Abstract
Vascularization is among the top challenges that impede the clinical application of engineered tissues. This challenge has spurred tremendous research endeavor, defined as vascular tissue engineering (VTE) in this article, to establish a pre-existing vascular network inside the tissue engineered graft prior to implantation. Ideally, the engineered vasculature can be integrated into the host vasculature via anastomosis to supply nutrient to all cells instantaneously after surgery. Moreover, sufficient vascularization is of great significance in regenerative medicine from many other perspectives. Due to the critical role of vascularization in successful tissue engineering, we aim to provide an up-to-date overview of the fundamentals and VTE strategies in this article, including angiogenic cells, biomaterial/bio-scaffold design and bio-fabrication approaches, along with the reported utility of vascularized tissue complex in regenerative medicine. We will also share our opinion on the future perspective of this field.
Keywords: vascular tissue engineering, stem cell, biomaterial, advanced biofabrication, regenerative medicine
Introduction
Blood vessels are part of the circulatory system that deliver oxygen and nutrients to all tissues and organs in mammalian bodies. Blood vessels typically account for 8% of body mass of an adult human, and stretch more than 60 000 miles in total length [1]. The development of the vascular system is one of the earliest events in organogenesis, where two distinct and finely orchestrated processes occur to generate functional blood vessel network: vasculogenesis and angiogenesis [2]. Vasculogenesis takes place in the early mammalian embryo and yolk sac through the aggregation of angioblasts into a primitive vascular plexus [2]. Angiogenesis is the expansion of new vessels from an existing vascular network, in which the endothelial cell growth, migration, polarization, sprouting and lumenization lead to the formation of a functional circulatory system [3]. The vascularization process is partly recapitulated in adults during physiological conditions that require nutrient and oxygen supply, such as tissue regeneration, wound healing, or in some cases disease progression such as tumor growth.
Since one of the major goals of tissue engineering (TE) is to create functional artificial replacement of damaged or diseased tissues and organs [4], tissue engineered grafts are often generated in clinically relevant sizes (0.1–10 cm). Owing to the oxygen and soluble nutrient consumption of cells, particularly, in dense tissues, this size far exceeds the tissue dimensions (200 μm) where species diffusion is the main transport mechanism [5]. Lacking any functional vasculature, the cells impregnated in the engineered tissue are subjected to hypoxia and insufficient nutrient supply until full ingrowth of functional blood vessels from the surrounding host tissue is achieved. However, it can take weeks to completely vascularize an implant of several millimeters [5], during which the nutrient-starving cells run the risk of loss of phenotype, loss of cellular functionality, and even cell death. In addition, vascular invasion from host tissue can cause nutrient and oxygen gradient along the radial axis of the graft, which might result in undesired patterning of cell distribution and differentiation [6].
Vascularization is thereby among the top challenges to be addressed to translate tissue engineering to clinical applications [7]. This challenge has spurred a tremendous research endeavor, defined as vascular tissue engineering (VTE) in this article, to establish a pre-existing vascular network inside the tissue engineered graft prior to implantation. Ideally, an optimal tissue engineered vasculature should meet the following criteria: (i) cells must be in close proximity to the patterned vascular network to ensure sufficient oxygen and nutrient availability, (ii) the vascular lumen should be lined with a functional endothelium for homeostasis and selective material exchange, and (iii) the network should be easily integrated into surrounding host vasculature via surgical or natural anastomosis to function instantaneously after surgery.
Moreover, sufficient vascularization is of critical significance in regenerative medicine from many other perspectives. Angiogenesis is known to be a prerequisite for many regeneration events, meaning the tissue engineered graft can augment and accelerate spontaneous regeneration by regulating the angiogenesis as long as the graft is properly pre-vascularized [8, 9]. Additionally, pre-vascularized, engineered tissue can be employed to create a wide range of complex organoids, which offers unprecedented opportunity to interrogate the role of angiogenesis in human organ development [10, 11], as well as human vascular disease progression [12]. Moreover, bioreactors and chips composed of tissue engineered, perfusable microvascular networks may serve as a potent tool of high-throughput drug screening targeting vascular system for the pharmaceutical industry [13, 14].
Given the significance of vascularization in TE, in this review we recapitulate the fundamental components of VTE as well as strategies used to engineer vascularized complex tissues in regenerative medicine. Special attention is paid to the findings published in the past five years that advance 3D printing-based VTE, spheroid-based VTE, and the utility of VTE for the regeneration of various tissues and organs. We will also share our opinion on the future perspective of this field based on existing findings.
The development of the vascular system—what have we learned from embryonic blood vessel formation?
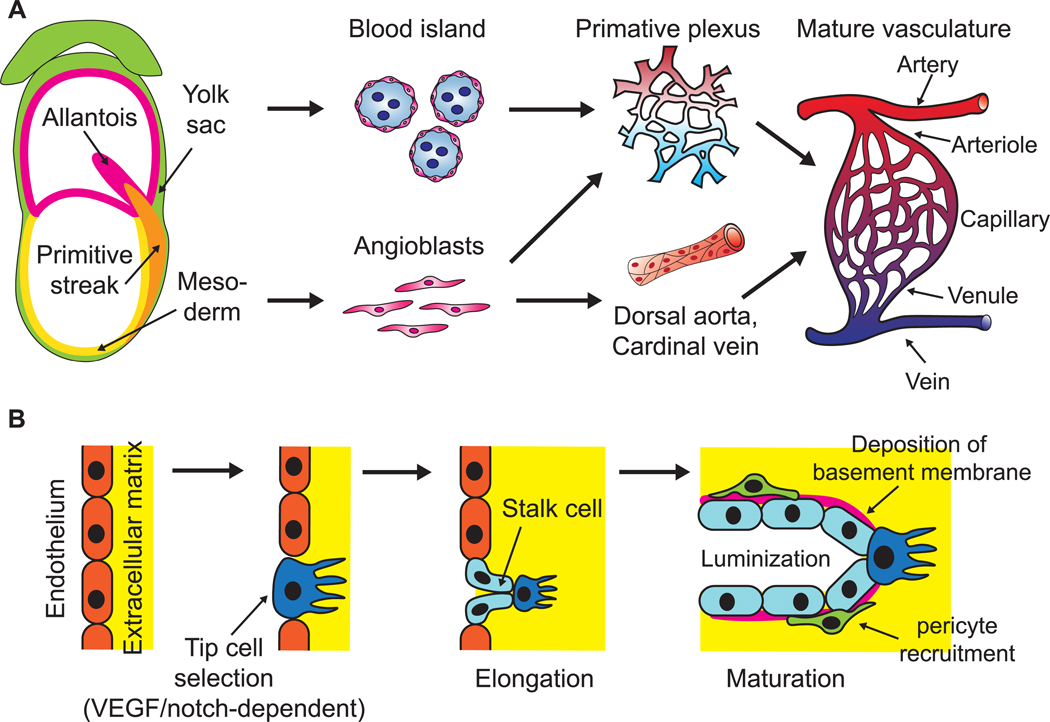
Blood vessels arise in both the embryo and the yolk sac. During early embryonic development, embryonic stem cells (ESCs) generate mesoderm that gives rise to several vascular system-related lineages such as vascular endothelial cells and primitive hematopoietic cells [15]. Endothelial precursor cells known as angioblasts derive from multiple mesodermal sources during embryonic development to generate intraembryonic vasculature, including the dorsal aorta, vitelline vessels, and local primary vascular plexus for most organs, neural tubes, and limbs [1, 16]. In the meantime, blood islands are formed around the visceral yolk sac, consisting of both vascular and hematopoietic precursor cells that give rise to the complex yolk sac vasculature [17] (figure 1(A)). After primary intraembryonic blood vessel formation via vasculogenesis within avascular tissue, expansion of vessel networks and the formation of new connections occurs via a process known as angiogenesis, in which activated endothelial cells undergo division and sprouting migration. In 2007–2010, Jakobsson et al and Hellstrom et al revealed a VEGF/Notch-based regulatory mechanism of the tip-stalk cell specification for angiogenic sprouting [18, 19] (figure 1(B)). The angiogenesis process is intensively associated with organogenesis, presenting morphogenetic cues required for organ formation in embryonic development and in the adult, such as pancreas, kidney, bone and placenta [20–24].
Figure 1.
Schematic of physiological development of mammalian vasculature. (A) Mesodermal-derived angioblasts in early mammalian embryos give rise to dorsal aorta, cardinal vein and various local primary vascular plexus. In the meantime, endothelial precursor cells in the yolk sac aggregate into blood islands and generate primary vascular plexus. The primary vessels then remodel and mature to form a hierarchical, functional vasculature. (B) When an existing blood vessel initiates expansion, some endothelial cells are activated to adopt a tip cell phenotype that can sprout and invade the surrounding basement membrane. Adjacent stalk cells follow the tip cells, proliferate to support sprout elongation and lumenize. Stalk cells also deposit basement membrane and recruit mural cells to stabilize newly formed vessels.
Newly sprouted blood vessels are lumenized by stalk cells before the onset of blood flow via various mechanisms [25–27](figure 1(B)) and undergo intense remodeling, leading to the specification of arteries and veins. Arteries experience high pressure and shear stress of blood flowing from the heart and are thereby lined with thick layers of tunica media and adventitia, whereas veins have thinner vessel walls and lower stiffness compared to arteries, to convey low pressure blood flow. Although the vascular remodeling is largely responsive to the blood flow, the initial differentiation of these two sub-lineages precedes and is independent of blood flow [28]. Various signaling cascades, including ephrinB2-EphB4, VEGF, Notch, Dll4 and COUP-TF II are involved in the arteriovenous specification [29–31].
The elongation and maturation of blood vessels are closely associated with mural cells (MCs), which was described for the first time in the late 19th century as contractile cells lining up around the endothelium [32]. MCs are a heterogeneous cell population that are classically divided into two distinct subtypes: the vascular smooth muscle cells (VSMCs) surrounding the entire arterioles and venules in a perpendicular fashion, and the pericytes attached to the capillaries in the longitudinal axis [33] (figure 2(C)). These cells support EC migration in the angiogenesis via MMP secretion [34], regulate endothelium permeability [35], and contribute to the basement membrane formation and vessel contractility. It is noteworthy that a variety of MC phenotypes derived from diverse developmental and adult origins can coexist in response to specific tissue demands, physiological stage, and disease state [36]. The versatile and indispensable role of MCs in the angiogenesis indicates their potential utility to advance VTE.
