abstract
Pericardial decompression syndrome, defined as paradoxical hypotension and pulmonary edema after pericardiocentesis, is a rare complication of…
History of Presentation
A 70-year-old man presented with 2 weeks of worsening dyspnea on exertion. On physical examination, the patient was not in any acute distress. He had regular tachycardia and decreased heart sounds. Jugular venous distention and bilateral 2+ lower extremity edema were present. Electrocardiography revealed sinus tachycardia (104 beats/min) with low voltage and no electric alternans or ischemic changes. Chest computed tomography without contrast demonstrated large pericardial effusion. Cardiac enzymes (CEs) were negative. Transthoracic echocardiography (TTE) confirmed the large circumferential pericardial effusion with diastolic right heart collapse (Figure 1, Video 1). No wall motion abnormalities were noted, and left ventricular ejection fraction (LVEF) was preserved. On therapeutic pericardiocentesis, 2,060 ml of sanguineous fluid was removed. A few minutes after the procedure, the patient reported sudden-onset chest pressure and nausea, became hypotensive (blood pressure 88/60 mm Hg), and developed worsening tachycardia (heart rate 120 to 130 beats/min) and hypoxia (oxygen saturation 80% to 85%).
Learning Objectives
-
•
To recognize paradoxical hemodynamic instability after pericardiocentesis as a potential complication of the procedure, as well as its differential diagnosis.
-
•
To distinguish the possible mechanisms of paradoxical hemodynamic instability after pericardiocentesis, including the infrequent PDS.
-
•
To investigate the potential overlap between PDS and SCM given potentially similar causative mechanisms.
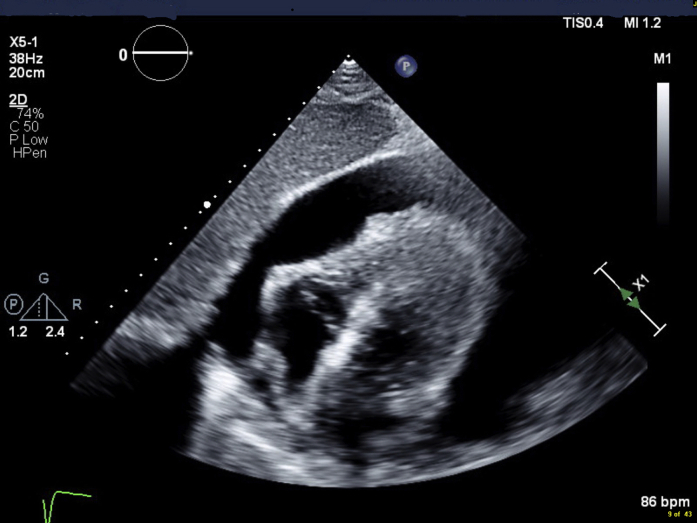
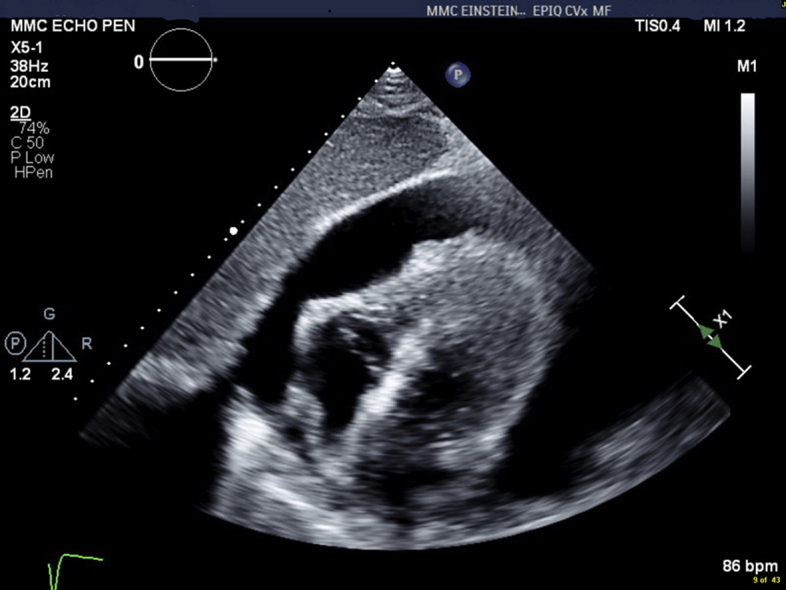
Figure 1.
