Abstract
Our patient presented in her third trimester of pregnancy with new onset of heart failure. A thorough workup in the initial postpartum period with detailed past medical history, advanced imaging modalities, and a multidisciplinary approach revealed a rare and treatable etiology of cardiomyopathy. (Level of Difficulty: Intermediate.)
Key Words: cardiomyopathy, genetic disorders, lipid metabolism disorders, pregnancy
Abbreviations and Acronyms: GDMT, guideline-directed medical therapy; LVEF, left ventricular ejection fraction; MTPD, mitochondrial trifunctional protein deficiency; RV, right ventricle/ventricular; TTE, transthoracic echocardiogram
Graphical abstract
Our patient presented in her third trimester of pregnancy with new heart failure. A thorough workup in the initial postpartum period with detailed past…
History of Presentation
A 33-year-old G1P1 woman presented 4 months postpartum for heart transplant evaluation.
Learning Objectives
-
•
To be able to make a differential diagnosis of cardiomyopathies in the peripartum/postpartum period.
-
•
To understand the role of fatty acid oxidation disorders in the pathophysiology of cardiomyopathy.
Following an uneventful early pregnancy, the patient presented during the third trimester with lower extremity edema to her thighs, shortness of breath with minimal exertion, and decreased urine output. She was admitted to her community hospital where transthoracic echocardiogram (TTE) showed left ventricular ejection fraction (LVEF) 20% to 25%, a restrictive filling pattern, left ventricular end-diastolic diameter 4.4 cm, intraventricular septum 1.06 cm, mild right ventricular (RV) enlargement and reduced systolic function, moderate mitral and tricuspid regurgitation, and estimated pulmonary artery systemic pressure 59 mm Hg. She had a successful vaginal delivery with hemodynamic monitoring briefly requiring milrinone. Her laboratory results were remarkable for mildly elevated creatinine kinase and creatinine 1.3 mg/dl. She was discharged home 10 days after delivery on guideline-directed medical therapy (GDMT). She lost 90 lbs during this hospitalization.
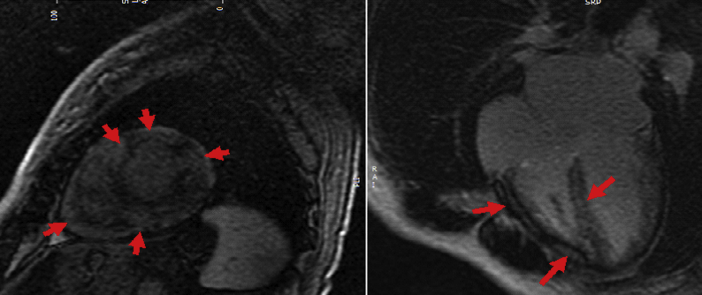
One month later, cardiac magnetic resonance imaging (CMR) showed mildly dilated left ventricle, LVEF 37% with diffuse hypokinesis, normal RV size and moderately reduced function, biatrial enlargement, and moderate mitral and tricuspid regurgitation. Most notably, imaging showed diffuse and patchy late gadolinium enhancement in the left and right ventricles, consistent with infiltrative or inflammatory disease (Figure 1).
Figure 1.
CMR 1 Month Postpartum
Short-axis and 4-chamber post-contrast images, demonstrating diffuse, predominantly mid-myocardial late gadolinium enhancement involving both the left and right ventricular suggestive of a diffuse infiltrative myocardial process (red arrows).
She was referred for heart transplant evaluation given her persistent New York Heart Association functional class III symptoms.
Past Medical History
Her past medical history is significant for 5 childhood hospitalizations for rhabdomyolysis precipitated by infection or exercise. Multiple muscle biopsies were negative for glycogen storage disease or fatty acid oxidation disorders. She had no episodes in adulthood.
At 8 years of age, she had viral myocarditis presumed secondary to coxsackievirus with full recovery.
Electrocardiogram at 12 years of age showed normal sinus rhythm with incomplete right bundle branch block, left atrial enlargement, and possible RV enlargement.
Her sister had similar episodes of recurrent rhabdomyolysis in childhood.
Differential Diagnosis
-
•
Peripartum cardiomyopathy
-
•
Glycogen storage disease/fatty acid oxidation disorder
-
•
Malonic aciduria
-
•
Fabry disease
-
•
Danon disease
-
•
Friedreich’s ataxia
-
•
Amyloidosis
-
•
Other: human immunodeficiency virus, thyroid disease, autoimmune etiology
Investigations
On the initial visit, the patient’s blood pressure was 110/64 mm Hg, pulse was 77 beats/min, respiratory rate was 16 breaths/min, and oxygen saturation was 98% on ambient air. Physical examination demonstrated S3 on cardiac auscultation, jugular venous distension to 12 cm, and lower extremity edema bilaterally.