Figure 2.
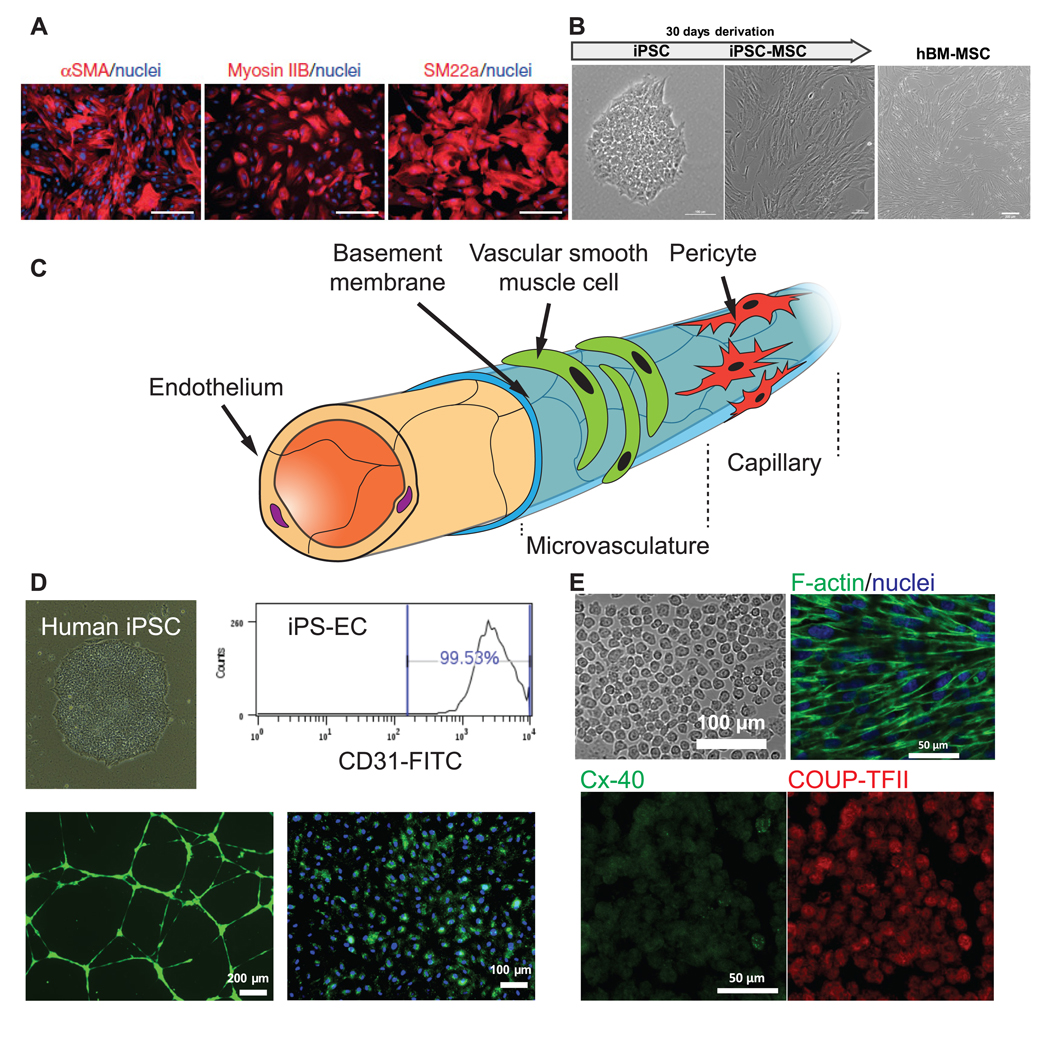
Summary of cell sources for VTE. (A) Human embryonic stem cell line can assume a vascular smooth muscle cell phenotype that express SMA, Myosin IIB, and SM22a. (B) Human mesenchymal stem cells, either derived from induced pluripotent stem cells (left) or adult tissue (right), can serve as mural cells to stabilize engineered blood vessel via differentiation, cell–cell contact, or paracrine effect. (C) Schematic of cellular composition of a blood vessel. (D) Endothelial cells can be derived from patient-specific induced pluripotent stem cells (upper left), uniformly expressing CD31 (upper right), forming capillary-like network on Matrigel (lower left, green) and uptaking AC-LDL (lower right, green). (E) EPCs are another promising source of ECs. They are aligned to laminar shear stress (upper right). These cells can undergo sub-lineage specification in response to different degree of shear stress, expressing arterial (Cx-40) and veinous markers (COUP-TFII), respectively. (A) is adapted from [56] Copyright 2015. With permission of Springer. (B), (D) and (E) are unpublished data acquired in our lab.
Cell source for VTE
To date, many tissue engineering approaches that aim to create the de novo vascular tree with functional endothelium utilizes vascular endothelial cells (ECs) due to their inherent angiogenic behaviors. Given the proper microenvironment and treatments, the ECs self-assemble into functional capillary networks that can be anastomosed with host vasculature post-implantation, and thereby significantly reduce the time to revascularize the implanted tissue graft [23, 37]. Despite the identified pivotal utility of ECs in VTE, there are two major challenges to be addressed in terms of cell source for VTE. Firstly, terminally differentiated ECs, such as human primary aortic endothelial cells (HAEC) and human primary dermal microvascular endothelial cells (HDMVEC), are limited in expansion potential and requires additional invasive surgeries to isolate from patients. Additionally, donor variability, tissue site specificity, and loss of functionality in in vitro cultures limits their TE applications. Secondly, ECs alone are not sufficient to constitute a prolonged, self-sustaining and functional cell-based vasculature. In other word, resolving the source of supportive cells such as pericytes, fibroblasts, and VSMCs is equally imperative to the field of VTE.
To this end, a diverse array of cell types have gained research interest as potential cell sources for VTE, including pluripotent stem cells such as ESCs and induced pluripotent stem cell (iPSCs) (figures 2(A) and (D)); as well as various types of multipotent adult stem cell/progenitor cell such as mesenchymal stem cells (MSCs) (figure 2(B)), umbilical cord blood-derived stromal cells (UCBSCs), and endothelial progenitor cells (EPCs) (figure 2(E)). Due to their high self-renewal capacity, these cell types can be easily expanded in vitro, thereby enabling clinically relevant applications. Additionally, each of these cell sources presents unique differentiation and angiogenesis potential to function as alternatives to primary ECs and mural cells for VTE.
Endothelial progenitor cell
The discovery of EPCs is credited to Asahara et al for the identification of a CD34-postive population in adult peripheral blood [38]. Seven days after in vitro plate culture, attached CD34-positive mononuclear blood cells (MBCCD34+) lost expression of leukocyte common antigen CD45 and progressed to adopt an EC-like phenotype: Expression of CD34, CD31, Flk-1, Tie-2, and E selectin—all markers of the EC lineage—was dramatically upregulated. Equally important, MBCCD34+ injected into ischemic hindlimbs of a mouse model augmented neovascularization and clearly integrated into the capillary vessel walls [38]. These findings have led to the ideation of minimally-invasive isolating autologous adult EPCs from patient peripheral blood for vascular regeneration. To date, the criteria that define EPCs include clonal proliferation potential of single cells, over 90% expression of endothelial markers CD31, CD105, CD144, CD146, and the absence of hematopoietic markers CD14, CD45, CD115 expression [39, 40]. These cells are in constant circulation within the vascular system and play a primary role in the repair and re-cellularization of an injured vascular endothelium [41]. Despite the ubiquity of EPCs in human peripheral blood, it is noteworthy that the availability and quality of EPCs may be impaired by many risk factors, such as cardiovascular disease and diabetes. The reduced number of circulating EPCs is strongly correlated with the increase in Framingham risk factor score [42], and deemed as an indicator of coronary artery disease progression [43]. Likewise, clinical studies suggest that the levels of EPCs were significantly lower in Type I diabetic children compared to non-diabetic controls [44], as well as in Type II diabetic patients [45]. Additional caution should be exercised when EPCs are isolated from or delivered to patients with these risk factors.
Late outgrowth endothelial cells (EOC), also known as endothelial colony forming cells, represent another subtype of the EPCs with promising regenerative potential [46]. Although EOCs are a rare population within the circulating cells, they possess a high in vitro proliferative capacity, responsiveness to flow-induced shear, and have the ability to endothelialize lumens in vivo [47, 48]. Moreover, their angiogenic potential has also been leveraged to treat clinical pathologies such as ischemic retinopathy [49], and coronary artery disease [50]. In contrast, early outgrowth endothelial cells, despite being a circulating endothelial cell population, present a limited proliferative capacity and endothelial characteristics such as endothelial nitrics oxide synthase (eNOS) and nitric oxide (NO) expression.
EPCs possess key functional activities to generate lumenized capillary-like network in vitro, as well as de novo blood vessel formation anastomosed with host vasculature in vivo. For instance, EPCs derived from human cord blood formed vessel-like structures on Matrigel, and self-assembled into lumenized, partly functional capillary network inside a modified poly(ethylene glycol) (PEG) hydrogel in the presence of mural cells [51]. Human EPCs also formed prevascular structures in the 3D spheroid co-culture setting with undifferentiated human MSCs, demonstrating a higher degree of organization compared to HUVECs [52]. When human blood-derived EPCs and MSCs were suspended in Matrigel and injected subcutaneously into nude mice for 1 week, histological staining on explant revealed numerous lumenized vessels lined with implanted human EPCs and containing erythrocytes [53]. Interestingly, in spite of the similar success of vascularization in each experiment setting, these studies draw different conclusions on the optimal EC to supportive cell ratio for the maximum vessel growth. EPC to SMC co-culture ratio of 1:1 best supported microvessel formation than the 1:4 or 4:1 ratio [51], whereas EPC to MSC ratio ranging from 3:2 to 1:4 showed negligible difference in microvessel growth [53]. The in vitro spheroid co-culture model of HUVEC/MSC and EPC/MSC revealed significant difference in area of CD31 staining at low EC ratio (1%–2%) [52]. These findings remind us that the optimal EPC to pericyte ratio must be evaluated on a cell-type and culture strategy-specific context. EPCs have also been successfully used to cellularize biodegradable vascular grafts for their endothelialization and in vitro maturation [54]. Taken together, all these pioneering studies validate the utility of EPCs as a promising cell source to pre-vascularize tissue engineered grafts.