Transthoracic Echocardiography
Subcostal view showing large pericardial effusion causing diastolic right heart collapse.
Online Video 1.
Transthoracic Echocardiography, Subxiphoid View, Showing a Large Pericardial Effusion With Right Heart Collapse and Preserved Left Ventricular Systolic Function.
Medical History
The patient’s medical history included hypertension and obesity.
Differential Diagnosis
The differential diagnosis included cardiac perforation, coronary laceration, vasovagal response, pericardial decompression syndrome (PDS), and stress cardiomyopathy (SCM).
Investigations
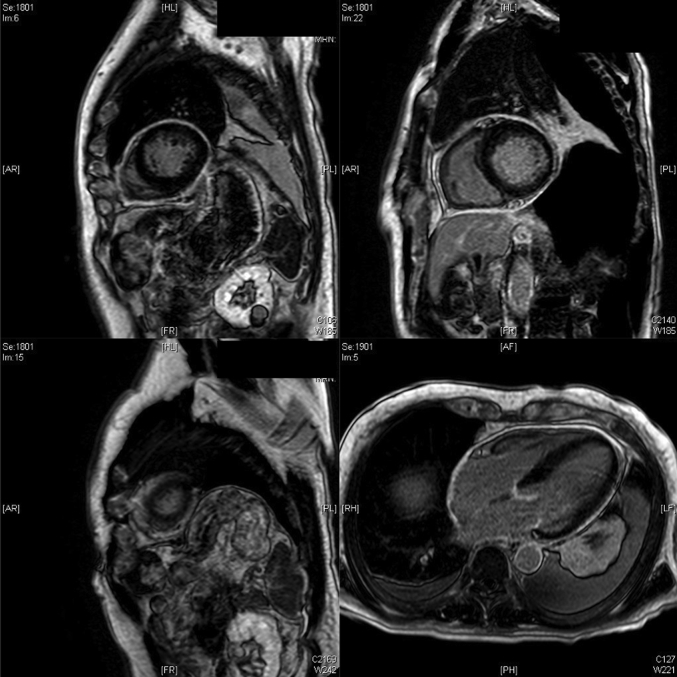
Emergent TTE was performed a few minutes after the patient’s change in clinical status and showed a newly reduced LVEF of 30%, with apical, septal, and anterolateral akinesis. All other walls were hypokinetic except for the basal left ventricular (LV) segments, which had preserved contractility (Videos 2 and 3). Troponin T and creatine kinase-MB were newly elevated to 1.04 ng/l and 238 U/l, respectively. Electrocardiography showed interval development of Q waves in the inferior leads. CEs peaked the following day (troponin T 2.0 ng/l, creatine kinase-MB 435 U/l), and repeat TTE showed persistent biventricular dysfunction, despite complete resolution of the pericardial effusion. LVEF was 25% with new apical thrombus. Pericardial drain output decreased to a minimum, and the drain was removed. Coronary angiography revealed mild nonobstructive coronary artery disease. Cardiac magnetic resonance imaging showed mildly reduced LVEF of 49% and apical hypokinesis extending into the mid anterior wall, with no evidence of myocardial fibrosis, infarction, or myocarditis (Figure 2). Repeat TTE prior to discharge (13 days after initial TTE) showed improved LVEF of 50% and moderate-sized apical akinesis (Video 4). Pericardial fluid analysis demonstrated an exudative effusion by Light’s criteria (pericardial fluid lactate dehydrogenase 557 U/l, serum lactate dehydrogenase 281 U/l). Histopathologic analysis showed grossly bloody fluid without malignant cells and reactive inflammatory cells. Other infectious, oncological, and rheumatologic work-up was negative.
Online Video 2.
Emergent Transthoracic Echocardiography Showing New Systolic Dysfunction With Apical, Septal, and Anterolateral Akinesis
All other walls were hypokinetic except for the basal left ventricular segments, which had preserved contractility
Online Video 3.
Emergent Transthoracic Echocardiography, Short-Axis View, Showing New Systolic Dysfunction After Large-Volume Pericardiocentesis
Figure 2.
Cardiac Magnetic Resonance Imaging
Mildly reduced left ventricular ejection fraction of 49%, apical hypokinesis extending into the mid anterior wall, and no evidence of myocardial fibrosis, infarction, or myocarditis.
