Abstract
Objective
To test the hypothesis that fundamental relationships along the amyloid, tau, and neurodegeneration (A/T/N) cascade depend on synaptic integrity in older adults in vivo and postmortem.
Methods
The 2 independent observational, cross-sectional cohorts included (1) in vivo community-dwelling, clinically normal adults from the University of California, San Francisco Memory and Aging Center who completed lumbar puncture and MRI (exclusion criteria, Clinical Dementia Rating score >0) and (2) postmortem decedents from the Rush Memory and Aging Project (exclusion criteria, inability to sign informed consent). In vivo measures included CSF synaptic proteins (synaptotagmin-1, synaptosome associated protein-25, neurogranin, and growth associated protein-43), β-amyloid (Aβ42/40), tau phosphorylated at amino acid 181 (ptau181), and MRI gray matter volume (GMV). Postmortem measures captured brain tissue levels of presynaptic proteins (complexin-I, complexin-II, vesicle associated membrane protein (VAMP), and SNARE complex) and neuritic plaque and neurofibrillary tangle (NFT) counts. Regression models tested statistical moderation of synaptic protein levels along the A/T/N cascade (synaptic proteins × amyloid on tau, and synaptic proteins × tau on GMV).
Results
Sixty-eight in vivo older adults (age 71 years, 43% female) and 633 decedents (age 90 years, 68% female, 34% clinically normal) were included. Each in vivo CSF synaptic protein moderated the relationship between Aβ42/40 and ptau181 (−0.23 < β < −0.12, p < 0.05) and the relationship between ptau181 and GMV (−0.49 <β < −0.32, p < 0.05). Individuals with more abnormal CSF synaptic protein demonstrated expected relationships between Aβ-ptau181 and ptau181-brain volume, effects that were absent or reversed in those with more normal CSF synaptic protein. Postmortem analyses recapitulated CSF models. More normal brain tissue levels of complexin-I, VAMP, and SNARE moderated the adverse relationship between neuritic plaque and NFT counts (−0.10 <β < −0.08, p < 0.05).
Conclusions
Pathogenic relationships of Aβ and tau may depend on synaptic state. Synaptic markers may help identify risk or resilience to AD proteinopathy.
Synaptic failure is a core feature of Alzheimer disease (AD) and tracks closely with cognition.1 β-Amyloid (Aβ) oligomers disrupt synaptic signaling before amyloid plaque or tau tangle formation,2-4 and aberrant synaptic firing promotes tau hyperphosphorylation.5-8 Synaptic dysregulation may therefore fundamentally contribute to AD pathogenesis.
Furthermore, preserved synaptic integrity may protect cognitive functioning. Restoration of select synaptic components rescues cognition despite the presence of amyloid or tau.9-11 Autopsy studies of adults demonstrating cognitive resilience to neuropathologic AD show increased synaptic molecules12,13 and structures13,14 compared to nonresilient peers with AD. Synaptic maintenance may modulate neurotoxicity of amyloid and tau.
Advances in CSF synaptic protein quantification have opened the window to capture in vivo dynamics. When synapses lose function, synaptic proteins are released and increase in CSF.15 High levels (more abnormal) of CSF synaptic proteins differentiate cognitively normal adults from those with clinical AD, relate to memory performances, predict rapidity of cognitive decline, and differentiate AD mutation carriers >10 years before symptoms.16-23 In vivo capture of synaptic markers alongside amyloid and tau in humans may advance our basic understanding of how AD unfolds.
The A/T/N framework posits that amyloid (A) aggregation, tau (T) hyperphosphorylation, and neurodegeneration (N) are fundamental to the AD cascade.24 We tested how synaptic proteins affect the A/T/N cascade in humans both in vivo and postmortem using distinct methodologies (CSF or brain tissue protein levels) across 2 independent cohorts. We hypothesized that synaptic proteins would moderate the adverse relationship between amyloid and tau and between tau and neurodegeneration.
Methods
Study Design
This 2-cohort, cross-sectional study was designed to test the conceptual hypothesis that synaptic markers play an important role in the A/T/N cascade using 2 distinct methodologies. CSF metrics were prospectively quantified in the University of California, San Francisco (UCSF) Memory and Aging Center (MAC). Synaptic and AD proteins were retrospectively available as a validation sample in the Rush Memory and Aging Project (MAP). Protein markers were not directly harmonized and instead represent complementary markers along conceptually similar pathways, i.e., amyloid, tau, and synaptic functioning. This design aimed to provide converging evidence for our overarching hypothesis. Demonstration of conceptually consistent models across cohorts despite distinct methodologies helps support the robustness and generalizability of the proposed relationships yet is limited in protein-specific comparisons or inferences.
Participants
UCSF MAC
Sixty-eight community-dwelling participants in the Hillblom Aging Network at the UCSF MAC who completed a baseline lumbar puncture were included. Participants completed comprehensive neurologic and neuropsychological evaluations and study partner interview (Clinical Dementia Rating score 0). All participants were reviewed and determined to be within normative standards at interdisciplinary case conferences by board-certified neurologists and neuropsychologists (further cohort description is given in reference 25). This study focused on clinically normative older adults to better characterize the role of synaptic markers in the earliest stages of the AD spectrum (i.e., before overt neurodegeneration). This is particularly relevant given the larger body of prior literature demonstrating overt CSF synaptic marker changes in AD disease state.2,3,26
Rush MAP
We also selected 633 decedents who had undergone brain autopsy with neuropathologic evaluation and brain tissue previously analyzed for presynaptic proteins from the Rush MAP.27 Exclusion criterion was inability to sign an informed consent and Anatomical Gift Act. All participants signed a repository consent to allow their data to be repurposed.
Participants were selected for inclusion from larger parent studies according to availability of the primary metrics of interest (i.e., synaptic protein; amyloid and tau marker quantification).
Both studies were conducted in accordance with the latest Declaration of Helsinki and approved by local institutional review boards. Participants provided written informed consent to participant in either study.
UCSF MAC: Clinical Evaluations
CSF Analytics
CSF was collected in the morning after a 12-hour fast, processed, and stored following standard procedures.23
AD Proteins
Samples were analyzed for the 40 and 42 amino acid forms of Aβ (Aβ1–40, Aβ1–42, respectively) and tau phosphorylated at amino acid 181 (ptau181) via Lumipulse.28 Aβ42/40 and ptau181 were selected to represent AD-related pathologic forms of amyloid and tau, respectively.29
Synaptic Proteins
Proteins reflecting presynaptic vesicle machinery (synaptosome associated protein-25 [SNAP-25], synaptotagmin-1 [SYT-1]), postsynaptic calcium modulation (neurogranin [Ng]), and growth factor regulation (growth associated protein-43 [GAP-43]) were measured with previously described assays.17-21,23 SNAP-25 and SYT-1 were analyzed with an in-house–developed assay at the University of Gothenberg, Sweden, that consisted of simultaneous enrichment of both proteins with immunoprecipitation (KingFisher Flex System) followed by digestion, addition of heavy isotope-labeled standards, and quantification with liquid chromatography/selected reaction monitoring mass spectrometry (Agilent 6490 QQQ MS, Santa Clara, CA). For SNAP-25, we quantified the N-terminal amino acids 32 through 40, which are present in only the longest soluble forms of SNAP-25. Previous works have demonstrated increased sensitivity of this peptide (compared to levels of total soluble N-terminal levels) differentiating cognitive impairment.30 Ng and GAP-43 concentrations were measured using in-house sandwich ELISAs. CSF synaptic protein levels are expressed in picograms per milliliter (Ng and GAP-43) or picomoles (SYT-1 or SNAP-25).
