Abstract
We discuss a patient who presented with cardiogenic shock secondary to massive pulmonary embolism and right ventricular failure. She was managed by a multidisciplinary heart team and treated with catheter-directed thrombectomy, followed by ProtekDuo (Tandem [Liva Nova], London, United Kingdom) heart percutaneous right ventricular support leading to complete recovery from this often fatal condition. (Level of Difficulty: Intermediate.)
Key Words: pulmonary hypertension right-sided catheterization, thrombosis
Abbreviations and Acronyms: AKI, acute kidney injury; CT, computed tomography; ED, emergency department; IVC, inferior vena cava; PA, pulmonary artery; PE, pulmonary embolism; PERT, pulmonary embolism response team; RV, right ventricular; TTE, transthoracic echocardiography
Graphical abstract
We discuss a patient who presented with cardiogenic shock secondary to massive pulmonary embolism and right ventricular failure. She was managed by a…
History of Presentation
A 72-year-old woman presented to the emergency department (ED) with symptoms of rapidly worsening shortness of breath over the past 36 to 48 h. Her symptoms had progressed rapidly from her usual state of health to not being able to take a couple of steps and to significant dyspnea at even at rest. She had no significant chest pain. Physical examination revealed the following: blood pressure, 103/56 mm Hg; heart rate, 118 beats/min; respiratory rate, 22 breaths/min; and hypoxia with oxygen saturation in the low 80s with respiratory distress. Her lung fields were clear to auscultation, and there was no significant evidence of volume overload by examination.
Learning Objectives
-
•
To make a rapid diagnosis of PE with hemodynamic instability by multidisciplinary team (PERT approach), reducing invasive diagnostic tests, allowing rapid diagnosis, and applying advanced therapies with likely reduction in morbidity and mortality.
-
•
To understand catheter-directed mechanical thrombectomy use in the setting of massive PE without the risk of critical and fatal hemorrhage associated with thrombolysis.
Medical History
She had a known history of immunoglobulin G kappa multiple myeloma treated with lenalidomide and dexamethasone, previous history of pulmonary embolism (PE), hypertension, hypothyroidism, and diabetes mellitus. She had completed 1 year of anticoagulant therapy for a previous episode of PE approximately 9 years earlier and was not currently receiving anticoagulant therapy. Her other significant medications included amlodipine, glimepiride, levothyroxine, and losartan.
Differential Diagnosis
The differential diagnosis included PE, decompensated heart failure, pneumonia, and atypical presentation of acute coronary syndrome.
Investigations
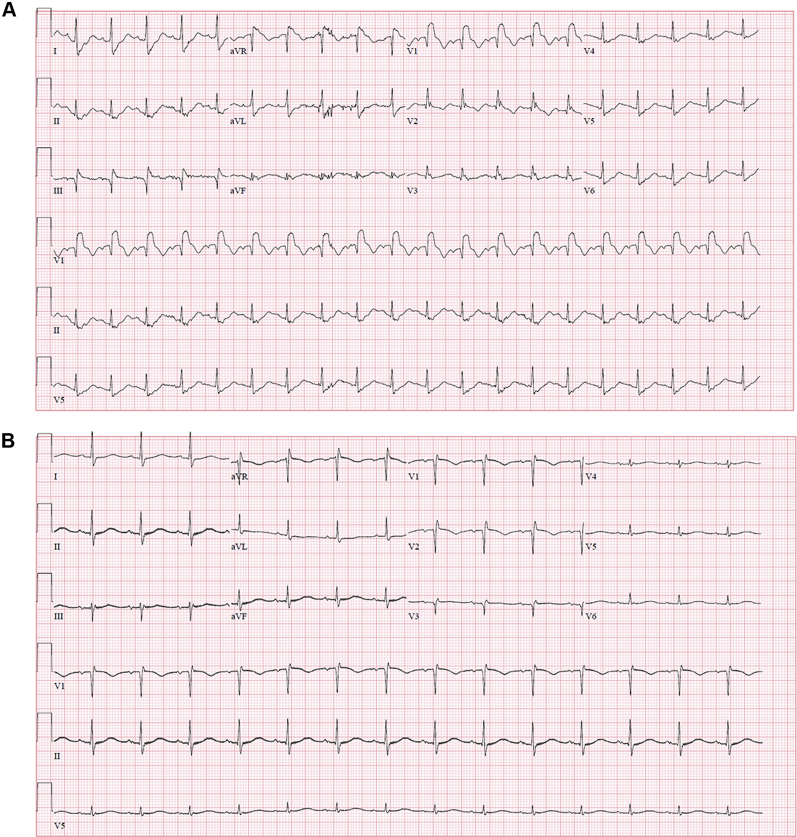