Human pluripotent stem cell
Human pluripotent stem cells (hPSCs) can be derived from the inner cell mass of human blastocysts. These human embryonic stem cells (hESCs) possess unlimited proliferation capacity and the potential to differentiate into all somatic cell types. However, ethical controversies of using human embryos has impeded the applications of ESCs. In addition, it is difficult to generate patient- or disease-specific ESCs for tissue engineering applications. In contrast to ESCs, iPSCs can be derived from a patient’s somatic cells, which circumvents concerns such as embryonic tissue availability and immune rejection.
iPSCs are somatic cells reprogrammed into a pluripotent stem cell state through the expression of key transcriptional factors [55]. Human iPSCs resemble many pluripotent properties of ESCs in their self-renewal capacity and differentiation potential into cells of all three germ layers, including EC lineage. Using a two-step method similar to the protocol reported by Patsch et al [56], we and others have succeeded in differentiating iPSCs into ECs with high efficiency and purity (~1.5 week, 75%–90%) [12, 57] that expresses CD31, VE-cadherin, and eNOS. These cells form tube-like structures in matrigel, uptake acetylated low density lipoprotein (LDL) and produce NO, which are all functional characteristics of bona fide ECs. The identification of human iPSC-derived ECs (iPSC-ECs) has contributed tremendously to the advances of VTE in the past decade. Rufaihah et al reported functional improvement in limb perfusion and angiogenesis upon injection of iPSC-ECs in mice with femoral artery ligation to induce hindlimb ischemia [58]. The pro-angiogenic effect of iPSC-ECs was further strengthened when pre-impregnated in a protective hydrogel that prevents membrane damage during syringe flow [59].
Moreover, the iPSCs committed to a mesodermal fate are also capable of differentiating into VSMCs via exposure to VEGF-A or PDGF-BB. The iPS-VSMCs prepared in this manner respond to vasoconstrictive stimuli, exhibiting a key functional feature of maturated VSMCs in vivo [56]. A more recently study reveals the commitment of hPSCs to brain pericyte–like cells. These cells closely resembled primary human brain pericytes, self-assembled with endothelial cells, and induced blood-brain barrier properties [60]. The endothelia–pericyte interactions was confirmed in an in vitro vascular plexus formation model, in which the co-culture of hiPSC-ECs and hiPSC-derived pericytes/MSCs led to organized, tube-like structure of hiPSC-ECs in 7 d [61]. Taken together, hPSCs represent great potential as an inexhaustible cell source to generate pre-existing vascular system comprised of ECs and mural cells possessing identical, patient specific genomic background. While pioneering clinical applications of human iPSCs are moving forward [62, 63], there is growing concern that implantation of differentiated iPSC might lead to the formation of tumors in the recipients due to the ‘unsafe’ iPSC lines and residual undifferentiated iPSCs in the final product [64–66]. Tumorigenicity tests are thereby particularly important in the case of iPSC-based cell therapies. Additionally, track of the genetic stability of iPSC to eliminate genetic abnormality is of major interest for both research and clinical use of iPSCs.
Human mesenchymal stem cell
Human adult MSCs have been identified in different tissues, including bone marrow, adipose tissue, and dental tissues. Traditionally, the multi-lineage potential of these cells are examined with defined differentiation media that induces osteogenesis, adipogenesis and chondrogenesis. Moreover, the International Society for Cellular Therapy has proposed a set of minimum surface marker criteria that define human MSCs in an effort to standardize MSC studies [67].
Endothelial differentiation of MSCs via defined chemical factors has been widely reported. Exposure to 50 ng ml−1 VEGF and 10 ng ml−1 bFGF for 7 d led to the acquisition of EC markers in human bone marrow-derived MSCs including von willebrand factor (vWF), Flk-1, Flt-1, and VE-cadherin [68]. Likewise, human adipose-derived MSCs treated with endothelial growth medium-2 (EGM-2) containing VEGF acquired ECs markers CD31, vWF and eNOS, and formed capillary-like network on Matrigel [69]. Human MSCs were also found responsive to shear stress in a 3D culture platform to express EC markers Flk-1, vWF, and VE-cadherin [70]. The discovery of EC potential in MSCs has spurred pioneering studies on MSC-based vascular regeneration. The intravenous injection of EC-differentiated MSCs into mouse hindlimb ischemia models improved muscular angiogenesis and arteriogenesis composed of human ECs, and the restoration of blood perfusion [71, 72].
In addition to endothelial lineage commitment, MSCs may act as supportive cells that sustain the functionality of engineered vasculature through various mechanisms. The pro-anigogenic secretome profile of MSCs, which includes HGF, bFGF, IGF-1, and VEGF, is a critical factor for vascularization [73]: VEGF secreted by MSCs mediates the differentiation of EPCs into ECs, as well as the angiogenesis by ECs [74, 75]. Bone marrow-derived MSCs were also found to improve angiogenesis in a rabbit ischemic limb model via an increase in NO release [76]. Given the high similarity between MSCs and native pericytes [77], it has been widely noted that the proper spatial proximity and patterning of MSCs facilitate angiogenesis of ECs from various sources. Au et al found that undifferentiated MSCs function as pericytes to wrap around and stabilize HUVECs to constitute functional vascular tubes in an in vivo graft for more than 130 d without sign of regression [78]. Similarly, EPC implants can form human microvessels in a mouse model only in the presence of MSCs [53]. Thirdly, MSCs are capable of transdifferentiating into VSMCs. TGF-β treatment on bone-marrow MSCs has been shown to increase early SMCs markers α smooth muscle actin (SMA) and H1-calponin expression [79]. The impact of MSC on angiogenesis is mostly likely exerted in a ratio-dependent manner, including angiogenic gene expression [80], cell metabolic activity [81], and alteration of inflammatory response by ECs stimulated with TNF or with IL-1 [82]. The heterogeneity nature of MSCs and individual to individual variance are the major obstacles that impede the clinical translation of MSCs. Human MSCs from different anatomical sites were found differ widely in their transcriptomic signature, in vivo differentiation potential and reparative properties [83, 84]. Elucidating the heterogeneity MSC pool is the key to the field of MSCs-based VTE through utilizing optimal donor cells to maximize vascularization outcome.
Understanding the microenvironmental cues for angiogenesis
Physiological vasculogenesis and angiogenesis occurs in a highly dynamic and orchestrated microenvironment. The activation of quiescent vascular ECs to migrate and invade surrounding tissues is strictly dependent on cell adhesion to the extracellular matrix (ECM) and sensing of the microenvironment. To date, a wide spectrum of biophysical and biochemical factors are identified to participate in vascularization, highlighting the significance of recapitulating key environmental cues to elicit proper EC behavior for successful VTE.
Bioactive materials
Type I collagen is a ubiquitous structural protein of many tissues that interact with active ECs during embryonic and pathological angiogenesis [85]. The endothelial capillary-like lumen formation requires endothelial α2β1 integrin engagement of a single type I collagen integrin-binding site, possibly signaling via p38 MAPK and the inactivation of focal adhesion kinase (FAK) [86, 87]. Moreover, collagen I-integrin interaction was found to mediate the matrix type 1-metalloproteinase (MMP1)-dependent EC lumenogenesis in a 3D collagen hydrogel [88]. Due to the critical role of type I collagen in the early stages of the angiogenic process [89], it has been employed to induce angiogenesis in vitro. When cultured in microchannels inside a type I collagen hydrogel, ECs formed a confluent monolayer on the walls of the microchannels with appropriate morphology and cell-cell junctions, and sprouted new branches in respond to the presence of pericytes [90]. Apical culture of ECs on collagen hydrogel led to cell reorganization and tube-like network formation within 24 h [86].
Fibrin is a fibrous protein formed after thrombin cleavage of fibrinopeptide A from fibrinogen, which causes fibrin molecules to align in a staggered overlapping end-to-middle domain arrangement to polymerize [91]. As a constituent of provisional matrix in wound healing, fibrin is naturally angiogenic, and thereby has been long used as a biomaterial for the development of microvasculature in various culture platforms [92]. Human ECs sprouted from the surface of beads and formed capillaries when embedded in fibrin gels [93]. Similarly, human ECs encapsulated in fibrin microbeads sprouted initially inside the microbead matrix and then invaded into the surrounding fibrin hydrogel [94]. Benning et al defined fibrin as a suitable bio-ink for the 3D printing of ECs due to the improved vasculogenesis-related EC parameters compared to other materials [95].
Natural ECM extract derived from decellularized native tissues comprise tissue-specific microenvironment that invokes the adhesion, migration, differentiation and regenerative potential of resident cells home to that tissue [96–98]. As far as vascular tissue is concerned, this hypothesis has been evidenced by the finding that ECM deposited by HUVECs promotes endothelial differentiation of adult stem cells [99]. The pro-angiogenic potential of vascular ECM extract has gained recent research interest in VTE, despite the complexity in composition that hinders our understanding on the underlying mechanism. Digested ECM extract from vascular adventitia augmented the tube-like network formation of human ECs in vitro, and elicited vascular invasion from surrounding chorioallantoic membrane of the chick embryo [100]. Likewise, decellularized rat aorta ECM conjugated with heparin were found to reduce whole blood clotting, and stabilize long-term EC attachment to the lumen of ECM-derived vascular conduits for a prolonged durability of endothelialization in vitro [101].