Neuroimaging
A subset (n = 51) of participants completed 3T Magnetom Vision TIM Siemens Trio (Malvern, PA) MRI within 180 days of lumbar puncture. Participants who completed brain MRI did not differ in age (70.0 years vs 72.8 years, p = 0.18), sex (43.1% vs 41.2% female), education (17.0 years vs 17.8 years, p = 0.25), or Mini-Mental Status Examination score (29.1 vs 29.1, p = 0.93) compared to those who did not. T1-weighted images were acquired and processed with the unified segmentation procedure in SPM12 into International Consortium of Brain Mapping space following standard procedures previously used.31 Total intracranial volume was estimated for each participant in Montreal Neurological Institute space. Given its relevance in AD, we selected bilateral medial temporal lobe substructures (summing all modulated gray matter within hippocampal, parahippocampal, entorhinal regions) and total gray matter volume as indicators of neurodegeneration. Total intracranial volume was statistically regressed before analyses.
Rush MAP: Clinical Evaluation
Neuropathologic Evaluation
Brain hemisections were cut into 1-cm coronal slabs, and both hemispheres were evaluated for gross pathology (e.g., macroscopic infarcts). The hemisphere with visible pathology was prepared for histologic evaluation (see elsewhere32 for in-depth details). Fresh slabs were fixed in 4% paraformaldehyde. Tissue blocks from predetermined regions were dissected, embedded in paraffin, and cut into 6-μm sections.
AD Proteins
Neuritic plaque and neurofibrillary tangle (NFT) burdens were determined by microscopic examination of silver-stained slides from 5 regions (midfrontal, midtemporal, inferior parietal, and entorhinal cortices and hippocampus). Count of each region was scaled by dividing the corresponding SD and averaged to obtain summary neuritic plaque and NFT burden counts. We selected neuritic plaque and NFT counts because they are gold-standard markers of pathologic AD proteinopathy and because they most closely paralleled the CSF Aβ42/40 and ptau181 markers in the UCSF MAC cohort.29 To determine specificity to the pathologic forms of Aβ (plaques vs total amount), we also quantified the total fraction of Aβ protein of the cortex via immunohistochemistry (quantified by image analysis).27 Values represent the percent area of the cortex occupied by Aβ (averaged across hippocampus, entorhinal, midfrontal, inferior temporal, angular gyrus, anterior cingulate cortex, superior frontal cortex).
Other common neuropathologies were quantified following previously described methods, including hippocampal sclerosis, Lewy body disease, TAR DNA-binding protein 43, cerebral amyloid angiopathy, and cerebrovascular disease (macroinfarcts, microinfarcts, arteriosclerosis, atherosclerosis).32
Presynaptic Proteins
Frozen gray matter samples were obtained from 6 regions (hippocampus, middle frontal gyrus, inferior temporal gyrus, calcarine cortex, ventromedial caudate, and posterior putamen) and used to prepare homogenates at a consistent protein concentration, followed by serial dilution for ELISA, as described previously.33 We chose a regionally global index of synaptic proteins given that measures of each synaptic protein are highly correlated across brain regions and that prior reports demonstrate meaningful associations between global indices with cognition and to reduce multiple comparisons.33,34 In addition, given that this is the same approach as other MAP analyses, a regionally global index allows direct comparison across reports. Individual synaptic proteins were selected on the basis of prior associations with cognition in MAP.33,34
Monoclonal antibodies quantified complexin-I, complexin-II, and vesicle associated membrane protein (VAMP) levels. Values were expressed in log10 units, standardized, and averaged across regions within each participant. Individual protein abundances provide information regarding integrity of the presynaptic compartment. We also queried protein-protein interactions among SNARE proteins (SNAP-25, syntaxin-1, and VAMP) to provide information regarding the relative functional capacity of presynaptic proteins.35 High-throughput immunoprecipitation was implemented with heterologous capture ELISA. Purified antibody directed against SNAP-25 or syntaxin-1 was immobilized on the ELISA plate to capture these proteins from serially diluted brain homogenates as “bait”. Captured bait-target complexes (SNAP-25–syntaxin, SNAP-25–VAMP, syntaxin–SNAP-25, syntaxin-VAMP) were detected with monoclonal antibodies of a different subclass, directed to a SNARE protein other than the capture antibody. For each of the 4 protein-protein interactions, values were expressed in log10 units, standardized, and averaged across the same 6 brain regions. Mean SNARE protein-protein interaction complex levels reflect the average of the 4 protein-protein interactions. Higher values indicate more SNARE protein-protein interactions and have shown sensitivity to cognitive aging and psychiatric-related brain changes in previous works.33 Brain tissue synaptic protein levels are expressed in z score units for all markers assessed according to the Rush MAP cohort.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all patients (or guardians of patients) participating in the study (consent for research).
Statistical Analyses
Pairwise exclusion was used for missing data in UCSF MAC analyses (total n = 68 for Ng and GAP-43 analyses; total n = 53 for SNAP-25 and SYT-1 analyses; total n = 51 for all MRI analyses); there were no missing data in the Rush MAP analyses (total n = 633). We evaluated univariate correlations among AD and synaptic proteins in each cohort separately, including visual investigation for nonlinear trends. Based on shape on visualization, nonlinear trends were statistically tested via regression entering both linear (X) and quadratic (X2) terms.
UCSF MAC
A/T/N framework posits that Aβ, tau hyperphosphorylation, and neurodegeneration are fundamental events in the AD cascade.24 We therefore aimed to determine the statistical modification of synaptic protein markers at each of these pathogenic junctures. Statistical moderation posits that the slope of the relationship between X and Y (e.g., amyloid and tau) differs depending the level of a third variable (e.g., synaptic marker). To do so, we conducted a series of regression interaction models, all of which covaried for age, sex, and education. First, we tested the interaction between each CSF synaptic protein and Aβ42/40 (synaptic protein × Aβ42/40) on ptau181 levels. Next, we examined the interaction between each CSF synaptic protein and ptau181 (synaptic protein × ptau181) on gray matter volumes (marker of neurodegeneration). We also tested the effect of individual Aβ isoforms separately in models showing significant Aβ42/40 ratio effects.
