Abstract
A 25-year-old African-American woman with end-stage renal disease presented with new-onset heart failure. Transthoracic echocardiography indicated a significantly hyperechoic myocardium, and computed tomography noted a circumferential hyperattenuated myocardium. Endomyocardial biopsy revealed focal interstitial and intramyocyte calcium deposition in the heart, confirming a rare diagnosis of massive myocardial calcium deposition. (Level of Difficulty: Beginner.)
Key Words: cardiomyopathy, disorders of calcium metabolism, restrictive
Abbreviations and Acronyms: CT, computed tomographic; EMB, endomyocardial biopsy; ESRD, end-stage renal disease; HF, heart failure
Graphical abstract
A 25-year-old African American woman with end-stage renal disease presented with new-onset heart failure. Transthoracic echocardiography indicated a…
History of Present Illness
A 25-year-old African-American woman presented with chest pain during hemodialysis. She was diagnosed with new-onset heart failure (HF) complicated by respiratory failure. Her physical examination was notable for respiratory distress, fine bibasilar crackles, and jugular vein distension. She was also found to have scattered atypical, full-thickness, painful dermal lesions.
Learning Objectives
-
•
HF secondary to massive myocardial calcification is a rare but significant etiology.
-
•
To expand the differential diagnosis of new-onset HF in patients with chronic kidney disease.
-
•
To highlight the role of calcium-phosphorus metabolism in the development of a cardiomyopathy.
Medical History
The patient had a medical history of systemic lupus erythematosus on long-term hydroxychloroquine and end-stage renal disease (ESRD) on hemodialysis for approximately 4 years.
Differential Diagnosis
The patient presented with new-onset HF of unclear etiology. In addition to more typical etiologies of HF in a young patient (e.g., viral, familial, and idiopathic cardiomyopathies), a unique differential diagnosis including systemic lupus erythematosus myocarditis and hydroxychloroquine toxicity was included because of the patient’s reported history.
Investigations
Electrocardiography revealed sinus tachycardia, right-axis deviation, and poor R-wave progression (Figure 1). Transthoracic echocardiogram indicated a significantly hyperechoic myocardium with mild, concentric left ventricular hypertrophy, and an ejection fraction of 25% (Videos 1A, 1B, and 1C). Pulsed-wave Doppler through the mitral valve revealed fusion of the E and A waves. Although this E- and A-wave fusion limited accurate calculation of the E-wave deceleration time, this metric appeared qualitatively shortened, consistent with restrictive physiology (Figure 2A). However, tissue Doppler of both the medial and lateral mitral valve annuli revealed elevated e′ values, which would not be consistent with restriction, as it is normally associated with reduced e′ velocities (Figures 2B and 2C). Computed tomographic (CT) imaging of the chest revealed circumferential hyperattenuated myocardium in the left ventricle, discrete areas of the right ventricular free wall, and both atria concerning for calcium deposition (Figures 3A to 3C).
Figure 1.
Electrocardiogram
Online Video 1A.
Transthoracic Echocardiography, Parasternal Long-Axis View
Online Video 1B.
Transthoracic Echocardiography, Parasternal Short-Axis View
Online Video 1C.
Transthoracic Echocardiography, Apical 4-Chamber View
Figure 2.
Echocardiogram
(A) Pulsed-wave Doppler through the mitral valve. (B) Tissue Doppler of the medial mitral valve annulus. (C) Tissue Doppler of the lateral mitral valve annulus. DT = E-wave deceleration time; E = E wave; e′ = e′ mitral velocity.
Figure 3.