On presentation, the electrocardiogram showed sinus tachycardia, new right bundle branch block, a qR pattern in lead V1, and T-wave inversions in leads V1 and V2 (Figure 1A). Chest radiography showed no acute cardiopulmonary disease. Laboratory study results were significant for the following: mild troponin T elevation, 0.19 ng/ml (reference range <0.005 ng/ml); elevated lactate, 5.1 mmol/l, with combined metabolic and respiratory acidosis on arterial blood gas determination with pH 7.19; partial pressure of carbon dioxide, 42 mm Hg; serum bicarbonate, 14 mEq/l; and creatinine, 2.7 mg/dl, compared with baseline creatinine of 1.0 mg/dl, indicating acute kidney injury (AKI). Urgent bedside transthoracic echocardiography (TTE) showed a small hyperdynamic left ventricular cavity with hyperdynamic systolic function with an estimated ejection fraction >75%, a severely dilated right ventricle with severely reduced right ventricular (RV) systolic function and a hyperdynamic RV apex (the McConnell sign), moderate to severe tricuspid regurgitation with elevated estimated RV systolic pressure of 50 to 55 mm Hg, and a dilated inferior vena cava (IVC).
Figure 1.
Initial and Post-Procedure ECGs
(A) Electrocardiogram (ECG) on presentation showing sinus tachycardia, right bundle branch block, and a qR pattern in lead V1. (B) Electrocardiogram 24 h post-procedure showing resolution of sinus tachycardia, right bundle branch block, and the qR pattern in lead V1.
Management
The patient was evaluated by a multidisciplinary heart team comprising ED, pulmonary and critical care, and interventional cardiology physicians. On the basis of clinical presentation and TTE imaging, a diagnosis of cardiogenic shock secondary to RV failure resulting from massive PE was made. Because of the patient’s AKI and unstable hemodynamics, the multidisciplinary team decided to not pursue confirmatory computed tomography (CT) with angiography. She was treated with intravenous heparin for anticoagulation and underwent emergency pulmonary angiography and intervention in the cardiac catheterization laboratory. She was evaluated by the multidisciplinary team within 60 min, and bedside TTE was performed within 90 min. The time from presentation to the ED and the start of mechanical thrombectomy was <120 min.
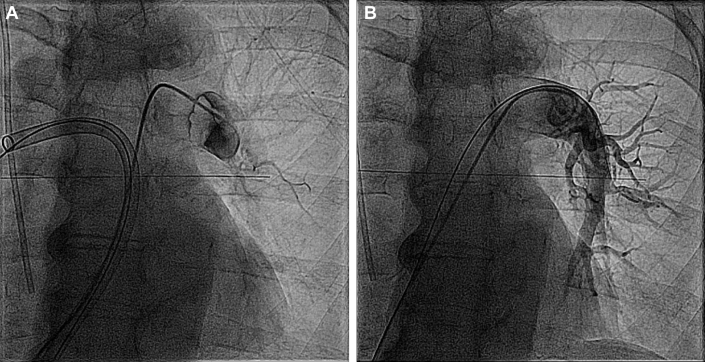
Right-sided heart catheterization was performed through a right femoral vein approach. The pulmonary angiogram showed a large clot burden in the right pulmonary artery (PA). The right femoral venous sheath was up-sized to a 22-F DrySeal sheath (W.L. Gore & Associates, Newark, Delaware). An Amplatz Super Stiff wire (Boston Scientific, Natick, Massachusetts) was placed under fluoroscopic guidance in the right PA, and a FlowTriever catheter (Inari Medical, Irvine, California) was advanced, followed by successful mechanical aspiration of large clot fragments and excellent flow noted on the angiogram. Subsequently, a pulmonary angiogram of the left PA showed complete obstruction of flow (Figure 2A). Several clot aspirations were similarly performed. A large clot was noticed on the tip of the FlowTriever cannula; this clot was unable to be moved despite disk deployment. With negative suction maintained, the clot was pulled down to the iliac vein, and an infrarenal IVC filter was placed through a left jugular vein approach. A repeat angiogram of the left PA showed the establishment of significant flow (Figure 2B).