For illustration purposes only (all interactions modeled continuous terms), we plotted synaptic protein levels by tertile. To estimate directionality and effect sizes of interaction models, we also calculated bivariate correlations of the relationship of interest in participants with low vs high CSF synaptic protein levels (lower vs upper tertile).
Rush MAP
We tested parallel models in Rush MAP. With adjustment for age at death, sex, and education, regression interaction models tested the statistically modifying effect of each individual presynaptic protein (complexin-I, complexin-II, VAMP) on the relationship between neuritic plaque and NFT burden. We additionally tested the interaction of the SNARE protein-protein complex levels on the relationship between neuritic plaque and NFT burden, adjusting for average SNARE protein abundance, as well as demographics. Finally, to determine specificity to pathologic forms of amyloid, we evaluated the same models described above, covarying for or substituting total Aβ fraction (vs neuritic plaques).
Data Availability
All data included in this report will be made available on request. UCSF MAC data requests can be sent to the corresponding author, while Rush MAP resources can be requested at radc.rush.edu.
Results
Participants
UCSF MAC in vivo participants averaged 71 years of age; ≈40% were female; and they performed well on a global cognitive screening (average Mini-Mental Status Examination score mean 29 of 30). Consistent with age-related prevalences,36,37 18% demonstrated abnormal CSF Aβ42/40 and 13% demonstrated abnormal ptau181 in this cohort of clinically normal older adults.
Rush MAP participants averaged 90 years of age at death. One-third were cognitively normal; one-third met clinical criteria for AD dementia; and 64% met Reagan criteria for neuropathologic AD (intermediate or high likelihood). Table 1 summarizes participant characteristics.
Table 1.
Clinical and Demographic Characteristics of the UCSF MAC and Rush MAP Study Samples
UCSF MAC: CSF Results
Associations Among CSF Synaptic and AD Proteins
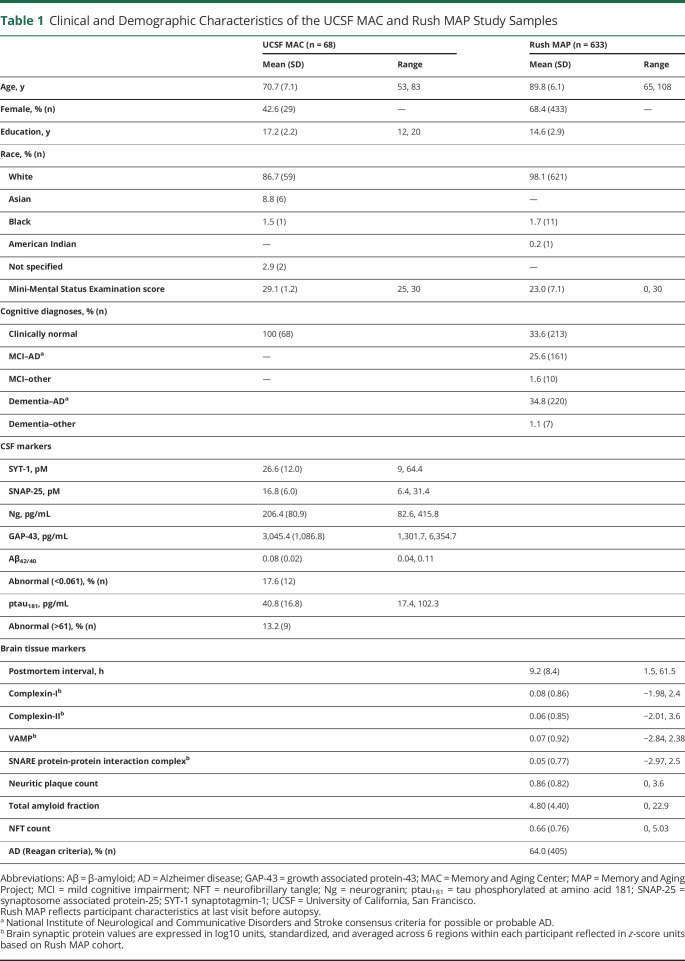
All synaptic proteins demonstrated linear, positive associations with ptau181 (r range = 0.61–0.88, all p < 0.001; figure 1B), as well as among each other (r range = 0.62–0.89, all p < 0.001). The relationship between CSF synaptic proteins and Aβ42/40 was negative but modest (r range = −0.39 to −0.27, all p < 0.03). On further evaluation, a quadratic relationship was present between postsynaptic proteins Ng (Ng2 B = −1.2, p = 0.03) and GAP-43 (GAP-432 B = −1.7, p = 0.003) with CSF Aβ42/40 such that low to medium synaptic protein concentrations were associated with the highest (more normal) CSF Aβ42/40 (figure 1A). Presynaptic proteins SNAP-25 and SYT-1 did not show a statistically significant quadratic relationship with Aβ42/40 (p > 0.10).
Figure 1. Relationship Between CSF Synaptic Proteins and (A) Aβ42/40 or (B) ptau181.
Aβ = β-amyloid; GAP-43 = growth associated protein-43; ptau181 = tau phosphorylated at amino acid 181; SNAP-25 = synaptosome associated protein-25.
Aβ1-40 is not as efficient as Aβ1-42 in forming neurotoxic fibrils.38 While Aβ1-40 is the primary isoform released with synaptic firing and evident in overall higher concentrations, Aβ1-42 is overrepresented in amyloid plaques.38 Given their differential roles and potentially differing neurotoxicity, we also evaluated relationships with individual Aβ isoforms. CSF synaptic proteins related more strongly with CSF Aβ40 compared to Aβ42 (Aβ40: r range = 0.62–0.87; Aβ42: r range = 0.21–0.41). The quadratic relation between Ng or GAP-43 with amyloid was most pronounced for the Aβ42/40 ratio, although statistically present for both individual Aβ isoforms (Ng2 or GAP2 all p < 0.001).
Models examining the quadratic relationship between synaptic proteins and ptau181 did not suggest superior fit (all quadratic parameter p > 0.12). Aβ42/40 and ptau181 demonstrated a modest, linear correlation (r = −0.49, p < 0.001).
