Abstract
Primary cardiac liposarcomas are rare tumors with a poor prognosis and no well-defined imaging characteristics or treatment guidelines. Here, we present a case of primary pleomorphic liposarcoma of the heart and pericardium with multimodality imaging findings and our institution’s treatment approach. (Level of Difficulty: Intermediate.)
Key Words: cardiac imaging, cardiac pleomorphic liposarcoma, neoplasm of the pericardium
Abbreviations and Acronyms: CT, computed tomography
Graphical abstract
Primary cardiac liposarcomas are rare tumors with a poor prognosis and no well-defined imaging characteristics or treatment guidelines. Here, we prese…
A 72-year-old woman presented to her primary care physician with symptoms of coughing and shortness of breath for the past 2 weeks. Heart rate, blood pressure, and respiratory rate were 58 beats/min, 128/75 mm Hg, and 26 breaths/min, respectively. Pulse oximetry done in the office showed 90% oxygen saturation on room air.
Learning Objectives
-
•
To learn the differential diagnosis of circumferential tumor encasing the heart.
-
•
To understand that, similar to extracardiac pleomorphic liposarcomas, pericardial pleomorphic liposarcomas may also contain only limited fat foci that may not be visualized on CT and cardiac magnetic resonance imaging.
-
•
To explore the importance of multimodality imaging in pericardial liposarcoma cases.
Medical History
The patient had a history of hypertension, hyperlipidemia, and alcohol consumption.
Differential Diagnosis
The patient’s presenting symptoms were nonspecific and suggestive of either primary cardiac or pulmonary disease.
Investigations
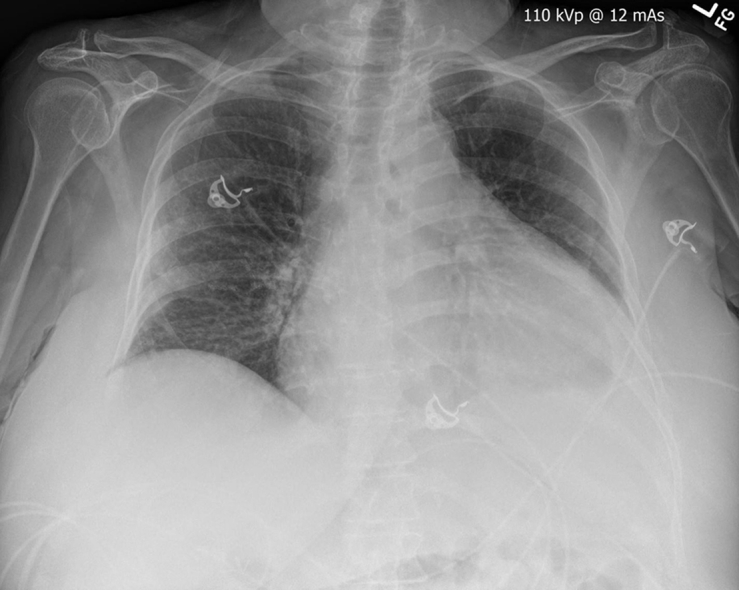
Initial chest radiograph was performed (Figure 1) and she was admitted to the hospital for further work-up. Transthoracic echocardiogram revealed a partially mobile echogenic mass in the pericardium, septal, and inferior cardiac walls. Because of poor visualization of the mass within the right ventricular outflow tract, transesophageal echocardiogram was performed (Figure 2). A small pericardial effusion was noted with no tamponade physiology. Once again the mass was seen in the right ventricular outflow tract but visualization of the pulmonic valve on transesophageal echocardiogram was limited.
Figure 1.
Anteroposterior Chest Radiograph
An enlarged cardiac silhouette, a mild central vascular congestion, and a small left pleural effusion.
Figure 2.

Cardiac Mass
Transesophageal echocardiogram showing an echogenic mass (arrow) in the septal and inferior walls, extending into the right ventricular outflow tract and throughout the pericardium. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
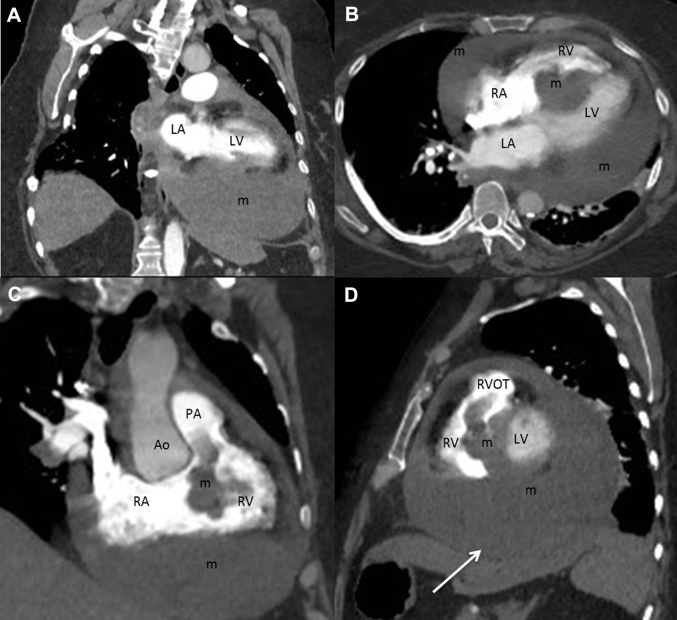
A computed tomography angiogram of the chest was performed to further characterize the relationship of the mass with the pericardium and left hemidiaphragm and for prebiopsy planning. The mass appeared to extend from the pericardium into the left basilar pleural space. No fat was visualized within the mass on computed tomography angiogram (Figure 3).