Interestingly, the pro-angiogenic property of ECM seems not restricted to vascular tissue. Matrigel is the trade name of a solubilized basement membrane prepared from the Engelbreth–Holm–Swarm mouse sarcoma. ECs seeded atop or within Matrigel rapidly reorganize into capillary-like network within 24 h. Due to this excellent pro-angiogenic property, Matrigel has been designated as the standard substrate material in the EC tube formation assay [102], as well as for in vivo angiogenesis tests. Equally important, Matrigel can exert pro-angiogenic effect when used as a supplement to other biomaterials. For instance, the lumenized angiogenic sprouting of human cerebral ECs were significantly improved in collagen hydrogel supplemented with 2% (v/v) Matrigel [103]. Small intestinal submucosa (SIS) is another typical non-vascular ECM origin that possesses pro-angiogenic properties. SIS gel is capable of evoking neovascularization and angiogenic secretome as determined by tube formation in vitro, the mouse aortic ring assay, and animal models [104, 105].
Synthetic biomaterials offer an alternative category for VTE that share two common advantages: (1) highly tunable in mechanical properties and chemical composition; and (2) compatible with natural pro-angiogenic agents for enhanced bioactivity. Poly(l-glutamic acid) (PLGA) hydrogels promoted vascularized adipose units in a mouse subcutaneous model [106]. Polycaprolactone (PCL) scaffolds modified with chitosan showed a significantly increased vascularization compared to unmodified PCL fiber mats in a rat model [107]. Pro-angiogenic peptides were incorporated into poly(ethylene glycol) (PEG) hydrogels as crosslinking peptides flanked by matrix metalloproteinase (MMP) degradable substrates. The resulting enzymatically-responsive pro-angiogenic hydrogels promoted vascularization in a mouse model [108]. Modified natural materials is another potent subcategory of artificial material for VTE. Methacrylated gelatin (GelMA) and methacrylated hyaluronic acid (HAMA) comprised a novel hydrogel carrier of MSCs that increased vascularization in vivo by up to 3 fold compared to cell free controls [109]. Recently, an engineered recombinant protein (C7) is developed as a component of hybrid shear-thinning hydrogel to encapsulate ECs and maintains cell-matrix adhesions through integrin binding motif [59]. Subcutaneous implantation of composite elastomeric polyurethane (PU) scaffolds incorporating SIS augmented the vascularization from surrounding tissue, and ultimately tissue regeneration in rats [110]. Composite scaffold that combines a PEGylated fibrin hydrogel with a decellularized ECM scaffold supported robust and stable vascularization in a rodent volumetric muscle loss (VML) model [111].
Hypoxia
Low oxygen tension, or hypoxia, is another crucial environmental cue for vasculogenesis and angiogenesis [112]. Numerous studies demonstrate that hypoxia induces a wide array of transcriptional responses in EC that regulate proliferation, ECM degradation, pericyte recruitment, and sprouting [113–116]. For instance, hypoxia-inducible factor-1 (HIF-1) is a primary transcriptional mediator expressed by EC in response to oxygen stress. Infection of endothelial cells with HIF-1α under non-hypoxic conditions was sufficient to induce increased basement membrane invasion and tube formation [113]. Hypoxia-induced HIF-1𝛼 were also found to regulate the arterial-venous specification of EPCs through upregulating Notch target genes Hey1 and Hey2 and subsequent repression of Coup-TFII [117]. Moreover, hypoxic environment upregulates the principal proangiogenic factor vascular endothelial growth factor (VEGF) isoforms, which is a major transcriptional target for HIF-1. The VEGF-VEGFR axis is activated to stimulate the EC proliferation, sprouting, and angiogenesis when oxygen level is disrupted [118, 119].
Inducing the angiogenic process through hypoxia is a powerful tool for VTE that harnesses the innate natural angiogenesis mechanism in physiological and pathological states. HIF-1α expressing EPCs delivered to ischemic mouse hind limb significantly reduced limb and toe necrosis, augmented neovascularization and exogenous EPC homing [120]. Repeated, short-term hypoxia (2% for 8 h) was recently established as opposed to long term hypoxia to promote vascularization in the co-culture of primary human osteoblasts and outgrowth endothelial cells for the regeneration of bone tissue constructs [121]. Hypoxic culture (2%) was also found to promote differentiation of adipose-derived stem cells into ECs through demethylation of ephrinB2, which then improved limb salvation in a mouse hind limb ischemic model [122].
Biophysical properties
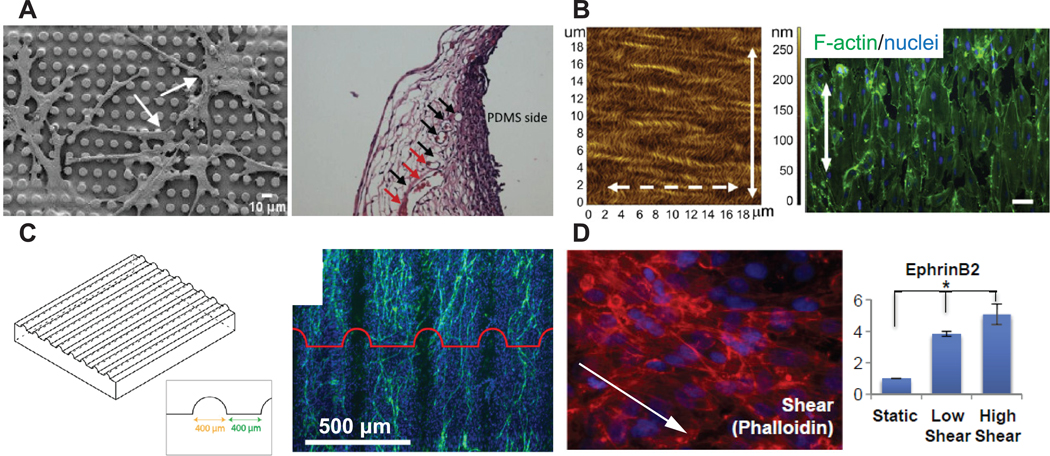
Surface micro-topography has great implications in regulating the behavior of a wide variety of cells [123, 124]. Numerous recent studies have begun to reveal the role of micro-topography on angiogenesis or neovascularization. When hMSCs on PDMS micro-textured substrates were treated with osteogenic medium and implanted subcutaneously in mice, histological data revealed increased vascular invasion [125] (figure 3(A)). Nanofibrillar alignment is another intensively characterized topographical feature for angiogenesis. ECs were elongated along the direction of aligned collagen nanofibrils and migrated with greater directionality and higher velocity [126]. EC-seeded aligned nanofibrillar collagen scaffolds improved blood perfusion and arteriogenesis in a mouse hindlimb ischemia model (figure 3(B)) [127]. Interestingly, in addition to micro-topography in the scale below tens of microns, a curvature scale much larger than any single cell was found to significantly impact the angiogenesis behavior of ECs: bioceramic patterned with grooves (330 μm in width and 300 μm in depth) resulted in the formation of individual, highly aligned microcapillary-like structures in vitro [128] (figure 3(C)). Taken together, these studies exemplify the implication of micro-topography on angiogenesis independently from the selection of biomaterial. However, the optimal micro-topography for angiogenesis remains to be identified.
Figure 3.
Biophysical cues to be involved in VTE. (A) When hMSCs on PDMS micro-textured substrates were implanted subcutaneously in mice, histological analysis revealed increased vascular invasion on micro-textured substrates. Reprinted from [125], Copyright 2015, with permission from Elsevier. (B) ECs were elongated along the direction of aligned collagen nanofibrils in vitro. Reprinted from [127], Copyright 2013, with permission from Elsevier. (C) Curvature in scale larger than single cell was found to significantly influence the angiogenesis behavior of ECs [128]. (D) Laminar, unidirectional shear stress regulated arterial-veinous specification of hPSC-derived ECs. Reprinted from [142], Copyright 2015, with permission from Elsevier. All pictures are adapted from published data with permission.
The impact of substrate stiffness on stem cell fate via cellular mechano-sensing came into view since Engler et al discovered matrix elasticity-mediated stem cell lineage specification [129]. It has been long noted that stiffening of blood vessel wall precedes many pathological behaviors of the endothelium, indicating the regulatory role of ECM stiffness in the homeostasis and activity of ECs [130, 131]. Derricks et al have recently reported that EC responses to VEGF-A are ECM stiffness dependent [132]. Substrate stiffness coordinates the interaction of VEGF-matrix binding and β1 integrin binding/activation to regulate EC behavior [133]. Moreover, substrate stiffness were found to regulate arterial-venous specification of EPCs [134]. However, scaffold elasticity is yet to be harnessed to favor VTE and has shown controversial results. Softer gels (2 kPa) of modified PEG exhibited greater vascularization compared to stiffer gels (18 kPa) in a rat subcutaneous implantation study [135], whereas stiff bone scaffold (24 kPa) composed collagen and hydroxyapatite augmented vascularization compared to softer scaffolds (7 kPa) in a similar rat model [136]. This subject is further complicated by the fact that ECs and stromal support cells can inherently alter the surrounding ECM elasticity during capillary morphogenesis [137]. The modulus and cell binding properties of the material were thereby decoupled in many studies. For instance, proteolytically degradable PEG hydrogel scaffolds were created for decoupled gradients of elastic modulus and immobilized RGD concentration to study vascular sprout invasion in vitro [138].
In addition to static biophysical features, hemodynamic tension affects EC behavior profoundly. The vascular endothelium is in direct contact with blood flow and is thereby subjected to consistent shear stress. Such hemodynamic shear stress is crucial for the maintenance of the phenotype, transcriptome, homeostasis and activation of the endothelial cells comprising the vascular endothelium [139, 140]. There is a growing body of evidence suggesting the utility of shear stress in vitro for VTE as an attempt to recapitulate the mechanical environment that favors ECs. In vitro assays revealed expression of endothelial markers vWF and CD31 in late EPCs upregulated by shear stress via cytoskeletal remodeling, leading to subsequently increased in vivo re-endothelialization after arterial injury [141]. Furthermore, hPSC-derived ECs aligned rapidly and adopted an arterial-like phenotype in vitro when exposed to high shear stress (~10 dynes cm−2) that mimics the blood flow in arteries (10–20 dynes cm−2) compared to low shear stress [142–144] (figure 3(D)). The pro-maturation effect of shear stress has been reported over a wide spectrum of cell sources. For example, MSCs from various species are able to differentiate into endothelial-like cells when subjected to physiological shear stress conditions [145–147]. Recently, stem cells from human exfoliated deciduous teeth were found responsive to shear stress by endothelial differentiation via the VEGF-Notch signaling [148].