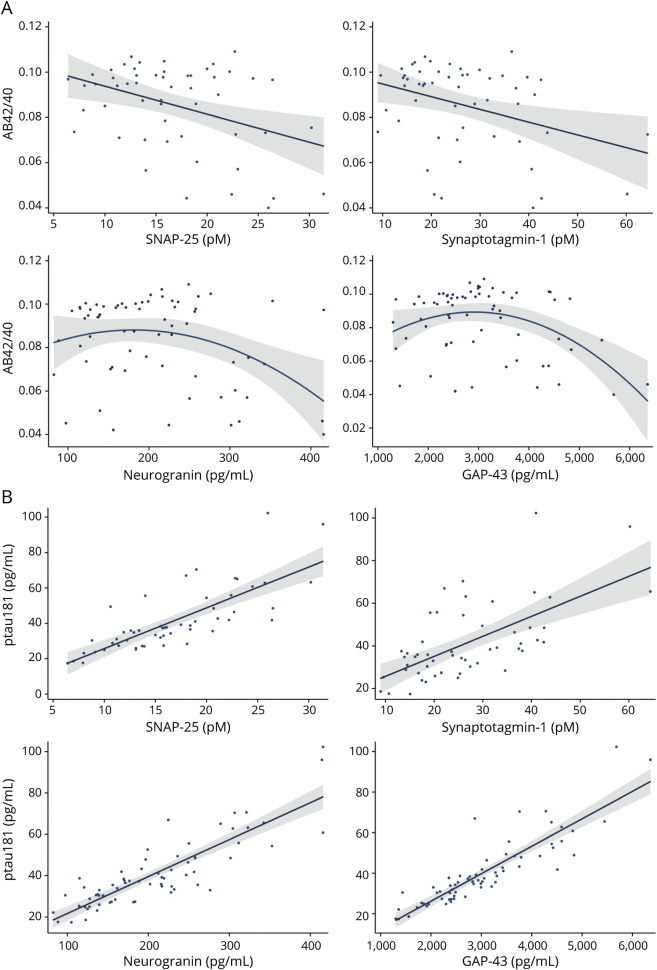
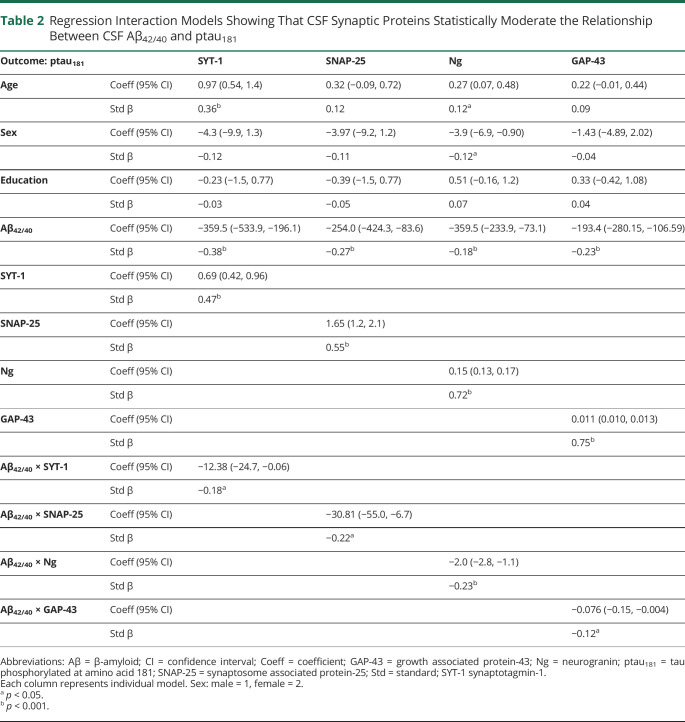
CSF Synaptic Proteins Statistically Modify the Relationship Between Aβ42/40 and ptau181
With adjustment for demographics, there was an interaction with each CSF synaptic protein on the relationship between Aβ42/40 and ptau181 (figure 2 and table 2). Split by tertiles, adults with high CSF synaptic protein (more abnormal) showed the expected strong, negative relationship between CSF Aβ42/40 and ptau181 (r = −0.70 [SNAP-25], r = −0.66 [SYT-1], r = −0.71 [Ng], r = −0.67 [GAP-43]), while participants with low synaptic protein CSF release (more normal) showed small to even positive associations between CSF Aβ42/40 and ptau181 (r = 0.57 [SNAP-25], r = 0.48 [SYT-1], r = 0.01 [Ng], r = −0.18 [GAP-43]).
Figure 2. CSF Synaptic Protein Levels Statistically Moderate the Relationship Between CSF AB42/40 and ptau181.
Raw values plotted. All interactions were modeled continuously. Synaptic protein levels were split into sample-based tertiles for illustration purposes. Aβ = β-amyloid; GAP-43 = growth associated protein-43; ptau181 = tau phosphorylated at amino acid 181; SNAP-25 = synaptosome associated protein-25.
Table 2.
Regression Interaction Models Showing That CSF Synaptic Proteins Statistically Moderate the Relationship Between CSF Aβ42/40 and ptau181
When Aβ isoforms were tested separately, only Aβ42 demonstrated a significant interaction with CSF synaptic proteins on ptau181 levels (SNAP-25: B = −0.23, p = 0.009; SYT-1: B = −0.27, p = 0.005; Ng: B = −0.19, p < 0.001; GAP-43: B = −0.11, p = 0.04). Models testing the interaction between Aβ40 and CSF synaptic proteins on ptau181 did not reach statistical significance (SNAP-25: B = 0.02, p = 0.83; SYT-1: B = 0.06, p = 0.58; Ng: B = −0.04, p = 0.49; GAP-43: B = −0.10, p = 0.09).
CSF Synaptic Proteins Statistically Modify the Relationship Between ptau181 and Gray Matter Volume
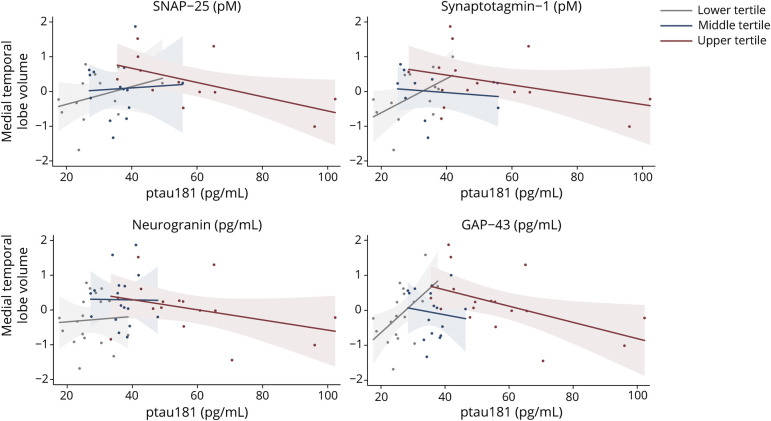
After adjustment for demographics, Aβ42/40 or ptau181 was not strongly associated with medial temporal (Aβ42/40: B = 0.24, p = 0.09; ptau181: B = −0.08, p = 0.57) or total gray matter volumes (Aβ42/40: B = 0.09, p = 0.52; ptau181: B = 0.15, p = 0.26). However, when entered into the model, each of the synaptic proteins demonstrated a significant interaction on the relationship between ptau181 and medial temporal or total gray matter volumes (SYT-1: B = −0.49 to −0.46, p < 0.02; SNAP-25: B = −0.40 to −0.32, p < 0.10; Ng: B = −0.48 to −0.42, p < 0.03; GAP-43: B = −0.43 to −0.39, p < 0.06). An inverse-U relationship between ptau181 and gray matter emerged that was dependent on synaptic marker levels (figure 3). Only participants with high CSF synaptic protein (more abnormal) demonstrated the expected negative association between ptau181 and medial temporal lobe volume (r = −0.59 [SNAP-25], r = −0.37 [STY-1], r = −0.37 [Ng], r = −0.52 [GAP-43]), an effect that reversed and showed positive associations between ptau181 and gray matter volume in those with low CSF synaptic protein (more normal) (r = 0.32 [SNAP-25], r = 0.53 [SYT-1], r = 0.06 [Ng], r = 0.54 [GAP-43]).