Figure 2.
Initial and Post-Procedure Angiograms
(A) Pulmonary angiogram showing complete obstruction of the left pulmonary artery. (B) Pulmonary angiogram showing re-establishment of flow in the left pulmonary artery following mechanical thrombectomy.
The procedure was complicated by hemoptysis requiring reversal of anticoagulation with protamine. Emergency intubation, mechanical ventilation, and flexible bronchoscopy were performed, with no active bleeding. Small to moderate-sized blood clots were seen in the airways, without obstruction. These clots were removed with saline wash and suctioning.
The patient continued to have persistent hypotension, tachycardia with narrow pulse pressure, and RV dysfunction noted on TTE. RV failure was treated with a ProtekDuo (Tandem [Liva Nova], London, United Kingdom) heart percutaneous RV assist device. The device was placed through a right internal jugular access, with inotropic support and an epoprostenol infusion. She had an episode of atrial fibrillation with rapid ventricular response requiring DC cardioversion, and sinus rhythm was maintained with an amiodarone infusion.
Follow-Up
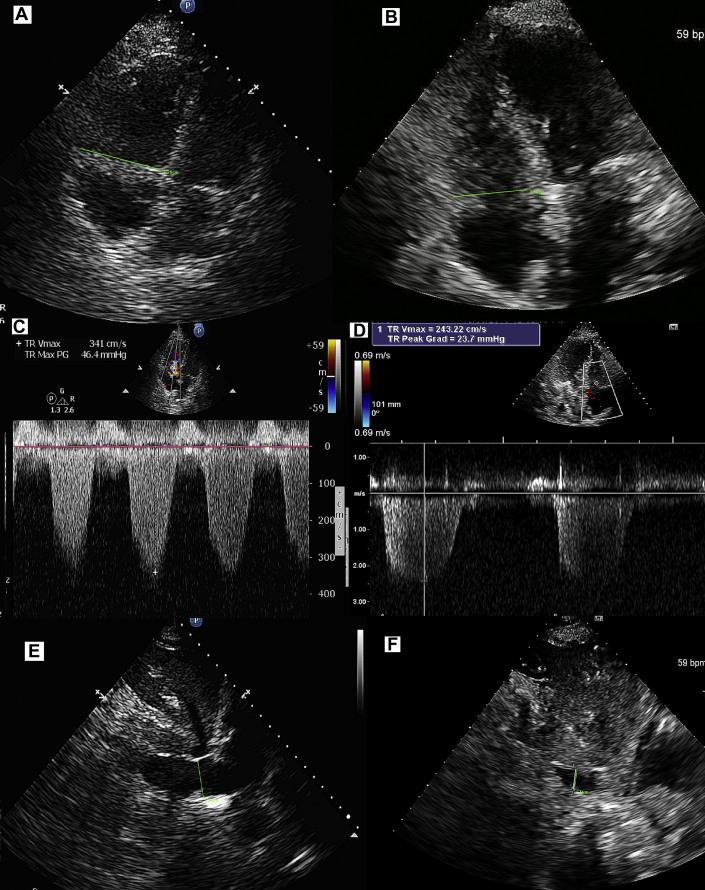
Follow-up TTE within 24 h showed significant improvement, with only mild RV dilation and moderate systolic dysfunction (Figure 3B). Her hemodynamics improved over 48 h, and she was successfully weaned from the RV assist device and epoprostenol. Repeat bronchoscopy showed no evidence of bleeding, and she was extubated in 72 h, followed by significant improvement in clinical status. She was discharged within 1 week of admission on an anticoagulant regimen with rivaroxaban. Follow-up TTE 5 weeks post-discharge showed normal RV size and function with mild tricuspid regurgitation and RV systolic pressure of 26 mm Hg (Figures 3A to 3F, which also show the patient’s initial findings for comparison). An IVC filter was removed in 3 months.
Figure 3.