Computed Tomography Imaging of the Chest
(A) Computed tomographic (CT) imaging of the chest. Arrows denote calcified myocardium. (B) Contrast-enhanced CT imaging of the chest with multiplanar reformatting in the 4-chamber view. (C) Contrast-enhanced CT imaging of the chest with multiplanar reformatting in the short-axis view. A = calcified myocardium; B = noncalcified endocardium; LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
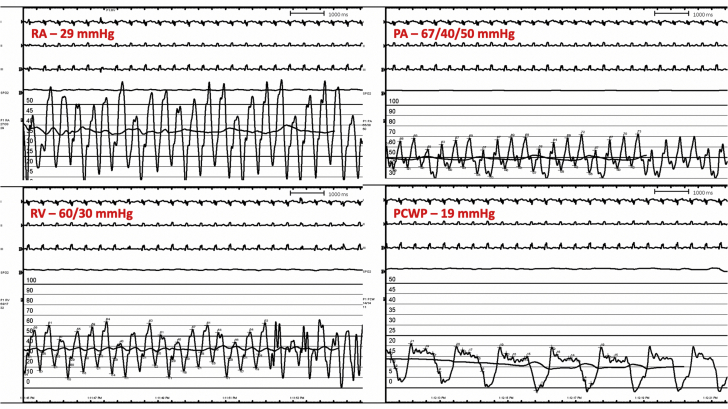
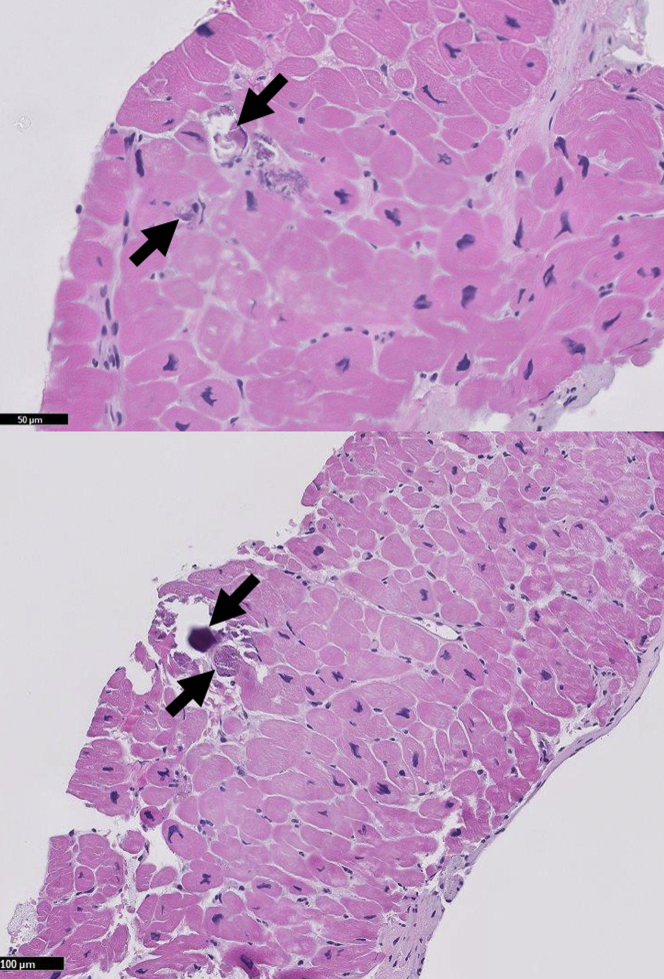
Right heart catheterization exhibited elevated biventricular pressures, equalization of diastolic pressures, and preserved cardiac output consistent with a restrictive physiology (Figure 4). Coronary angiography did not show any significant coronary artery disease. Serum was notable for calcium at 9.0 mg/dl (normal range: 8.4 to 10.2 mg/dl), phosphorus at 6.6 mg/dl (normal range: 2.5 to 4.4 mg/dl), and a parathyroid hormone level of 3,043 pg/ml (normal range: 15 to 75 pg/ml). A native heart endomyocardial biopsy (EMB) showed focal interstitial and intracellular calcium deposition with mild myocyte hypertrophy (Figure 5). Additionally, Von Kossa staining was used to highlight the calcium deposits (Figure 6). Finally, there was no histological evidence of vacuolated myocytes on hematoxylin and eosin staining or presence of cytoplasmic deposits, called “myeloid bodies,” within the cardiac myocytes on electron microscopy, both of which are specific to hydroxychloroquine toxicity (1). Biopsy of the dermal lesions noted superficial to deep perivascular and periadnexal lymphoplasmacytic infiltrate, with a fibrotic papillary dermis. The biopsy was not consistent with calciphylaxis.
Figure 4.
Right Heart Catheterization Tracings
PA = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; RA = right atrial pressure; RV = right ventricular pressure.