Diagnostic testing was notable for a creatinine of 1.28 mg/dl (estimated glomerular filtration rate 48 ml/min/1.73 m2), mild microcytic anemia secondary to iron deficiency, and N-terminal pro–B-type natriuretic peptide elevated to 3,100 pg/ml (0 to 178 pg/ml). Results of hemoglobin A1C, autoimmune panel, human immunodeficiency virus, troponin, creatinine kinase, hepatobiliary panel, lipid panel, light chains, and thyroid function studies were normal.
Repeat TTE showed severely reduced LVEF at 15%, left ventricular end-diastolic diameter 4.6 cm, normal wall thickness, RV mildly to moderately increased in size with severely reduced systolic function, moderate biatrial enlargement, and estimated pulmonary artery systemic pressure 60 mm Hg. Cardiopulmonary testing showed respiratory exchange ratio 1.13, VO2 15.4 (56% of age- and size-predicted maximum, moderate to severe functional impairment), and VE/VCO2 elevated to 42. Holter monitoring showed a wandering atrial pacemaker arrhythmia.
Management
Given her childhood history and unusual CMR findings, the patient was referred to a geneticist. GDMT was optimized with metoprolol succinate, spironolactone, sacubitril/valsartan, and furosemide.
Her biochemical genetics evaluation was significant for low total and free carnitine with normal acylcarnitine profile, urine organic acids, and serum lactic acid. Next-generation sequencing showed 2 variants in the HADHB gene: 1 likely pathogenic (F430S) and 1 a variant of uncertain significance (D372V). One variant was inherited from each of her parents. A skin fibroblast culture was sent for fatty acid oxidation probe assay. Results were consistent with HADHB deficiency, which results in mitochondrial trifunctional protein deficiency. She was started on a low-fat diet supplemented with medium chain triglycerides and L-carnitine.
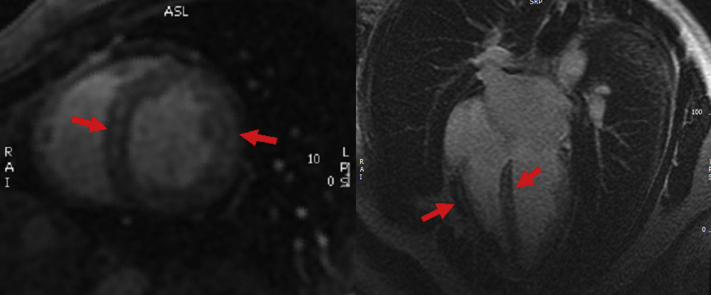
After 6 months of dietary modification, TTE shows regained cardiac function with LVEF 60% to 65%. CMR continues to show diffuse infiltrative disease with recovery of myocardial function (Figure 2). Repeat cardiopulmonary testing shows improvement in peak VO2 25 (87% of age- and size-predicted maximum, mild functional impairment), VE/VCO2 26.4.
Figure 2.
CMR After Dietary Modification, 1 Year Later
Short-axis and 4-chamber post-contrast images, demonstrates partial improvement in the diffuse and patchy late gadolinium enhancement (red arrows).
Discussion
The patient originally presented to our advanced heart failure clinic for cardiac transplant evaluation with a working diagnosis of peripartum cardiomyopathy, a diagnosis of exclusion. CMR can be used on an individual basis if alternative etiologies of cardiomyopathy are suspected, as seen in our patient. Her childhood history of recurrent rhabdomyolysis in both the patient and sister, “viral myocarditis,” and abnormal CMR with diffuse hyperenhancement gave us clinical pause to suspect an alternative etiology of cardiomyopathy and referral to a genetic specialist to identify an unusual genetic disorder and modify treatment.
Mitochondrial trifunctional protein deficiency (MTPD) is a rare autosomal recessive fatty acid oxidation disorder (1). The mitochondrial trifunctional protein is an inner mitochondrial membrane-bound protein complex encoded by HADHA and HADHB genes (2,3). The protein complex catalyzes the last 3 steps in mitochondrial beta-oxidation of long chain fatty acids (4). There are <100 cases of MTPD reported in the published data, and 2 known mutations that can lead to a broad spectrum of disease manifestations (5). The most severe cases are usually fatal and present in the neonatal period with hepatic steatosis, skeletal myopathy/neuropathy, and cardiomyopathy. The mild form presents from infancy to adolescence with peripheral neuropathy, episodic myalgias, and rhabdomyolysis (6). There are only case reports of first-time diagnoses made in adulthood. To our knowledge, there are no reported cases of MTPD-related cardiomyopathy diagnosed in adulthood.