Online Video 4.
Transthoracic Echocardiography, 4-Chamber View, After Improvement of Left Ventricular Function
Management
The initial hypotensive episode after pericardiocentesis was transient and improved after a small fluid bolus. The patient was started on guideline-directed medical therapy for heart failure including a beta-blocker and an angiotensin-converting enzyme inhibitor. He was started on apixaban for treatment of LV thrombus.
Discussion
Pericardiocentesis is a lifesaving therapeutic procedure for patients presenting with cardiac tamponade. It is a relatively safe procedure, but physicians must be aware of post-procedural complications. In particular, hypotension after pericardiocentesis should raise suspicion for 2 under-recognized and under-reported diagnoses: PDS and SCM.
PDS has been defined as paradoxical hypotension, often with pulmonary edema and ventricular dysfunction, after pericardial drainage. Onset can vary from immediately after drainage to up to 48 h later, and mortality can be as high as 29% (1). In one case series, 8 of 10 patients showed elevations of CEs (1), but in other series, a majority of patients with PDS showed no increases in CEs (2,3). Full recovery of LV function is expected in most patients. The true incidence of PDS is unknown: in a large retrospective analysis of 1,164 consecutive pericardiocentesis procedures, PDS was reported in only 1 patient, whereas in other series it has been reported in up to 4.8% (4). As is evident, this syndrome has no uniform clinical presentation and may be associated with both surgical pericardiostomy and pericardiocentesis, whereas the cause of pericardial effusions and clinical scenarios varies widely (5,6).
Various hypotheses have been proposed to explain the mechanism of PDS: hemodynamic shifts (increased venous return and right ventricular expansion after pericardial fluid removal causes LV compression and pulmonary edema) (2), ischemic changes (diminished coronary perfusion caused by compression of epicardial vessels by the pericardial effusion that results in transiently impaired myocardial function and inability to handle the sudden shifts in volume that occur when the pericardial effusion is drained too quickly) (7), or autonomic imbalances (increased sympathetic tone caused by the tamponade results in an inotropic effect that increases LV contractility and heart rate and, once suddenly removed by pericardiocentesis, leads to the unmasking of underlying LV dysfunction) (8).
In SCM, it has been hypothesized that perhaps cardiac tamponade can act as the inciting catecholamine surge (7). A review of 25 patients with heart failure after pericardial drainage showed 12 patients with echocardiographic features consistent with SCM (3).
Our case supports the autonomic imbalance hypothesis: systolic dysfunction with apical ballooning and preserved basal function, chest pain, elevated CEs, and normal coronary arteries, with subsequent improvement of LV function, are suggestive of SCM. It is possible that the sympathetic overdrive stimulated by the tamponade-induced reduction in cardiac output causes PDS and/or SCM after pericardiocentesis. After the abrupt removal of the tamponade physiology, and thus the source for the sympathetic overdrive, with pericardiocentesis, a reduction in sympathetic stimulation can result in systolic dysfunction, shock, and pulmonary edema with or without CE rise (Figure 3).
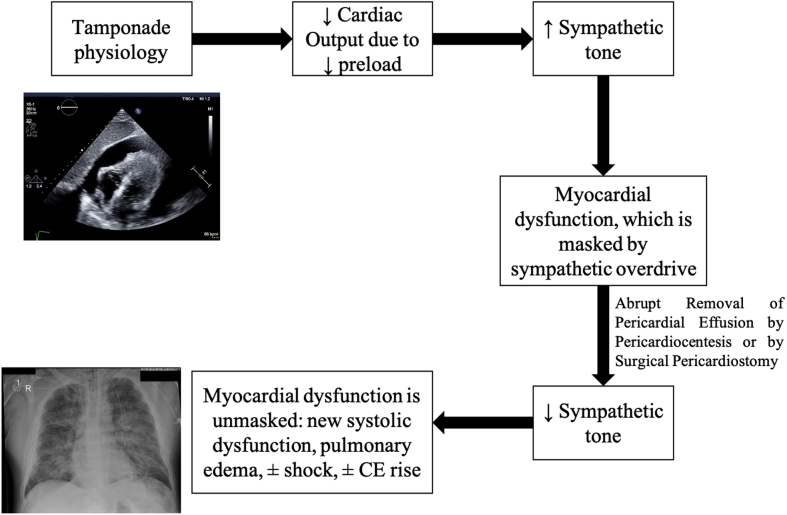
Figure 3.