Vascularization and angiogenesis strategies in tissue engineering: state of art and new perspective
The tremendous efforts made to understand endothelial biology from multiple perspectives has paved the way to create vascularization in tissue engineered grafts, which is currently regarded as one of the main hurdles for the translation of tissue engineering to clinical applications [7]. In this section, we will review the state of art and new perspectives in engineering vascularization strategies based on the latest findings (Summarized in figure 4).
Figure 4.
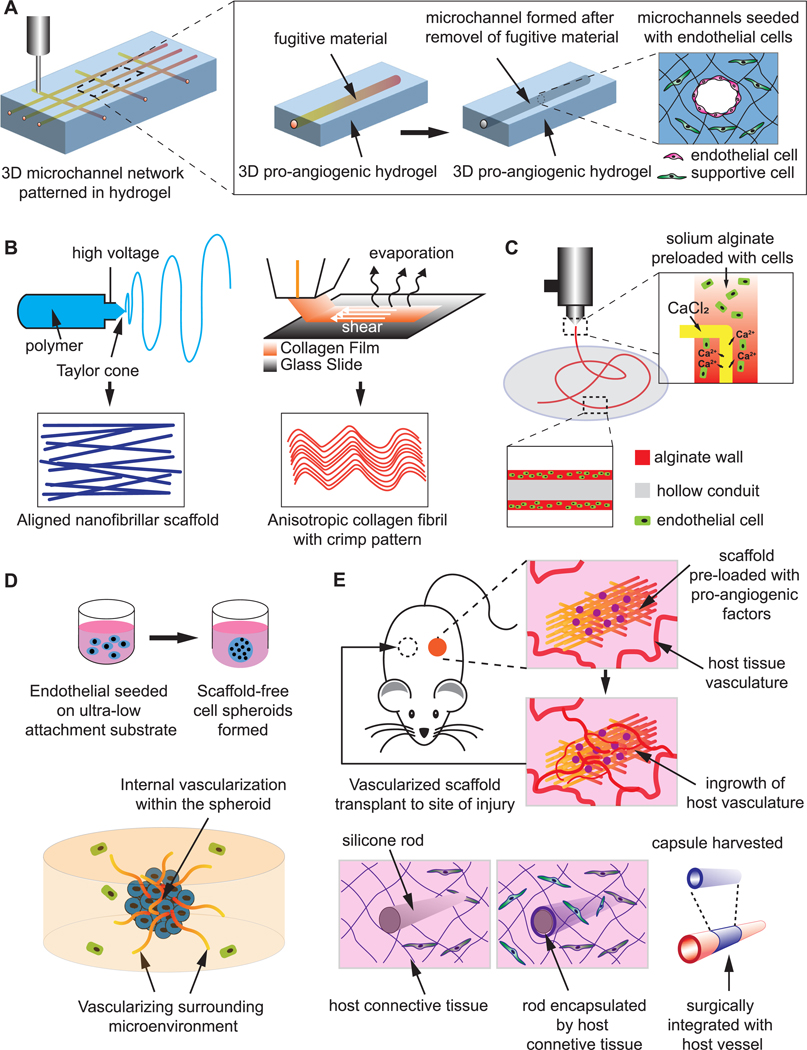
Overview of present strategies to engineer vasculature. (A) Hollow microchannels can be created within 3D hydrogel after removal of the fugitive material pre-patterned by casting or 3D printing. The ECs are then seeded onto the inner wall of the microchannels and thereby patterned by the pre-designed channel geometry and shape. (B) Nano/microfibrillar scaffolds prepared by electrospinning and shear-extrusion can render fibril alignment that regulates EC orientation and augment angiogenic behaviors. (C) The cells and biomaterials can be patterned concurrently by an extrusion-based printer featuring core-shell coaxial nozzles to print long, hollow microfibers with a cell-laden alginate wall to resemble the architecture of a native blood vessel. (D) The scaffold free, 3D EC spheroids may act as individual vascular units to vascularize internally, as well as pre-vascularize the surrounding tissue, and thus enhance the survival, homing and functionality of other impregnated cells within the construct. (E) Scaffolds that present angiogenic factors or resembles blood vessel morphology can be pre-implanted in a vascular site to be conditioned/vascularized in vivo through vessel ingrowth or encapsulation by host tissue, and then harvested and transplanted to the site of injury.
Patterning the vascular network by engineered Scaffold
Many endeavors in VTE have relied on the spontaneous angiogenic behavior of ECs from various sources. To date, a wide spectrum of 3D scaffolds have been developed using novel fabrication technologies to pattern and control the organization of vascular cells via different mechanisms. Such scaffold-cell complex has great potential to render vascular networks with high fidelity to ensure sufficient nutrient supplementation to surrounding cells, and enable anastomosis with surrounding host tissue after implantation. Apart from vascularizing thick tissue-engineered constructs, the recapitulation of native vascular structures in vitro may serve as a good research platform for high-throughput drug screening and disease models for pathological studies.
Hollow channels patterned within 3D hydrogel is a common scaffold design to generate vascular structures in vitro. The ECs are seeded onto the inner wall of the microchannels, thereby initially patterned by the pre-designed channel geometry and shape. Channels prepared in bioactive hydrogels offers a micro-environment tunable to cell remodeling, where endothelial cells can invade, sprout and lumenize inside the matrix to augment interconnectivity of patterned vascular network (figure 4(A)). Nichols et al cured gelatin methacrylate (GelMA) in a rectangular PDMS mold containing micro-needle as the space occupier, followed by infusion with HUVECs to create endothelialized microvascular channels [149]. Recently, further study using the similar microvascular channel design revealed retention of EC phenotype and confluency for 6 weeks after active perfusion with endothelium medium [150]. Using carbohydrate glass as a sacrificial material, Miller et al were able to create interconnected, orthogonal vascular networks within cell-laden fibrin hydrogels. Injected ECs formed single and multicellular sprouts from patterned vasculature [151].
An alternative approach to create perfusable, endothelialized microchannels in vitro is the perfusion of cell-seeded scaffolds by adjacent microfluidic channels. When seeded with fibrin inside a microfluidic device, HUVECs self-assembled into a continuous capillary network. The capillaries then anastomosed with adjacent microfluidic channels, allowing subsequent perfusion of the vessel network in vitro [152, 153]. Moreover, after initial formation of a capillary network via vasculogenesis, the inner wall of microfluidic channels can be lined with ECs to serve as arterial input and veinous output, respectively. Sprouting angiogenesis was induced, leading to complete interconnections between artery/vein and the capillary network [154].
Nano/microfibrillar scaffold is another widely reported scaffold design for VTE to generate organized vascular network. 3D porous microfibrous scaffolds with controllable fiber orientation were prepared by electrospinning of various poly-ester materials (figure 4(B)). Electrospun polycaprolactone (PCL) scaffold with parallel aligned microfibers and interconnected pores were found to improve the endothelial differentiation of human iPSCs, which led to elongated vascular-like network within the scaffold [57]. Likewise, when iPS-ECs were seeded on electrospun composite scaffold consisting of PCL and gelatin, improved survival and retention of phenotype was noted up to 3 d, as well as enhanced engraftment and improved blood perfusion in vivo [155]. In addition to electrospinning, nanofibrillar collagen scaffold can be fabricated via self-assembly from collagen film to render desired porosity and anisotropy to primary ECs and iPS-ECs (figure 4(B)). The seeded cells demonstrated improved outgrowth and survival in vitro, and augmented blood perfusion in a mouse hindlimb ischemia model [127, 156].
With advances in 3D bio-printing technologies in the past decade, the 3D vascular architecture can also be prepared directly by printing, which offers profound flexibility and precision in the spatial patterning of cells and micro-channels. Vascular-like structure printed by fugitive ink can be easily removed after printing to create lumen inside the 3D construct (figure 4(A)). Wu et al succeeded in 3D printing a perfusable microvascular network within a hydrogel reservoir [157]. The bioactivity of the vasculature prepared in this manner was validated in a more recent study by Bertassoni et al, in which bio-printed agarose template fibers created vascular-like networks within cell-laden hydrogel constructs to improve mass transport, cellular viability and differentiation of the surrounding cells. Successful formation of endothelial monolayers within the fabricated channels was also noted [158]. Using a similar extrusion based printing approach, intricate, heterogeneous 3D vascular structures consisting of various cell types can be created by precisely co-printing multiple cell-laden bioinks in three dimensions in a layer-by-layer manner [159]. Digital light projection (DLP) based stereolithography is another widely used printing technology for 3D vascular architectures. Three-dimensional, interconnected networks created by this approach were employed as a perfusable vascular scaffold for tissue engineering [160–162].
Equally important, the placement of cells and biomaterials can be performed concurrently by a printer utilizing proper cell-sustaining configuration and bio-ink without the subsequent cell infusion or seeding (figure 4(C)). For instance, extrusion-based systems featuring core-shell coaxial nozzles have been used to directly print long, hollow microfibers with a cell-laden alginate wall to resemble the architecture of a native blood vessel [163]. Likewise, cell-compatible bioink consisting of GelMA, sodium alginate, and 4-arm poly(ethylene glycol)-tetraacrylate (PEGTA) was developed in combination with a multilayered coaxial extrusion system to achieve direct 3D bioprinting of cell-laden, perfusable vascular-like tubes [164]. DLP stereolithography was implemented to successfully fabricate 3D Y-shaped tubular constructs with living cells encapsulated that exceeded 75% vitality [162]. 3D printing has offered unprecedented new avenues to create VTE products that represents great potential in the rapid, personalized, and bed-side production of patient specific vascularized implant.