Figure 3. CSF Synaptic Protein Levels Statistically Moderate the Relationship Between CSF ptau181 and Medial Temporal Lobe Volume.
Raw values plotted. Medial temporal lobe volumes represent gray matter residualized for total intracranial volume. All interactions were modeled continuously. Synaptic protein levels were split into sample-based tertiles for illustration purposes. GAP-43 = growth associated protein-43; ptau181 = tau phosphorylated at amino acid 181; SNAP-25 = synaptosome associated protein-25.
There was also a moderating effect of synaptic proteins on the relationship between Aβ42/40 and gray matter volumes, but the effect only neared or reached statistical significance for presynaptic proteins SYT-1 (medial temporal lobe: B = 0.35, p = 0.044; gray matter volume: B = 0.34, p = 0.06) and SNAP-25 (medial temporal lobe: B = 0.39, p = 0.02; gray matter volume: B = 0.49, p = 0.005) (Ng and GAP-43 p < 0.12). Comparing lower and upper CSF tertiles showed that participants with higher (more abnormal) SYT-1 and SNAP-25 levels demonstrated a stronger link between Aβ42/40 and gray matter volumes (r range = 0.25–0.46), while their peers with lower (more normal) presynaptic protein levels showed small relationships between Aβ42/40 and gray matter (r range = 0.15–0.19). Neither of the individual Aβ isoforms (1–40 or 1–42) significantly interacted with presynaptic protein levels on medial temporal lobe or total gray matter volume when tested separately (all p > 0.07).
Rush MAP: Postmortem Brain Tissue Results
To provide converging support using distinct methods for our overarching hypothesis that synaptic functioning plays an important role in the A/T/N cascade, we tested parallel analyses using brain tissue markers of presynaptic and AD proteins in autopsied adults.
Associations Among Brain Tissue Presynaptic and AD Proteins
Levels of complexin-I, complexin-II, VAMP, and SNARE protein-protein complex demonstrated small inverse relationships with neuritic plaque (r range = −0.18 to −0.07, all p < 0.08) and NFT burden (r range = −0.21 to −0.07, all p < 0.08). Models evaluating quadratic effects demonstrated an inverse-U between VAMP and neuritic plaque and NFTs (VAMP2 all p < 0.02). Models testing the quadratic relationship between the complexins or SNARE protein-protein complex and AD protein indicators did not suggest superior fit (p > 0.07).
Levels of complexin-I, complexin-II, VAMP, and SNARE protein-protein complex showed similar small, inverse associations with total amyloid fraction (r range = −0.18 to −0.10, all p < 0.001). SNARE protein-protein complex abundance demonstrated a quadratic (inverse-U) relationship with total amyloid fraction (SNARE2 p = 0.036).
Neuritic plaque burden demonstrated a positive relationship with NFT burden (r = 0.59, p < 0.001).
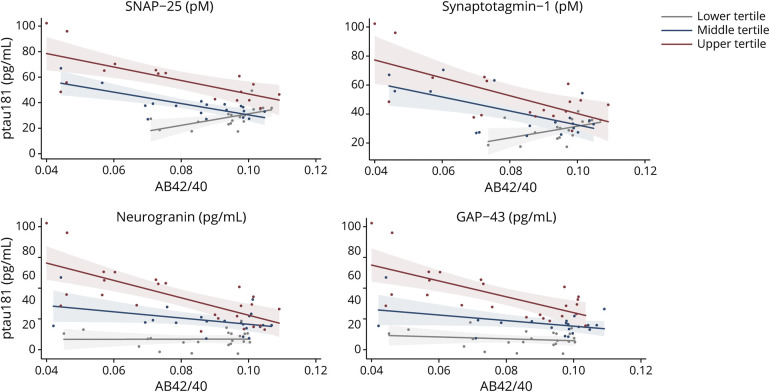
Brain Tissue Presynaptic Proteins Statistically Modify the Relationship Between Neuritic Plaques and NFTs
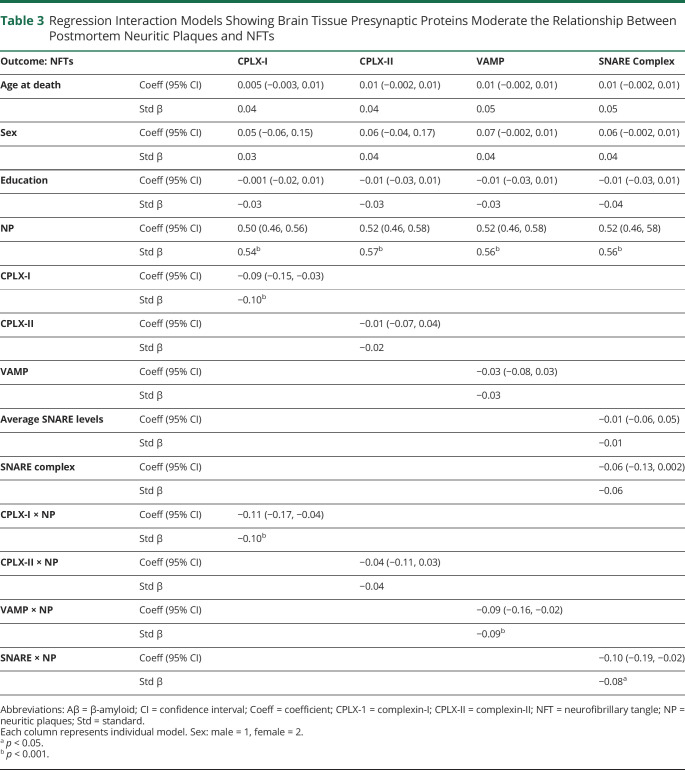
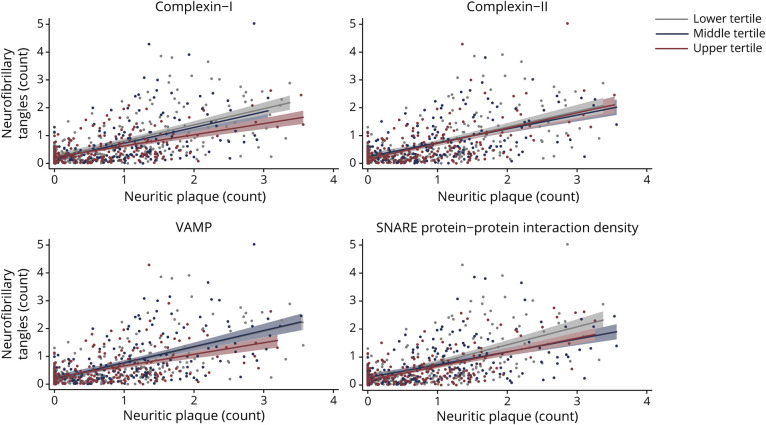
After adjustment for age at death, sex, and education, there was an interaction with complexin-I and VAMP on the relationship between neuritic plaque and NFT burden, but not with complexin-II (table 3). The relationship between neuritic plaque and NFT burden was weaker in adults with greater complexin-I and VAMP levels in brain tissue (figure 4).