Figure 5.
Endomyocardial Biopsy With Routine Stain
Arrows denote intracellular calcium deposits.
Figure 6.
Endomyocardial Biopsy With Von Kossa Stain
Arrows denote intracellular calcium deposits.
Given the findings of intracellular calcium deposition found on CT imaging and EMB, the patient was diagnosed with restrictive cardiomyopathy due to massive myocardial calcium deposition.
Management
Although parathyroidectomy was indicated, the patient was not hemodynamically stable for surgery. Cinacalcet 30 mg/day was started to lower circulating phosphate level. Three doses of sodium thiosulfate 12.5 mg, a calcium chelating agent increasingly used in the treatment of calciphylaxis to promote calcium excretion, were given following hemodialysis sessions while dermal biopsy results were pending and calciphylaxis was ruled out, after which it was stopped (2). Throughout the hospital course, the patient had a pulmonary arterial catheter for invasive hemodynamic monitoring. Her hemodynamic status was difficult to manage because of the restrictive cardiac physiology and poor biventricular contractility secondary to the myocardial calcification, as well as low systemic vascular resistance due to sepsis. Norepinephrine was the predominant vasoactive medication used. Volume status was managed by continuous hemodialysis.
Discussion
There have been only a few case reports of myocardial calcification, which has been associated with HF, arrhythmias, and sudden cardiac death (3, 4, 5, 6). Myocardial calcification can be either dystrophic, occurring in infarcted, nonviable tissue, or metastatic, due to abnormalities in calcium-phosphorus metabolism, as well as elevated parathyroid hormone (3,7). This patient had severe derangements in her calcium-phosphorus metabolism, consistent with secondary hyperparathyroidism.
Secondary hyperparathyroidism results from derangements in calcium-phosphorus metabolism in the setting of chronic kidney disease. In chronic kidney disease, reduced excretion of phosphate leads to elevated serum phosphate levels, resulting in reduced renal activation of vitamin D and subsequent gastrointestinal malabsorption of calcium. Reduced calcium levels trigger increased parathyroid hormone release, leading to increased calcium release from the bones, renal calcium reabsorption, and intestinal mucosal cell calcium absorption (Figure 7) (8).
Figure 7.
Pathophysiology of Secondary Hyperparathyroidism
CKD = chronic kidney disease; GI = gastrointestinal; PTH = parathyroid hormone.
The exact mechanisms for massive myocardial calcification due to secondary hyperparathyroidism are incompletely understood. Calcium deposition in cardiac soft tissues in the form of mitral annular or aortic valve calcification has long been described in patients with ESRD. This type of soft tissue deposition has been attributed to derangements in calcium and phosphate metabolism as well as the coalescing of focal calcific deposits in regions of microinjury and lipoprotein accumulation over time (9). However, the myocardium is presumably not subject to the same degree of microinjury as valvular soft tissues. Therefore, we suspect that myocardial calcium deposition likely results from a unique mechanism.
The cycling and storage of calcium are essential to myocardial excitation and contraction, in addition to playing essential roles in electrophysiological signaling, mitochondrial function, cell death, and transcription. Calcium is stored primarily in the sarcoplasmic reticulum, bound to the storage protein calsequestrin. During depolarization, calcium enters the cytosol through calcium channels, triggering the release of calcium from the sarcoplasmic reticulum. Increased cytosol levels of calcium result in calcium binding to troponin C, which initiates myocyte contraction. During diastole, the majority of cytosolic calcium is rapidly removed by an adenosine triphosphate–regulated calcium transporter and again stored within the sarcoplasmic reticulum. A smaller percentage of this calcium is also excreted from the cell via sodium and adenosine triphosphate–regulated exchangers. The resulting decrease in intracellular calcium allows dissociation of calcium from troponin C, causing myocardial relaxation (10).
It is possible that massive myocardial calcification results from derangements not only in systemic calcium-phosphorus metabolism but in the calcium cycling and storage system itself. Although mutations that result in reduced calcium release or in increased calcium uptake have previously been described, they have not been associated with myocardial calcium deposition. Perhaps such mutations, in the setting of ESRD and secondary hyperparathyroidism, result in an altered milieu which allows massive myocardial calcium deposition. Such a mechanism could help explain how massive myocardial calcium deposition remains a rare entity despite the wide prevalence of secondary hyperparathyroidism among patients with ESRD (8).