3D cell spheroid for VTE
The development of new blood vessels is a dynamic process dependent on the interaction between ECs, as well as between ECs and perivascular cells. Scaffold-free EC spheroid culture was introduced by Korff and Augustin in 1998 to mimic this close 3D cell-cell interaction and analyze the angiogenic process in vitro [165]. Since then the angiogenic potential of 3D EC spheroid has been extensively studied and reported. Compared to ECs in 2D culture, ECs on the surface of spheroids exhibit a quiescent state, which is sensitive to angiogenic stimulation [165]. Moreover, the dense aggregation of cells generates a hypoxia core in the center of each spheroid due to the limited oxygen diffusion [166]. This results in a pro-angiogenic shift of the secretome, as evidence by increased activity of HIF-1, angiopoietin-2 and VEGF-A. Accordingly, 3D cell spheroids exhibit a markedly higher potential as stimulators of angiogenesis. These unique features of the ECs primed in spheroidal culture are distinctly in favor of VTE, leading to numerous studies on the VTE utility of spheroids (figure 4(D)).
HUVEC-based spheroids embedded in a 3D collagen hydrogel developed elongated, partly lumenized sprouts by tip cells. These vascularized spheroids were then treated with drugs such as Perhexiline and Ellipticine to assess any angiogenesis-related adverse effects, indicating its potential as a high throughput drug-screening platform [167]. In accordance with this finding, Wimmer et al reported that EC spheroid from pluripotent stem cell self-assembled into a human blood vessel organoid when transplanted into mice and faithfully recapitulated the structure of a stable, perfused vascular tree, including arteries, arterioles and venules [12]. Thus, the EC spheroids may act as individual vascular units to pre-vascularize engineered tissue graft, and enhance the survival, homing and functionality of other impregnated cells within the construct. For instance, ECs rapidly self-assembled into tubular vessel-like network structures when they were co-cultured with osteoblasts in 3D spheroids, leading to the scalable production of prevascularized bone micro-tissue. The network was anastomosed to surrounding blood vessels after in vivo implantation [168]. Moreover, it should be noted that the prevascularized, co-culture spheroids has markedly potential to generate more complex microenvironments such as cardiac tissue [169] and hepatic tissue [170] in a bottom-up manner. In short, the versatility and ease of 3D spheroid-based studies has begun to see an increasing footprint in the fields of VTE and regenerative medicine.
Angiogenesis in vivo: let the body do the job
Most tissues are capable of invading and vascularizing a pro-angiogenic implant due to the inherent regenerative potential of our body. This ability has inspired researchers to propose another VTE strategy—the in vivo vascularization of TE grafts, in which the implanted biomaterial and cells are designed to rapidly integrate with the surrounding host tissue and vascularize under in vivo physiological conditions. After this initial stage, the construct is harvested and transplanted to the defect site using microsurgical vascular anastomosis techniques. A major advantage of in vivo VTE is the vascular maturation under anatomical and functional relevant native microenvironment of the host, thus skipping the in vitro culture of implant that may entail the exposure to immunogenic products or adverse microenvironment.
A proof of concept study showed that when 1000 spheroids of 100 HUVECs each were subcutaneously implanted into SCID mice, human EC–lined tube vasculature anastomosed with the mouse vasculature after 8 d, and the resulting vasculature was stable for at least 60 d and responded to exogenous cytokines [171]. The endothelialized filament polymeric scaffolds implanted in mice were invaded by blood vessels from host tissue and populated by a cellular component that displayed endothelial phenotype [172]. It is noteworthy that the in vivo angiogenesis is not limited to endothelial cells, bestowing greater flexibility to the choice of cells. Gelatin sponge scaffolds impregnated with HIF-1α overexpressing BMSCs showed dramatically improved blood vessel ingrowth in the tissue-engineered bone [173]. Apart from cell-laden grafts, cell-free scaffolds loaded with angiogenic factors is another important strategy for in vivo VTE. For instance, VEGF-incorporated polymeric scaffolds were markedly vascularized by the host animal vasculature in various in vivo models [174–177]. Likewise, a PEGDA hydrogel presenting interconnected pores was employed to deliver PDGF-BB in a murine subcutaneous model. Complete vascularization of the hydrogel was spotted 3 weeks post-implantation [178]. Well primed by native angiogenic cues, all these grafts are ready to be transplanted to the site of actual defect after extraction from the primary angiogenic site (figure 4(E)).
In vivo VTE has been applied towards several animal models in the past decade. Co-polymer rods were engineered and implanted subcutaneously to evoke encapsulation by a fibrocellular capsule. The harvested capsule/conduit was remodeled towards a vascular phenotype after grafting into the porcine vasculature as arterial interposition, in which the lumen was largely composed of collagen and lined by endothelial cells [179]. Likewise, Yamanami et al reported the preparation of ‘biotubes’ of extremely small caliber by placing silicone rods into dorsal subcutaneous pouches of rats for 4 weeks. Native-like vascular structure was regenerated with completely endothelialized luminal surface and regular circumferential orientation of collagen fibers and α-SMA positive cells after the biotubes were anastomosed to rat abdominal aortas [180] (figure 4(E)). These promising preclinical studies has envisioned the possible in vivo VTE strategy on patents using autologous cells to circumvent immunogenic response and disease transmission.
Non-invasive monitoring of vascularization in vivo: markers and imaging technology
Over the last decades, VEGF has been a global molecular marker for vasculogenesis and angiogenesis. May et al demonstrated a method to quantify VEGF levels by creating in vitro whole-cell based biosensor which reflects human umbilical vein endothelial cells (HUVECs) cultured on a cellulose triacetate membrane of an ion-selective electrode [181]. Other known endothelial markers include CD31, CD34 and nestin. Nestin is an intermediate filament protein detected in proliferating vascular endothelial cells that can serve as a novel angiogenic marker for microvessels [182]. In vivo molecular imaging of angiogenesis enables visualization and quantification of neovascularization. Traditional approach of evaluating vasculature in the clinical setting uses a blood-circulating agent with gadolinium to visualize vasculature through MRI contrast [183]. Recently, numerous long circulating MRI contrast and fluorescent agents have been identified for imaging vascular volume fraction (VVF) in clinical settings. For instance, long circulating dextrinated iron oxide (LCDIO) is used by Xu et al as a fluorescent labeled probe to detect vascular density by MRI [184]. Ferucarbotran-loaded red blood cells are reported as an angiogenic imaging agents for MRI [185].
Due to the importance of angiogenesis and vasculature development in tumor progression, research into high resolution imaging of tumor targets has led some exciting progression in the non-invasive track of angiogenesis. LED-based photoacoustic and ultrasound-based imaging has been successfully applied to visualize angiogenesis in scaffolds implanted in mice [186]. This technique enabled the researchers to detect perfusion in scaffolds and correlating it to vessel density and enable vascular mapping. In order to monitor the 3D architecture of in vivo vasculature, ultrafast Doppler tomography (UFD-T) has been recently used to actively monitor angiogenesis progression in tumors and quantifying vessel size and length in 3D [187]. Other novel imaging agents such as single-walled carbon nanotubes was also utilized to image murine hindlimb vasculature and blood flow [156, 188]. Although several in vitro strategies for monitoring vascularization have been identified, more effective and non-invasive approaches for tracking in vivo vascularization deeper into the tissues and over a long-term period still need to be developed. It is likely that the aforementioned techniques used in combination can provide additional information that complements each other in order to provide a robust and efficient methodology for vascular imaging.
Application of VTE in regenerative medicine: beyond angiogenesis
A large number of studies have indicated the positive role of natural angiogenesis in wound healing and regeneration process, which requires a dynamic, temporally and spatially regulated interaction between endothelial cells, angiogenesis factors and surrounding ECM. It is thereby reasonable to envision the utility of an engineered vascular graft as a driver of regeneration by augmenting the angiogenesis of host tissue at the site of injury, in addition to sustaining the survival and functionality of other therapeutic cells.
Despite the inherent regenerative capacity of bone tissue, adequate vascularization is still deemed as a critical factor for successful bone healing [189, 190]. In vitro study revealed augmented osteogenesis of mouse calvarial pre-osteoblasts cells when encapsulated in a bioprinted microchannel hydrogel pre-vascularized by HUVECs [158]. Evidence of vessel infiltration into the scaffold and a trend toward increased ectopic bone formation was noted in mouse subcutaneous implantation of macroporous scaffolds and co-cultured EPC/hMSC-scaffold grafts, indicating the potential of vascularized bone tissue engineering applications [191]. Skeletal muscle is a highly organized tissue, consisting of bundles of parallel-aligned myofibers interspersed with blood vessels. Studies have demonstrated the importance of the interactions between the capillaries and myogenic cells during muscle regeneration [192, 193]. From a VTE perspective, isolated tissue resident EPCs were found to contribute to angiogenesis of muscle regeneration via differentiating into blood vessels upon transplantation [194]. In a more recent study by Nakayama et al, human microvascular ECs were seeded with murine myoblasts on aligned nanofibrillar scaffolds to endothelialize the construct. Treatment of traumatically injured muscle with such endothelialized muscle grafts improves the post-injury regeneration of highly organized myofibers, microvasculature, and vascular perfusion [195].
In addition to the musculoskeletal system, recent studies have shed light on the potential participation of VTE in other key organ restoration. After brain stroke, many migrating neuroblasts from the subventricular zone (SVZ) are localized to the blood vessels at the infarct boundary. Blocking post-ischemic angiogenesis resulted in a 10-fold reduction in neuroblasts in the peri-infarct cortex 7 d after stroke [196–198]. Conversely, many studies have consistently shown that administration of human EPCs was associated with the reduction in infarct volume and brain atrophy in a mouse model of transient brain stroke, along with an increase in vessel density [199, 200]. Liver tissue repair/replacement is another prevailing challenge that relies on the advancement of VTE. Co-implantation of EC cords were found to support primary human hepatocytes in a mouse model: Tissue constructs containing human hepatocyte aggregates and EC cords exhibited significantly higher levels of albumin promoter activity compared with constructs without cords [201]. Moreover, VTE has the potential to salvage ischemic myocardium at early stages after myocardial infarction, and also to prevent the transition to heart failure through the control of cardiomyocyte hypertrophy and contractility [202]. Genetically modified EPC that overexpress Tβ4 significantly increased myocardial contractility and rates of cardiomyocyte survival in a rat myocardial infarction model [203].
Conclusion and future perspectives
Vascularization is the key challenge that hinders the clinical applications of tissue engineering product. To date, various strategies have been developed to vascularize tissue engineered grafts that comprises key fundamental elements of physiological vasculogenesis and angiogenesis, including vascular cells, angiogenic factors, biophysical cues and spatial organization.