Table 3.
Regression Interaction Models Showing Brain Tissue Presynaptic Proteins Moderate the Relationship Between Postmortem Neuritic Plaques and NFTs
Figure 4. Brain Tissue Levels of Presynaptic Complexin-I, VAMP, and SNARE Protein-Protein Interactions Statistically Moderated the Relationship Between Neuritic Amyloid Plaque and NFT Burden.
Raw values plotted. Synaptic protein values are expressed in log10 units, standardized, and averaged across 6 regions within each participant (z score units). Neuritic plaque and neurofibrillary tangle (NFT) counts were estimated across 5 regions region, scaled by dividing the corresponding SD, and averaged to obtain summary burden counts. All interactions were modeled continuously. Synaptic protein levels were split into sample-based tertiles for illustration purposes.
Individual protein levels provide information regarding presynaptic terminal integrity. We next evaluated the effect of the SNARE protein-protein complex to test a marker of relative presynaptic functionality. After adjustment for demographics and total SNARE protein level, the SNARE protein-protein complex also showed an interaction on the relationship between neuritic plaque and NFT burden (table 3). Again, the relationship between neuritic plaque and NFTs was weaker in those with higher SNARE complex abundances.
These relationships were specific to neuritic plaques. Models further adjusting for total Aβ fraction remained statistically significant. Models evaluating the interaction between presynaptic protein levels and total amyloid fraction on NFT reached significance only for complexin-I (B = 0.09, p = 0.01), but not VAMP or SNARE protein-protein complex. Models further adjusting for other common neuropathologies (i.e., Lewy body disease, TAR DNA-binding protein 43, hippocampal sclerosis, arteriosclerosis, cerebral amyloid angiopathy, atherosclerosis, macroinfarcts, and microinfarcts) and postmortem interval remained statistically significant (all p < 0.002).
To parallel the UCSF MAC cohort, we conducted sensitivity analyses restricting analyses to MAP participants diagnosed as clinically normal before autopsy. In clinically normal adults (n = 213), complexin-I (B = −0.12, p = 0.046) and the SNARE complex (B = −0.18, p = 0.003) continued to show a significant interaction on the relationship between neuritic plaques and NFTs (complexin-II: B = 0.08, p = 0.21; VAMP: B = −0.03, p = 0.62).
Discussion
In 2 independent cohorts applying distinct methods, we demonstrate that neurotoxic relationships along the A/T/N cascade may be dependent on synaptic state. Both in living clinically normal older adults and at postmortem, the adverse relationship between the 2 canonical AD proteins was strongest in individuals who demonstrated more abnormal synaptic markers. In both cohorts, this effect was most apparent when the more neurotoxic forms of amyloid (i.e., CSF Aβ1-42 > Aβ1-40, brain tissue neuritic plaques > total Aβ fraction) were examined. The pattern of results remained similar after adjustment for other neuropathologies and when the autopsy MAP cohort was restricted to those diagnosed as clinically normal, indicating the importance of this effect in the earliest, subclinical cognitive stage of illness potentially before overt neurodegeneration. In vivo CSF synaptic proteins further statistically modified the relationship between ptau181 and gray matter volumes. Adults with more abnormal CSF synaptic protein showed the expected inverse relationship between ptau and gray matter volume. Remarkably, the relationship reversed directionality in those with more normal CSF synaptic protein levels. Amyloid and tau accumulation is detectable as early as the second decade of life, and the majority (>65%) of older adults demonstrate neuropathologically significant levels of both in late life.37,39 Yet, at least a third of adults die with Aβ or ptau aggregates and never exhibit adverse clinical sequalae.34 Though observational, our results suggest that patients with the combination of AD proteinopathy and markers of synaptic dysfunction are at the most elevated risk. We suggest that fundamental relationships in the A/T/N framework may need to incorporate synaptic integrity to fully capture AD pathophysiology. Therapeutic approaches that directly support maintenance of synaptic integrity may be high yield targets to support preserved resilience to AD neuropathology.
In the context of the A/T/N framework, although structural breakdown of the synapse may be considered a component of neurodegeneration, increasing animal data suggest that molecular dysfunction at the synapse may occur before and potentially drive early amyloid and tau aggregation.2-4,26 Given our observational design, we are not able to determine whether synaptic dysfunction may be driving the relationship between Aβ-ptau or between ptau-neurodegeneration or is the result of AD protein aggregation and/or neurodegeneration. We highlight animal models demonstrating evidence for both. The synaptic hypothesis of AD posits that disrupted synaptic signaling and cognitive impairment are observable before the appearance of amyloid plaques or tau tangles, potentially in the context of soluble Aβ and tau oligomers.2,3 Furthermore, these early synaptic changes fundamentally contribute to AD pathogenesis and spread.2-4 In vitro studies additionally demonstrate a positive feedback loop between the synapse and Aβ such that synaptic firing releases Aβ and Aβ oligomers then promote synaptic firing itself.40,41 In the setting of chronic aberrant synaptic activity or poor clearance, excessive Aβ at the synaptic cleft leads to postsynaptic internalization of NMDA receptors and structural loss of dendritic spines.26,42 Consistent with this model are the findings that synaptic loss is most pronounced surrounding Aβ plaques and that synaptic proteins colocalize within Aβ plaques.5,6 Tau hyperphosphorylation is also stimulated by synaptic dysfunction and further propagates the spread of aberrant synaptic firing, independently of neurodegeneration.7,43 Our multimodal human synaptic data fit within this model. We show synergistic relationships among the synapse, amyloid, tau, and brain volume in clinically normal older adults (i.e., putatively before major neurodegeneration) and that markers of synaptic abnormalities may need to be present for tau to show adverse relationships with neurodegeneration. Together, these data suggest that synaptic functioning may play a biphasic role with importance in both early and late A/T/N pathways. However, it would be an overstatement of these data to firmly conclude where in the A/T/N cascade synaptic functioning belongs, and future experimental designs and longitudinal human data are needed.