In this case, diagnosis of massive myocardial calcium deposition relied heavily on noninvasive imaging. Transthoracic echocardiography revealed an unusually hyperechoic myocardium and a depressed ejection fraction. CT evaluation confirmed subendocardial, circumferential hyperattenuation, which is consistent with calcium deposition in the setting of severe secondary hyperparathyroidism.
This degree of myocardial calcification in a young patient after only 4 years of hemodialysis has not previously been reported. Only 3 prior case reports have reported pathology confirming myocardial calcification, but 2 were completed postmortem, and 1 was completed on an explanted heart following orthotopic heart transplantation (3,5,6). This is the first case reported of EMB completed in a living patient to confirm the diagnosis of massive myocardial calcification. It is worth noting that the EMB showed minimal calcification likely as a result of the endocardial sparing noted on imaging (Figures 3B and 3C). The calcification on EMB was found both in the interstitium and within the cardiac myocytes. Unfortunately, examination with electron microscopy was unable to characterize the localization of the calcium deposits further. Further examination was limited because the family declined autopsy.
Routine diagnostic evaluation for myocardial calcium deposition is not currently indicated in the ESRD population, but these cases have reported diagnoses on the basis of chest radiography, chest CT imaging, fluoroscopy, and echocardiography (4,6). In all reported cases, patients were diagnosed post-mortem, were too critically ill to receive treatment, or required orthotopic heart transplantation for advanced-stage cardiomyopathy (3, 4, 5, 6). Therefore, no specific treatments have been published for massive myocardial calcification.
Follow-Up
The patient continued to decompensate over the 3 weeks following diagnosis from a combination of cardiogenic and septic shock, in the setting of fungemia, due to Candida auris and Candida glabrata, as well as extended spectrum beta-lactamase Escherichia coli bacteremia. The patient was not a candidate for advanced cardiac therapies because of her active, resistant infections and multiorgan system failure, from which she died during the hospitalization.
Conclusions
Massive myocardial calcification is a rare, serious complication of abnormal calcium-phosphorus metabolism in patients with ESRD.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Joyce E., Fabre A., Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013;2:77–83. doi: 10.1177/2048872612471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Z., Gu L., Pang H., Fang Y., Yan H., Fang W. Sodium thiosulfate: an emerging treatment for calciphylaxis in dialysis patients. Case Rep Nephrol Dial. 2015;5:77–82. doi: 10.1159/000380945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Na J.Y. A heart of stone: an autopsy case of massive myocardial calcification. Forensic Sci Med Pathol. 2018;14:102–105. doi: 10.1007/s12024-017-9936-8. [DOI] [PubMed] [Google Scholar]
- 4.Matsui M., Okayama S., Takitsume A. Heart failure associated with metastatic myocardial calcification in a hemodialysis patient with progressive calcification of the hand. Cardiorenal Med. 2012;2:251–255. doi: 10.1159/000343497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isotalo P.A., Halil A., Green M., Tang A., Lach B., Veinot J.P. Metastatic calcification of the cardiac conduction system with heart block: an under-reported entity in chronic renal failure patients. J Forensic Sci. 2000;45:1335–1338. [PubMed] [Google Scholar]
- 6.Shackley B.S., Nguyen T.P., Shivkumar K., Finn P.J., Fishbein M.C. Idiopathic massive myocardial calcification: a case report and review of the literature. Cardiovasc Pathol. 2011;20:e79–e83. doi: 10.1016/j.carpath.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Thompson A.R., Fallon J., Nussbaum S. Evaluation of metastatic cardiac calcification in a model of chronic primary hyperparathyroidism. Surgery. 1990;108:1047–1051. [PubMed] [Google Scholar]
- 8.Cunningham J., Locatelli F., Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 9.Massera D., Kizer J.R., Dweck M.R. Mechanisms of mitral annular calcification. Trends Cardiovasc Med. 2020 doi: 10.1016/j.tcm.2019.07.011. In press. [DOI] [PubMed] [Google Scholar]
- 10.Bers D.M. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]