However, we must keep in mind that it is unlikely to find one universal approach to engineer vascular network for all tissue engineering purposes. Due to the pivotal role of tissue-specific cues and vascular-tissue interaction that drives vascularization, VTE is more likely to succeed when created in a tissue-dependent manner. On the other hand, the advancement of VTE in the near future depends on the integration of multiple, complimentary strategies depicted in this article for optimal in vivo functionality. For instance, vascularized cell spheroids may be 3D printed to generate composite grafts. Likewise, biophysical parameters such as matrix stiffness and shear stress can be easily integrated into 3D microchannel blocks to regulate EC behaviors that lines the inner wall of the channels.
Furthermore, the existing findings point to several future perspectives to further advance the clinical potential of VTE. To date, most of the VTE scaffolds fail to reproduce the native organization of the vascular tree in tissue engineered grafts. Future 3D fabrication approaches should be capable of generating a hierarchical vascular network that recapitulates the transition from macrovessels (arteries and veins) to microvessels (arterioles and venules) and finally capillaries. Additionally, more attention should be paid to the long term quality of engineered vessel system, which can be reflected by endothelium homeostasis, perfusability, permeability and contractility of the vessels. Finally, it is important to note that the intensive crosstalk between regional vasculogenesis and organogenesis indicates the possible synergistic tissue engineering of vascularization and organ differentiation, which might be the next breakthrough that leads to the production of vascularized whole organ and tissue.
Acknowledgment
The authors have no conflict of interest to disclose. This work was supported by the National Institute of Biomedical Imaging and Bioengineering/National Institutes of Health (NIBIB/NIH) Center for Engineering Complex Tissues (P41 EB023833).
References
- [1].Bautch VL and Caron KM 2015. Blood and lymphatic vessel formation Cold Spring Harb. Perspect. Biol. 7 a008268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coultas L, Chawengsaksophak K and Rossant J 2005. Endothelial cells and VEGF in vascular development Nature 438 937–45 [DOI] [PubMed] [Google Scholar]
- [3].Patel-Hett S and D’Amore PA 2011. Signal transduction in vasculogenesis and developmental angiogenesis Int. J. Dev. Biol. 55 353–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shafiee A and Atala A 2017. Tissue engineering: toward a new era of medicine Ann. Rev. Med. 68 29–40 [DOI] [PubMed] [Google Scholar]
- [5].Rouwkema J, Rivron NC and van Blitterswijk CA 2008. Vascularization in tissue engineering Trends Biotechnol. 26 434–41 [DOI] [PubMed] [Google Scholar]
- [6].Burr S et al. 2018. Oxygen gradients can determine epigenetic asymmetry and cellular differentiation via differential regulation of Tet activity in embryonic stem cells Nucleic Acids Res. 46 1210–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaklenec A, Stamp A, Deweerd E, Sherwin A and Langer R 2012. Progress in the tissue engineering and stem cell industry ‘are we there yet?’ Tissue Eng. B 18 155–66 [DOI] [PubMed] [Google Scholar]
- [8].Tsigkou O et al. 2010. Engineered vascularized bone grafts Proc. Natl Acad. Sci. USA 107 3311–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen L et al. 2017. Pre-vascularization enhances therapeutic effects of human mesenchymal stem cell sheets in full thickness skin wound repair Theranostics 7 117–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jakab K et al. 2008. Tissue engineering by self-assembly of cells printed into topologically defined structures Tissue Eng. A 14 413–21 [DOI] [PubMed] [Google Scholar]
- [11].Inamori M, Mizumoto H and Kajiwara T 2009. An approach for formation of vascularized liver tissue by endothelial cell-covered hepatocyte spheroid integration Tissue Eng. A 15 2029–37 [DOI] [PubMed] [Google Scholar]
- [12].Wimmer R A et al. 2019. Human blood vessel organoids as a model of diabetic vasculopathy Nature 565 505–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim S, Lee H, Chung M and Jeon N L 2013. Engineering of functional, perfusable 3D microvascular networks on a chip Lab Chip 13 1489–500 [DOI] [PubMed] [Google Scholar]
- [14].Bersini S and Moretti M 2015. 3D functional and perfusable microvascular networks for organotypic microfluidic models J. Mater. Sci., Mater. Med. 26 180. [DOI] [PubMed] [Google Scholar]
- [15].Keller G 2005. Embryonic stem cell differentiation: emergence of a new era in biology and medicine Genes Dev. 19 1129–55 [DOI] [PubMed] [Google Scholar]
- [16].Park C, Kim TM and Malik AB 2013. Transcriptional regulation of endothelial cell and vascular development Circ. Res. 112 1380–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Damert A, Miquerol L, Gertsenstein M, Risau W and Nagy A 2002. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation Development 129 1881–92 [DOI] [PubMed] [Google Scholar]
- [18].Jakobsson L et al. 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting Nat. Cell Biol. 12 943–53 [DOI] [PubMed] [Google Scholar]
- [19].Hellstrom M et al. 2007. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis Nature 445 776–80 [DOI] [PubMed] [Google Scholar]
- [20].Eremina V et al. 2003. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases J. Clin. Invest. 111 707–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maes C et al. 2002. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188 Mech. Dev. 111 61–73 [DOI] [PubMed] [Google Scholar]
- [22].Redman CW and Sargent IL 2005. Latest advances in understanding preeclampsia Science 308 1592–4 [DOI] [PubMed] [Google Scholar]
- [23].Levenberg S et al. 2005. Engineering vascularized skeletal muscle tissue Nat. Biotechnol. 23 879–84 [DOI] [PubMed] [Google Scholar]
- [24].Lammert E, Cleaver O and Melton D 2001. Induction of pancreatic differentiation by signals from blood vessels Science 294 564–7 [DOI] [PubMed] [Google Scholar]
- [25].Kamei M et al. 2006. Endothelial tubes assemble from intracellular vacuoles in vivo Nature 442 453–6 [DOI] [PubMed] [Google Scholar]
- [26].Blum Y et al. 2008. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo Dev. Biol. 316 312–22 [DOI] [PubMed] [Google Scholar]
- [27].Strilic B et al. 2010. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels Curr. Biol. 20 2003–9 [DOI] [PubMed] [Google Scholar]
- [28].Wang HU, Chen ZF and Anderson DJ 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4 Cell 93 741–53 [DOI] [PubMed] [Google Scholar]
- [29].Swift MR and Weinstein BM 2009. Arterial-venous specification during development Circ. Res. 104 576–88 [DOI] [PubMed] [Google Scholar]
- [30].You LR et al. 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity Nature 435 98–104 [DOI] [PubMed] [Google Scholar]
- [31].Adams RH et al. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis Genes Dev. 13 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].van Dijk CG et al. 2015. The complex mural cell: pericyte function in health and disease Int. J. Cardiol. 190 75–89 [DOI] [PubMed] [Google Scholar]
- [33].Armulik A, Genove G and Betsholtz C 2011. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises Dev. Cell 21 193–215 [DOI] [PubMed] [Google Scholar]
- [34].Carmeliet P and Jain RK 2011. Molecular mechanisms and clinical applications of angiogenesis Nature 473 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Daneman R, Zhou L, Kebede AA and Barres BA 2010. Pericytes are required for blood-brain barrier integrity during embryogenesis Nature 468 562–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Holm A, Heumann T and Augustin HG 2018. Microvascular mural cell organotypic heterogeneity and functional plasticity Trends Cell Biol. 28 302–16 [DOI] [PubMed] [Google Scholar]
- [37].Chen YC et al. 2012. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels Adv. Funct. Mater. 22 2027–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Asahara T et al. 1997. Isolation of putative progenitor endothelial cells for angiogenesis Science 275 964–7 [DOI] [PubMed] [Google Scholar]
- [39].Hirschi KK, Ingram DA and Yoder MC 2008. Assessing identity, phenotype, and fate of endothelial progenitor cells Arter. Thromb. Vasc. Biol. 28 1584–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Peters EB 2018. Endothelial progenitor cells for the vascularization of engineered tissues Tissue Eng. B 24 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yoder MC 2012. Human endothelial progenitor cells Cold Spring Harbor Perspect. Med. 2 a006692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hill JM et al. 2003. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk New Engl. J. Med. 348 593–600 [DOI] [PubMed] [Google Scholar]
- [43].Briguori C et al. 2010. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells FASEB J. 24 1981–8 [DOI] [PubMed] [Google Scholar]
- [44].Zahran AM et al. 2019. Circulating endothelial cells, circulating endothelial progenitor cells, and circulating microparticles in type 1 diabetes mellitus Clin. Appl. Thromb./Hemost. 25 1076029618825311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fadini GP et al. 2005. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus J. Am. Coll. Cardiol. 45 1449–57 [DOI] [PubMed] [Google Scholar]
- [46].Munoz-Pinto DJ et al. 2017. Evaluation of late outgrowth endothelial progenitor cell and umbilical vein endothelial cell responses to thromboresistant collagen-mimetic hydrogels J. Biomed. Mater. Res. A 105 1712–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stroncek JD et al. 2009. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers Tissue Eng. A 15 3473–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tura O et al. 2013. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow Stem Cells 31 338–48 [DOI] [PubMed] [Google Scholar]
- [49].Medina RJ, O’Neill CL, Humphreys MW, Gardiner TA and Stitt AW 2010. Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy Invest. Ophthalmol. Vis. Sci. 51 5906–13 [DOI] [PubMed] [Google Scholar]
- [50].Tagawa S et al. 2015. Determination of early and late endothelial progenitor cells in peripheral circulation and their clinical association with coronary artery disease Int. J. Vasc. Med. 2015 674213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Peters EB, Christoforou N, Leong KW, Truskey GA and West JL 2016. Poly(ethylene glycol) hydrogel scaffolds containing cell-adhesive and protease-sensitive peptides support microvessel formation by endothelial progenitor cells Cell. Mol. Bioeng. 9 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rouwkema J, Westerweel PE, de Boer J, Verhaar MC and van Blitterswijk CA 2009. The use of endothelial progenitor cells for prevascularized bone tissue engineering Tissue Eng. A 15 2015–27 [DOI] [PubMed] [Google Scholar]