Our data also demonstrate differentially shaped relationships between synaptic markers with amyloid (versus tau) that are novel in humans but consistent with animal and in vitro models. In animal models, Aβ potentiates neuronal activity at low levels but depresses synaptic firing at elevated levels.2-4 Our data are the first to suggest this curvilinear relationship in humans, an effect particularly prominent with proteins present in the postsynaptic compartment (Ng, GAP-43). As presynaptic Aβ rises, enhanced release of synaptic vesicles potentiates synaptic firing, leading to excess release of both extracellular Aβ and possibly synaptic protein.2-4 Postsynaptically, high Aβ then promotes internalization of AMPA and NMDA receptors26 and increases extracellular glutamate, causing desensitization of the postsynaptic membrane and long-term depression.26 Our data appear to reflect this delicate balance between Aβ and synaptic proteins even in subclinical older adults who are just beginning to show evidence for Aβ misfolding.
In contrast, tau is a microtubule stabilizing protein essential for bidirectional transport and multiple signaling pathways between the cell body and synapse. Although less well understood, the current view is that misfolding of tau may lead to both loss of microtubule structure and gain of toxic function via blocked axonal transport and signaling.44 At the synapse, tau can bind to presynaptic vesicles, inhibiting mobility and release,8 and postsynaptic hyperphosphorylated tau can mislocalize to dendritic spines, disrupting glutamate receptor anchoring.7 Synaptic activity itself also potentiates tau release from neurons both in vivo and in vitro, which may contribute to its prion-like spread along synaptically connected cells.45 Our data are consistent with this close, bidirectional relationship between tau and synaptic functioning and suggest that synaptic integrity tracks linearly with tau phosphorylation levels, regardless of synaptic compartment or posited pathway. CSF ptau181 demonstrated a nonlinear relationship with cortical volume, and each synaptic marker closely tracked along this relationship. That is, those with more abnormal CSF synaptic protein showed negative associations between ptau and cortical volume, an effect that reversed in adults with normal CSF synaptic protein. This finding converges with a recent study illustrating the temporal dynamics of CSF Ng in a mouse model of neurodegeneration.15 On induction of neurodegeneration (p25), rodents demonstrated rapid shedding of Ng into the CSF, which peaked at 2 weeks, declined, and leveled off, though remaining elevated.15 The observed positive associations among CSF ptau and cortical volume when CSF synaptic was more normal may reflect normal physiologic functions with tau or initial compensatory recruitment of these processes in the earliest stages of neurodegeneration—relationships that reversed directionality when CSF synaptic protein was more abnormal. Of note, few participants in our study had both high (abnormal) CSF ptau181 and low (more normal) synaptic protein, suggesting that ptau may be a hallmark of abnormal synaptic functioning.
Our data also indicate that the amyloid-tau relationship itself may be influenced by the synaptic state. We demonstrated that the moderating effect of synaptic markers was specific to the most pathogenic forms of Aβ (CSF Aβ42 and brain tissue neuritic plaques). These findings may place the synapse at the center of AD pathogenesis, suggesting that synaptic processes are intimately linked to Aβ-induced tau hyperphosphorylation pathways. Similarly, Zempel and colleagues46 found that spine loss induced by exogenous Aβ occurred only in dendritic regions in which tau was missorted and microtubules were disrupted, highlighting the tripartite relationship among these 3 processes. Synapses also play a role propagating disease spread through axonally connected cells and regions.45 The statistically interactive relationships observed among markers of Aβ, ptau, and synaptic integrity support these exponentially propagating processes. In brain tissue, complexin-I (but not complexin-II), VAMP, and SNARE protein-protein complex modified the Aβ-tau relationship. These data provide further specificity suggesting that inhibitory (complexin-I) vs excitatory (complexin-II) localizing synaptic proteins, as well as relative functional levels (SNARE complex), may play important roles in how early plaque-tangle relationships develop. Of note, prior MAP works have specifically linked complexin-I, VAMP, and the SNARE complex (but not complexin-II) levels to early changes in cognitive trajectories in life, highlighting their clinical relevance.33,34
Last, these findings converge with animal and human autopsy studies implicating the synapse in cognitive resilience to AD neuropathology. More than one-third of older adults show fulminant plaques and tangles at death without ever evidencing cognitive impairment in life.34 Converging human autopsy studies have identified preservation of synaptic structure,12-14 protein levels,12,13 and gene expression47 in adults showing cognitive resilience to neuropathologic AD compared to matched nonresilient peers with AD. Our data extend these findings into living humans, suggesting that Aβ and tau may not demonstrate adverse relationships when synaptic protein is better preserved. This suggests that synaptic maintenance could be at the crux of cognitive resilience and that therapies directly supporting synaptic functioning should be considered to stave off adverse manifestations of age-related neuropathologies.
Our study has several important limitations. First, the cross-sectional, observational design precludes conclusions regarding causal directionality of these relationships. Although we observed a tripartite link among Aβ, tau, and synaptic functioning in adults both in vivo and at postmortem, it is still unclear which of the processes or how these processes initiate development of pathogenic AD. That is, we show synaptic proteins closely relate to Aβ and ptau outcomes, but whether they promote disease or protect against neuropathology cannot be determined. It is certainly possible that other unmeasured processes (e.g., glial pathways, vascular integrity) or confounds play a critical role in how these relationships unfold. In addition, the synaptic proteins quantified across the 2 cohorts were not fully aligned. While the UCSF MAC markers were prospectively quantified, the Rush MAP synaptic proteins represented a convenience sample of a previously captured synaptic protein panel. The lack of harmonization across synaptic proteins precludes specific protein-by-protein comparisons and limits more pathway-specific interpretations. However, our overarching goal was to demonstrate converging evidence for the role of synaptic functioning along the A/T/N cascade, which appeared to be present largely regardless of quantification method or marker type. We also were not able to link antemortem in vivo with postmortem protein levels within the same individual due to lack of available data; future works using this design will be invaluable for shedding light on the dynamics of synaptic protein levels in humans. Integration of in vivo molecular markers reflecting other implicated pathways (e.g., glial, vascular), as well as PET synaptic ligands, would provide important context to these potentially highly dynamic processes. Further exploration of how these markers covary in culturally distinct backgrounds (e.g., lower socioeconomic status, educational diversity, health access disparity groups) will also be critically informative. Last, our CSF cohort was also relatively small, and only a subset completed brain MRI; although those who completed MRI did not statistically differ on demographics from those who did not complete MRI, they may not be representative of the larger sample. It is also important to note that such time- and procedure-intensive studies relying on clinicopathologic, imaging, or lumbar puncture outcomes may not inherently represent the broader aging population. Future replication across additional and diverse cohorts is needed.
Our study demonstrates very early dependence of AD proteinopathy on synaptic integrity in living, clinically normal older adults that is recapitulated in postmortem brain tissue. As molecular markers advance, we suggest a need to concurrently capture discrete synaptic processes to better diagnose, prognosticate, and understand AD pathophysiology in vivo. Given the high positivity of Aβ at older ages,36 markers of synaptic integrity may provide much needed specificity to indicate those most at risk of developing AD dementia. These findings further support efforts to preserve synaptic integrity via behavioral (e.g., exercise, cognitive stimulation) and pharmacologic approaches to buffer negative effects of common age-related proteinopathies.