Figure 3.
Reformatted, Contrast-Enhanced Computed Tomography Images of the Chest
(A) Vertical long axis, (B) horizontal long axis, (C) right ventricular (RV) inflow-outflow, and (D) short-axis projections showing a mass (m) centered in the interventricular septum and throughout much of the pericardial space 87 Hounsfield units. Ao = aorta; PA = pulmonary artery; other abbreviations as in Figure 2.
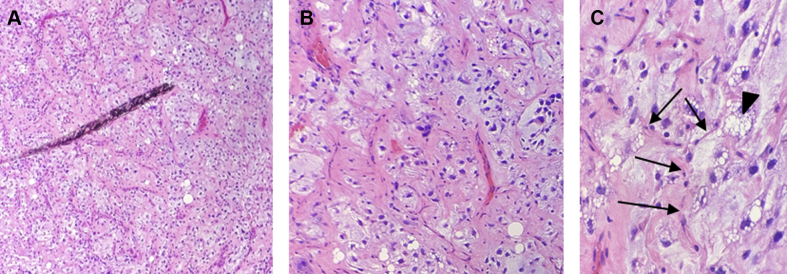
A subxiphoid pericardial window was performed, and the pericardial and epicardial portions of the mass were biopsied. Subsequent analysis demonstrated a pleomorphic malignant neoplasm likely originating from the pericardium (Figure 4). Using immunohistochemistry, S100 highlighted lipoblasts, and the tumor cells were strongly positive for p53, focally positive for myoD1, and negative for PD-L1, AE1/AE3, calretinin, WT1, myogenin, CAM5.2, desmin, BerEp4, CD31, CD68, S100, and CD15. Based on biopsy and pathology results, the expert histopathology opinion was that this was a primary pleomorphic liposarcoma.
Figure 4.
Biopsy Results
(A) 4×, (B) 10×, and (C) 40× microscopy slides of biopsy of mass from the pericardial space after hematoxylin and eosin stain, showing lipoblasts (arrowhead) with pleomorphic, atypical nuclei, over a background of myxoid stroma admixed with numerous lipoblasts and interspersed “chicken wire” capillaries (arrows).
Further staging work-up with positron emission tomography/computed tomography (CT) was performed (Figure 5A). Lack of involvement beyond the pericardium was confirmed.
Figure 5.
FDG-PET CT Before and After Treatment
(A) Before treatment: sagittal positron emission tomography (PET)-computed tomography CT) (a), whole body planar PET (b), coronal PET-CT (c), and axial PET-CT (d) images from initial fluorodeoxyglucose (FDG)-PET CT imaging showing a metabolically active mass. SUVmax: 12.8. (B) After treatment: FDG-PET CT (a to d) demonstrating significantly decreased metabolic activity. SUVmax: 4.6.
Limited cardiac magnetic resonance imaging without contrast was performed to clarify the anatomical relationship of the mass with the pulmonic valve and any functional consequence because this remained unclear from echocardiography and CT (Figure 6). However, further tissue characterization assessment with contrast was not performed as the study was stopped early due to shortness of breath and tissue diagnosis had already been confirmed based on biopsy.
Figure 6.
Cardiac Magnetic Resonance of the Pleomorphic Liposarcoma
Balanced steady-state free precession cardiac magnetic resonance images in (A) short axis, (B) vertical long axis, (C) axial planes of imaging showing the large mass (m) prior to treatment. The portion of the mass in the RVOT was mobile throughout the cardiac cycle and abutted the inferior surface of the pulmonic valve, with normal pulmonic valve function using phase contrast imaging (not shown). (D) Axial triple inversion recovery sequence of the mass within the pericardium. The mass has a mildly heterogenous increased signal with a few subtle foci of fat (arrows). Abbreviations as in Figure 2 and 3.