Possible Mechanism Behind Paradoxical Hypotension and Pulmonary Edema Following Pericardiocentesis
Diagram depicting the possible mechanism behind paradoxical hypotension and pulmonary edema following pericardiocentesis in some patients with large pericardial effusions with tamponade physiology: the tamponade physiology itself causes sympathetic stimulation to compensate for the decrease in cardiac output caused by a decrease in venous return. That same sympathetic overdrive can cause myocardial damage, which is masked by the sympathetic-predominant state. Once the pericardial effusion is removed, the sympathetic stimulation is lost, and the myocardial damage is unmasked. This damage has myriad clinical presentations (systolic dysfunction, shock, pulmonary edema, cardiac enzymes (CEs) normal or elevated) and might be the explanation behind the overlap seen in pericardial decompression syndrome and stress cardiomyopathy.
The management of PDS is generally supportive, and early intervention (fluids or diuretic agents depending on volume status, inotropes, and mechanical support devices if needed) is imperative, as mortality can be as high as 29% (1).
Risk factors for developing hemodynamic instability after pericardiocentesis are currently unknown. The only factor associated with increased mortality in PDS is surgical pericardiostomy when compared with needle pericardiostomy (1). Expert opinion suggests that pericardial fluid should be removed only until tamponade physiology is resolved, and the rest should be slowly drained to permit adaptive changes in coronary flow, wall stress, and myocardial mechanics (2). Currently, there are no guidelines to prevent or manage PDS. It is unclear if PDS and SCM are distinct entities or if they belong to the same spectrum of disease. Studies need to be conducted to investigate the possible association between PDS and SCM.
Follow-Up
The patient was discharged and was seen 1 month later at outpatient follow-up, without recurrence of symptoms.
Conclusions
The underlying mechanism of PDS is unknown. It is possible that sympathetic overdrive caused by the tamponade-induced reduction in cardiac output causes a SCM. A reduction in sympathetic stimulation after removal of the tamponade physiology can result in hypotension and even shock with pulmonary edema. It is important to recognize that SCM and PDS might not be entirely distinct processes and might actually belong to the same spectrum of disease. Further studies are needed to establish evidence-based guidelines to prevent and treat these entities.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Ritu Thanman, MD, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Pradhan R., Okabe T., Yoshida K., Angouras D.C., DeCaro M.V., Marhefka G.D. Patient characteristics and predictors of mortality associated with pericardial decompression syndrome: a comprehensive analysis of published cases. Eur Heart J Acute Cardiovasc Care. 2015;4:113–120. doi: 10.1177/2048872614547975. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan A., Mahajan N., Suri A., Mahajan K. Pericardiocentesis decompression syndrome: An uncommon complication of a common procedure. IHJ Cardiovasc Case Reports. 2019;3:18–20. [Google Scholar]
- 3.Ayoub C., Chang M., Kritharides L. A case report of ventricular dysfunction post pericardiocentesis: stress cardiomyopathy or pericardial decompression syndrome? Cardiovasc Ultrasound. 2015;13:32. doi: 10.1186/s12947-015-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoub C., Lekhakul A., Assawakawintip C. Abstract 17022: pericardial decompression syndrome: incidence in a large consecutive series of echocardiographic-guided pericardiocentesis procedures. Circulation. 2018;138:A17022. [Google Scholar]
- 5.Vandyke W.H., Jr., Cure J., Chakko C.S., Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med. 1983;309:595–596. doi: 10.1056/NEJM198309083091006. [DOI] [PubMed] [Google Scholar]
- 6.Kumar R., Sinha A., Lin M.J. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5:206–212. doi: 10.4103/2229-5151.165007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versaci F., Donati R., Mezzanotte R., Chiariello L., Ammirati F. An unusual complication following pericardiocentesis: reversible left ventricular dysfunction. J Cardiovasc Med (Hagerstown) 2015;16(Suppl 2):S133–S135. doi: 10.2459/JCM.0b013e32833cdbf1. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe M.W., Edelman E.R. Transient systolic dysfunction after relief of cardiac tamponade. Ann Intern Med. 1993;119:42–44. doi: 10.7326/0003-4819-119-1-199307010-00007. [DOI] [PubMed] [Google Scholar]