Glossary
- A/T/N
amyloid/tau/neurodegeneration
- Aβ
β-amyloid
- AD
Alzheimer disease
- GAP-43
growth associated protein-43
- MAC
Memory and Aging Center
- MAP
Memory and Aging Project
- NFT
neurofibrillary tangle
- Ng
neurogranin
- ptau181
tau phosphorylated at amino acid 181
- SNAP-25
synaptosome associated protein-25
- SYT-1
synaptotagmin-1
- UCSF
University of California, San Francisco
- VAMP
vesicle associated membrane protein
Appendix. Authors
Study Funding
This study was supported by NIH–National Institute on Aging (NIA) grants K23AG058752 (principal investigator [PI]: K.B.C.), R01AG032289 (PI: J.H.K.), R01AG048234 (PI: J.H.K.), and UCSF ADRC P30AG062422 (PI: B.L.M.). Our work was also supported by Larry L. Hillblom Network Grant (2014-A-004-NET; PI: J.H.K.) and Fellowship Grant (2017-A-004-FEL; PI: K.B.C.) and the Alzheimer’s Association (AARG-20-683875, PI: K.B.C.). H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (2018–02532), the European Research Council (681712), Swedish State Support for Clinical Research (ALFGBG-720931), the Alzheimer Drug Discovery Foundation, USA (201809-2016862), and the UK Dementia Research Institute at UCL. MAP was supported by NIA grant R01AG17917. MAP data can be requested at radc.rush.edu.
Disclosure
K. Casaletto, D. Bennett, K. Blennow, A. Brinkmalm, N. Djukic, S. Weiner-Light, M. You, C. Fonseca, B. Miller, and J.H. Kramer declare no disclosures relevant to the manuscript; H. Zetterberg has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program (outside submitted work). W. Honer has received consulting fees or sat on paid advisory boards for AlphaSights, Guidepoint, In Silico, Translational Life Sciences, Otsuka, Lundbeck, and Newron and holds shares in Translational Life Sciences and Eli Lilly. J. Schneider has served as scientific advisory at National Hockey League, Alnylam Pharmaceuticals, AVID radiopharmaceuticals, and Grifols. Go to Neurology.org/N for full disclosures.
References
- 1.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457-464. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789-791. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam MM. Neuroplasticity failure in Alzheimer's disease. Neuron. 1999;24(3):521-529. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE. The synaptic Aβ hypothesis of Alzheimer disease. Nat Neurosci. 2005;8(8):977-9. [DOI] [PubMed] [Google Scholar]
- 5.Masliah E, Mallory M, Hansen L, et al. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991;6(5):729-39. [DOI] [PubMed] [Google Scholar]
- 6.Koffie RM, Meyer-Luehmann M, Hashimoto T, et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106(10):4012-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoover BR, Reed MN, Su J, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, McInnes J, Wierda K, et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun. 2017;8(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mango D, Saidi A, Cisale GY, Feligioni M, Corbo M, Nisticò R. Targeting synaptic plasticity in experimental models of Alzheimer's disease. Front Pharmacol 2019;10:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracy TE, Sohn PD, Minami SS, et al. Acetylated tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy-related memory loss. Neuron. 2016;90(2):245-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cissé M, Halabisky B, Harris J, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469(7328):47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold SE, Louneva N, Cao K, et al. Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer's disease. Neurobiol Aging. 2013;34(1):157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Nievas BG, Stein TD, Tai HC, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology. Brain. 2013;136(pt 8):2510-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boros BD, Greathouse KM, Gentry EG, et al. Dendritic spines provide cognitive resilience against Alzheimer's disease. Ann Neurol. 2017;82(4):602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höglund K, Schussler N, Kvartsberg H, et al. Cerebrospinal fluid neurogranin in an inducible mouse model of neurodegeneration: a translatable marker of synaptic degeneration. Neurobiol Dis. 2020;134:104645. [DOI] [PubMed] [Google Scholar]
- 16.Galasko D, Xiao M, Xu D, et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer's disease. Alzheimer’s Dement Transl Res Clin Interv. 2019;5:871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blennow K. A review of fluid biomarkers for Alzheimer's disease: moving from CSF to blood. Neurol Ther. 2017;6(suppl 1):15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkmalm A, Brinkmalm G, Honer WG, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer's disease. Mol Neurodegener. 2014;23(9):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Öhrfelt A, Brinkmalm A, Dumurgier J, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer's disease. Alzheimers Res Ther. 2016;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson A, Willette AA; Alzheimer’s Disease Neuroimaging Initiative. Neuronal pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer's disease spectrum. Brain Behav Immun. 2016;58:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Headley A, De Leon-Benedetti A, Dong C, et al. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology. 2018;90(10):e887-e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindler SE, Li Y, Todd KW, et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer's disease. Alzheimers Dement. 2019;15(5):655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casaletto KB, Elahi FM, Bettcher BM, et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017;89(17):1782-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casaletto KB, Elahi FM, Staffaroni AM, et al. Cognitive aging is not created equally: differentiating unique cognitive phenotypes in “normal” adults. Neurobiol Aging. 2019;77:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimer’s Dis. 2018;64(s1):S161-S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcolea D, Pegueroles J, Muñoz L, et al. Agreement of amyloid PET and CSF biomarkers for Alzheimer's disease on Lumipulse. Ann Clin Transl Neurol. 2019;6(9):1815-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuperstein I, Broersen K, Benilova I, et al. Neurotoxicity of Alzheimer's disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29(19):3408-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke MTM, Brinkmalm A, Foiani MS, et al. CSF synaptic protein concentrations are raised in those with atypical Alzheimer's disease but not frontotemporal dementia. Alzheimers Res Ther. 2019;11(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staffaroni AM, Ljubenkov PA, Kornak J, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain 2019;142(2):443-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honer WG, Barr AM, Sawada K, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012;2(5):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74(3):478-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barakauskas VE, Beasley CL, Barr AM, et al. A Novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology. 2010;35(5):1226-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes. JAMA. 2015;313(19):1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960-969. [DOI] [PubMed] [Google Scholar]
- 38.Miller DL, Papayannopoulos IA, Styles J, et al. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993;301(1):41-52. [DOI] [PubMed] [Google Scholar]
- 39.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of Aβ(1-42) in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsia AY, Masliah E, Mcconlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96(6):3228-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82(4):756-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-β/fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci. 2011;31(2):700-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopeikina KJ, Polydoro M, Tai HC, et al. Synaptic alterations in the rTg4510 mouse model of tauopathy. J Comp Neurol. 2013;521(6):1334-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada K, Holth JK, Liao F, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211(3):387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30(36):11938-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White CC, Yang HS, Yu L, et al. Identification of genes associated with dissociation of cognitive performance and neuropathological burden: multistep analysis of genetic, epigenetic, and transcriptional data. PLoS Med. 2017;14(4):e1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this report will be made available on request. UCSF MAC data requests can be sent to the corresponding author, while Rush MAP resources can be requested at radc.rush.edu.