Cardiac involvement in long-chain fatty acid oxidation defects is a common manifestation and significantly contributes to disease mortality in infancy (7,8). The myocardium appears to be susceptible to either direct toxicity from metabolic accumulation of long-chain fatty acids or from the substrate deficiency itself (6,9,10). Most cases of MTPD present with cardiomyopathy (dilated or hypertrophic) by the time a child reaches infancy. This early manifestation within the first few months of life is likely in part due to the transition in cardiac energy substrate from glucose (in utero) to fatty acid metabolism.
Our patient likely presented with the mild phenotype of MTPD in childhood. In retrospect it is unclear if her episode of myocarditis was an independent event related to viral illness, or if it was related to her underlying defect in fatty acid metabolism.
Follow-Up
Reassuringly, our patient has recovered to New York Heart Association functional class I symptoms with normalization of LVEF. We plan to maintain her on GDMT, dietary restriction, and lifestyle modifications. Although there are very few reports of cardiac transplantation in MTPD patients, disease recurrence is not expected if she maintains her diet (10).
Conclusions
To our knowledge, our patient is the first reported case of a diagnosis of MTPD-related cardiomyopathy in adulthood. The combination of substrate depletion in addition to the imbalance between energy supply and demand and intravascular volume expansion during pregnancy may have led to her decompensated presentation in the third trimester. She will continue to be followed closely on GDMT with dietary modifications for signs of disease progression. She may require advanced heart failure interventions in the future.
Acknowledgments
The authors thank the support staff within the Cohen Children’s Medical Center (CCMC) Northwell Health Division of Medical Genetics and Human Genomics, and the administration and support staff at Columbia University Irving Medical Center for Advanced Cardiac Care.
Footnotes
Dr. Axsom has served as a consultant for Abbott Laboratories. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Boutron A., Acquaviva C., Vianey-Saban C. Comprehensive cDNA study and quantitative analysis of mutant HADHA and HADHB transcripts in a French cohort of 52 patients with mitochondrial trifunctional protein deficiency. Mol Genet Metab. 2011;103:341–348. doi: 10.1016/j.ymgme.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Uchida Y., Izai K., Orii T., Hashimoto T. Novel fatty acid β-oxidation enzymes in rat liver mitochondria. J Biol Chem. 1992;267:1034–1041. [PubMed] [Google Scholar]
- 3.Kamijo T., Aoyama T., Komiyama A., Hashimoto T. Structural analysis of cDNAs for subunits of human mitochondrial fatty acid β-oxidation trifunctional protein. Biochem Biophys Res Commun. 1994;199:818–825. doi: 10.1006/bbrc.1994.1302. [DOI] [PubMed] [Google Scholar]
- 4.Sander M.H., Wanders R.J. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Boer M.E., Dionisi-Vici C., Chakrapani A., van Thuijl A.O., Wanders R.J., Wijburg F.A. Mitochondrial trifunctional protein deficiency: a severe fatty acid oxidation disorder with cardiac and neurologic involvement. J Pediatr. 2003;142:684–689. doi: 10.1067/mpd.2003.231. [DOI] [PubMed] [Google Scholar]
- 6.Spiekerkoetter A., Sun B., Khuchua Z., Bennett M.J., Strauss A.W. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum Mutat. 2003;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
- 7.Vockley J., Marsden D., McCracken E. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment—a retrospective chart review. Mol Genet Metab. 2015;116:53–60. doi: 10.1016/j.ymgme.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baruteau J., Sachs P., Broué P. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study from 187 patients. J Inherit Metab Dis. 2013;36:795–803. doi: 10.1007/s10545-012-9542-6. [DOI] [PubMed] [Google Scholar]
- 9.Lehman J.J., Kelly D.P. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 10.Bursle C., Weintraub R., Ward C., Justo R., Cardinal J., Coman D. Mitochondrial trifunctional protein deficiency: severe cardiomyopathy and cardiac transplantation. J Inherit Metab Dis. 2018;40:91–95. doi: 10.1007/8904_2017_68. [DOI] [PMC free article] [PubMed] [Google Scholar]