Management
After presentation at an interdisciplinary tumor board, review of the literature, and discussion with the patient, the patient was started on eribulin mesylate (Halaven, Eisai Incorporated, Woodcliff Lake, New Jersey). Surgical resection was considered undesirable by both the surgical team and the patient due to the large size of the tumor, extensive cardiac and pericardial involvement, and multiple comorbidities.
Discussion
Primary cardiac liposarcoma is a rare subset of malignant cardiac sarcomas, with only a handful of case reports in the literature (1). Further differentiation of liposarcomas can be made into 1 of 4 subtypes: well differentiated, dedifferentiated, myxoid, and pleomorphic (2). Pleomorphic is the least common subtype and the prognosis is poor.
Because these tumors are rare, there are no established imaging criteria and the diagnosis is usually made based on biopsy. Imaging features correlate with pleomorphic liposarcomas found elsewhere in the body: infiltrative, heterogeneous, solid, soft-tissue masses causing mass effect on adjacent structures (3). CT and magnetic resonance of nonlipomatous regions of the mass have attenuation and signal similar to muscle. The mass in our case contained very little fat, a common finding with pleomorphic liposarcomas (4). One review of skeletal pleomorphic liposarcomas reported that only 62% contained fat visible on CT or magnetic resonance imaging (5), usually only small focal fatty foci.
Complete tumor encasement of the heart is an unusual and potentially helpful finding. Metastatic disease does not circumferentially involve the pericardium. The differential diagnosis of circumferential encasement of the heart should include primary pericardial sarcomas, non-Hodgkin lymphoma (usually diffuse, large B-cell lymphoma), pericardial primitive neuroectodermal tumor, and primary pericardial mesothelioma (6).
Just as there are no imaging criteria for this disease, there are also no established treatment regimens. Complications of these tumors are potentially severe and include valvular dysfunction, intracavity obstruction, peripheral embolization, arrhythmias, and tamponade (7). Surgical resection is the preferred treatment if possible. In our case, eribulin mesylate proved to be effective. Initial studies for this medication have shown promise in overall survival by targeting microtubules in soft tissues and pausing and inhibiting their growth (8).
Follow-Up
Post-treatment positron emission tomography/CT 1 month after initiation of treatment showed a decrease in the size of the intraseptal component from 4.9 cm to 4 cm and decreased metabolic activity (Figure 5B). At her 3-month follow-up, the patient had improved performance status compared with initial presentation, and improvement of her presenting symptoms.
Conclusions
Primary liposarcomas of the heart and pericardium are rare tumors without established diagnostic imaging criteria or treatment regimens. In our case, the tumor followed imaging characteristics of pleomorphic liposarcomas found elsewhere in the body. Useful and distinctive imaging findings include circumferential encasement of the heart within the pericardium and, in some cases, subtle foci of fat. Multimodal cardiac imaging is essential to define tumor characteristics and staging, guide surgical appropriateness and approach, and identify potential complications.
Acknowledgment
The authors thank Shaun Hinen, MD, for editorial assistance.
Footnotes
Supported by resources from and with the use of facilities at AdventHealth Orlando. Dr. Burt has equity ownership of YellowDot Innovations, LLC; and has intellectual property rights and is a medical consultant for Hyland Healthcare. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Wang J.G., Wang B., Hu Y. Clinicopathologic features and outcomes of primary cardiac tumors: a 16-year-experience with 212 patients at a Chinese medical center. Cardiovasc Pathol. 2018;33:45–54. doi: 10.1016/j.carpath.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Lee A.T.J., Thway K., Huang P.H., Jones R.L. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. 2018;362:151–159. doi: 10.1200/JCO.2017.74.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy A.D., Manning M.A., Miettinen M.M. Soft-Tissue sarcomas of the abdomen and pelvis: radiologic-pathologic features, part 2-uncommon sarcomas. Radiographics. 2017;37:797–812. doi: 10.1148/rg.2017160201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphey M.D., Arcara L.K., Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25:1371–1395. doi: 10.1148/rg.255055106. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek J.S., Kransdorf M.J., Shmookler B.M., Aboulafia A.J., Malawer M.M. Liposarcoma of the extremities: MR and CT findings in the histologic subtypes. Radiology. 1993;186:455–459. doi: 10.1148/radiology.186.2.8421750. [DOI] [PubMed] [Google Scholar]
- 6.Restrepo C.S., Vargas D., Ocazionez D., Martinez-Jimenez S., Betancourt Cuellar S.L., Gutierrez F.R. Primary pericardial tumors. Radiographics. 2013;33:1613–1630. doi: 10.1148/rg.336135512. [DOI] [PubMed] [Google Scholar]
- 7.Uemura S., Watanabe M., Iwama H., Saito Y. Extensive primary cardiac liposarcoma with multiple functional complications. Heart. 2004;90:e48. doi: 10.1136/hrt.2004.036707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoffski P., Chawla S., Maki R.G. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]