Transthoracic Echocardiograms
(A to F) Transthoracic echocardiograms on presentation and at follow-up. (A, C, E) Initial images: Severe right ventricular dilatation, severe tricuspid regurgitation with elevated pulmonary artery pressure, and dilated inferior vena cava. (B, D, F) Follow-up images: Normal right ventricular size, mild tricuspid regurgitation with normal pulmonary artery pressure, and normal-size inferior vena cava. Grad = gradient; Max = maximum; PG = peak gradient; TR = tricuspid regurgitation; Vmax = maximum velocity.
Discussion
The PE response team (PERT) approach to patients who present with intermediate- to high-risk PE has been described to provide rapid access to advanced therapies (1,2). Our patient presented with clinical manifestations consistent with massive PE with several features suggestive of adverse prognosis, including sinus tachycardia, new right bundle branch block, a qR pattern in lead V1, an elevated troponin T level, RV dilatation, hypokinesis, the McConnell sign, severe tricuspid regurgitation, and evidence of pulmonary hypertension on TTE. The McConnell sign has a 94% specificity for the diagnosis of acute PE, and with the clinical presentation suggesting a high pre-test probability of PE, confirmatory testing with CT angiography was not performed (3). Acute massive or submassive PE has an exceedingly high mortality rate, and an elevated lactate level is a strong independent predictor of mortality (4,5). The magnitude of lactate elevation correlates with the risk of death and is >35% for lactate levels higher than 5 mmol/l (5). Systemic thrombolysis has been the recommended treatment of choice in this setting; however, it is associated with major systemic bleeding, including a risk of intracranial hemorrhage (6,7).
Catheter-directed mechanical thrombectomy with the FlowTriever Retrieval/Aspiration System has been found to be beneficial in intermediate-risk PE (8). This case demonstrates the use of this system in a patient with PE with cardiogenic shock. Thrombectomy was performed by engaging the thrombus, disrupting it with self-expanding nitinol disks, and extracting it by simultaneously aspirating and withdrawing it through a 20-F guide catheter, with appropriate mechanical circulatory support used for shock. Hemoptysis is a known complication of PE as a result of ischemic pulmonary parenchymal necrosis. Although an obvious site of bleeding could not be identified on bronchoscopy, wire perforation as a cause of hemoptysis could not be ruled out and should be recognized as a potential complication of the procedure. In this case, the PERT approach with multidisciplinary experts allowed treatment without confirmatory CT angiography and immediate treatment with advanced therapies, thus reducing the time to treatment. A total of 70 ml of iodine contrast material was used for the procedure. The CT angiogram PE protocol requires approximately 60 to 150 ml of iodine contrast material (9). Hence possible harm from additional iodine contrast medium exposure–induced renal dysfunction in the setting of AKI was avoided.
Conclusions
This case highlights a multidisciplinary approach (PERT) to the use of catheter-directed thrombectomy in massive PE with cardiogenic shock, with an excellent long-term outcome. This approach avoids the risk of critical and fatal hemorrhage associated with thrombolysis.
Footnotes
Ashwin Ravichandran, MD, served as Guest Editor for this paper. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Rosovsky R., Zhao K., Sista A., Rivera-Lebron B., Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3:315–330. doi: 10.1002/rth2.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahar J.H., Haddadin I., Sadana D. A pulmonary embolism response team (PERT) approach: initial experience from the Cleveland Clinic. J Thromb Thrombolysis. 2018;46:186–192. doi: 10.1007/s11239-018-1686-2. [DOI] [PubMed] [Google Scholar]
- 3.McConnell M.V., Solomon S.D., Rayan M.E., Come P.C., Goldhaber S.Z., Lee R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 4.Kucher N., Rossi E., De Rosa M., Goldhaber S.Z. Massive pulmonary embolism. Circulation. 2006;113:577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 5.Vanni S., Viviani G., Baioni M. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Ann Emerg Med. 2013;61:330–338. doi: 10.1016/j.annemergmed.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Jaff M.R., McMurtry M.S., Archer S.L. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 7.Marti C., John G., Konstantinides S. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015;36:605–614. doi: 10.1093/eurheartj/ehu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu T., Toma C., Tapson V.F. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. J Am Coll Cardiol Intv. 2019;12:859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Moore A.J.E., Wachsmann J., Chamarthy M.R., Panjikaran L., Tanabe Y., Rajiah P. Imaging of acute pulmonary embolism: an update. Cardiovasc Diagn Ther. 2018;8:225–233. doi: 10.21037/cdt.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]