- [53].Melero-Martin JM et al. 2008. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells Circ. Res. 103 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Melchiorri AJ, Bracaglia LG, Kimerer LK, Hibino N and Fisher JP 2016. In vitro endothelialization of biodegradable vascular grafts via endothelial progenitor cell seeding and maturation in a tubular perfusion system bioreactor Tissue Eng. C 22 663–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Takahashi K et al. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131 861–72 [DOI] [PubMed] [Google Scholar]
- [56].Patsch C et al. 2015. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells Nat. Cell Biol. 17 994–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim JJ et al. 2017. Microfibrous scaffolds enhance endothelial differentiation and organization of induced pluripotent stem cells Cell. Mol. Bioeng. 10 417–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rufaihah AJ et al. 2011. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease Arter. Thromb. Vasc. Biol. 31 e72–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Foster AA et al. 2018. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease Biomater. Sci. 6 614–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stebbins MJ et al. 2019. Human pluripotent stem cell-derived brain pericyte-like cells induce blood-brain barrier properties Sci. Adv. 5 eaau7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Orlova V V et al. 2014. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts Arter. Thromb. Vasc. Biol. 34 177–86 [DOI] [PubMed] [Google Scholar]
- [62].Stoddard-Bennett T and Reijo Pera R 2019. Treatment of parkinson’s disease through personalized medicine and induced pluripotent stem cells Cells 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mandai M et al. 2017. Autologous induced stem-cell-derived retinal cells for macular degeneration New Engl. J. Med. 376 1038–46 [DOI] [PubMed] [Google Scholar]
- [64].Tsuji O. et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl Acad. Sci. USA. 2010;107 doi: 10.1073/pnas.0910106107. 12704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yasuda S. et al. Tumorigenicity-associated characteristics of human iPS cell lines. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205022. e0205022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Miura K et al. 2009. Variation in the safety of induced pluripotent stem cell lines Nat. Biotechnol. 27 743–5 [DOI] [PubMed] [Google Scholar]
- [67].Dominici M et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement Cytotherapy 8 315–7 [DOI] [PubMed] [Google Scholar]
- [68].Wang N et al. 2013. Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor—a signaling pathway Int. J. Biochem. Cell Biol. 45 1447–56 [DOI] [PubMed] [Google Scholar]
- [69].Zhang P et al. 2011. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease Stem Cells Dev. 20 977–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hasanzadeh E et al. 2017. The stability evaluation of mesenchymal stem cells differentiation toward endothelial cells by chemical and mechanical stimulation In Vitro Cell. Dev. Biol. Anim. 53 818–26 [DOI] [PubMed] [Google Scholar]
- [71].Cao Y et al. 2005. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo Biochem. Biophys. Res. Commun. 332 370–9 [DOI] [PubMed] [Google Scholar]
- [72].Xu Y et al. 2010. Umbilical cord-derived mesenchymal stem cells isolated by a novel explantation technique can differentiate into functional endothelial cells and promote revascularization Stem Cells Dev. 19 1511–22 [DOI] [PubMed] [Google Scholar]
- [73].Tao H, Han Z, Han ZC and Li Z 2016. Proangiogenic features of mesenchymal stem cells and their therapeutic applications Stem Cells Int. 2016 1314709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ge Q et al. 2018. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms Mol. Med. Rep. 17 1667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bussche L and Van de Walle GR 2014. Peripheral blood-derived mesenchymal stromal cells promote angiogenesis via paracrine stimulation of vascular endothelial growth factor secretion in the equine model Stem Cells Transl. Med. 3 1514–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mikami S. et al. Autologous bone-marrow mesenchymal stem cell implantation and endothelial function in a rabbit ischemic limb model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067739. e67739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].de Souza LE, Malta TM, Kashima Haddad S and Covas DT 2016. Mesenchymal stem cells and pericytes: to what extent are they related? Stem Cells Dev. 25 1843–52 [DOI] [PubMed] [Google Scholar]
- [78].Au P, Tam J, Fukumura D and Jain RK 2008. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature Blood 111 4551–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Narita Y, Yamawaki A, Kagami H, Ueda M and Ueda Y 2008. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage Cell Tissue Res. 333 449–59 [DOI] [PubMed] [Google Scholar]
- [80].Santoro M et al. 2019. Endothelial/mesenchymal stem cell crosstalk within bioprinted cocultures Tissue Eng. A ( 10.1089/ten.TEA.2019.0175) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [81].Bidarra S J et al. 2011. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells Stem Cell Res. 7 186–97 [DOI] [PubMed] [Google Scholar]
- [82].Luu NT et al. 2013. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment Stem Cells 31 2690–702 [DOI] [PubMed] [Google Scholar]
- [83].Naftali-Shani N et al. 2013. The origin of human mesenchymal stromal cells dictates their reparative properties J. Am. Heart Assoc. 2 e000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sacchetti B et al. 2016. No identical ‘mesenchymal stem cells’ at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels Stem Cell Rep. 6 897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ingber D and Folkman J 1988. Inhibition of angiogenesis through modulation of collagen metabolism Lab. Invest. 59 44–51 [PubMed] [Google Scholar]
- [86].Sweeney SM et al. 2003. Angiogenesis in collagen I requires alpha2beta1 ligation of a GFP * GER sequence and possibly p38 MAPK activation and focal adhesion disassembly J. Biol. Chem. 278 30516–24 [DOI] [PubMed] [Google Scholar]
- [87].Ilan N, Mahooti S and Madri JA 1998. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis J. Cell Sci. 111 3621–31 [DOI] [PubMed] [Google Scholar]
- [88].Stratman AN et al. 2009. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices Blood 114 237–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mongiat M, Andreuzzi E, Tarticchio G and Paulitti A 2016. Extracellular matrix, a hard player in angiogenesis Int. J. Mol. Sci. 17 1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Morgan JP et al. 2013. Formation of microvascular networks in vitro Nat. Protocols 8 1820–36 [DOI] [PubMed] [Google Scholar]
- [91].Mosesson MW 2005. Fibrinogen and fibrin structure and functions J. Thromb. Haemost. 3 1894–904 [DOI] [PubMed] [Google Scholar]
- [92].Morin KT and Tranquillo RT 2013. In vitro models of angiogenesis and vasculogenesis in fibrin gel Exp. Cell Res. 319 2409–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nakatsu MN et al. 2003. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1 Microvasc. Res. 66 102–12 [DOI] [PubMed] [Google Scholar]
- [94].Rioja AY, Tiruvannamalai Annamalai R, Paris S, Putnam AJ and Stegemann JP 2016. Endothelial sprouting and network formation in collagen- and fibrin-based modular microbeads Acta Biomater. 29 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Benning L et al. 2018. Assessment of hydrogels for bioprinting of endothelial cells J. Biomed. Mater. Res. A 106 935–47 [DOI] [PubMed] [Google Scholar]
- [96].McDevitt CA, Wildey GM and Cutrone RM 2003. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa J. Biomed. Mater. Res. A 67 637–40 [DOI] [PubMed] [Google Scholar]
- [97].Lin H, Yang G, Tan J and Tuan R S 2012. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential Biomaterials 33 4480–9 [DOI] [PubMed] [Google Scholar]
- [98].Yang G et al. 2013. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix Biomaterials 34 9295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gong T et al. 2017. Decellularized extracellular matrix of human umbilical vein endothelial cells promotes endothelial differentiation of stem cells from exfoliated deciduous teeth J. Biomed. Mater. Res. A 105 1083–93 [DOI] [PubMed] [Google Scholar]
- [100].Fercana GR et al. 2017. Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor Biomaterials 123 142–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jiang B, Suen R, Wertheim JA and Ameer GA 2016. Targeting heparin to collagen within extracellular matrix significantly reduces thrombogenicity and improves endothelialization of decellularized tissues Biomacromolecules 17 3940–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ponce ML 2009. Tube formation: an in vitro matrigel angiogenesis assay Methods Mol. Biol. 467 183–8 [DOI] [PubMed] [Google Scholar]
- [103].McCoy MG, Seo BR, Choi S and Fischbach C 2016. Collagen I hydrogel microstructure and composition conjointly regulate vascular network formation Acta Biomater. 44 200–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang W et al. 2016. Preparation and characterization of pro-angiogenic gel derived from small intestinal submucosa Acta Biomater. 29 135–48 [DOI] [PubMed] [Google Scholar]
- [105].Lin X et al. 2015. Small intestinal submucosa-derived extracellular matrix bioscaffold significantly enhances angiogenic factor secretion from human mesenchymal stromal cells Stem Cell Res. Ther. 6 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang K et al. 2017. Strategy for constructing vascularized adipose units in poly(l-glutamic acid) hydrogel porous scaffold through inducing in situ formation of ASCs spheroids Acta Biomater. 51 246–57 [DOI] [PubMed] [Google Scholar]
- [107].Gniesmer S et al. 2019. In vivo analysis of vascularization and biocompatibility of electrospun polycaprolactone fibre mats in the rat femur chamber J. Tissue Eng. Regenerat. Med. 13 1190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Van Hove AH, Burke K, Antonienko E, Brown E III and Benoit DS 2015. Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo J. Control. Release 217 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Eke G, Mangir N, Hasirci N, MacNeil S and Hasirci V 2017. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering Biomaterials 129 188–98 [DOI] [PubMed] [Google Scholar]
- [110].Da L et al. 2017. Composite elastomeric polyurethane scaffolds incorporating small intestinal submucosa for soft tissue engineering Acta Biomater. 59 45–57 [DOI] [PubMed] [Google Scholar]
- [111].Aurora A, Wrice N, Walters TJ, Christy RJ and Natesan S 2018. A PEGylated platelet free plasma hydrogel based composite scaffold enables stable vascularization and targeted cell delivery for volumetric muscle loss Acta Biomater. 65 150–62 [DOI] [PubMed] [Google Scholar]
- [112].Risau W 1997. Mechanisms of angiogenesis Nature 386 671–4 [DOI] [PubMed] [Google Scholar]
- [113].Manalo DJ et al. 2005. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1 Blood 105 659–69 [DOI] [PubMed] [Google Scholar]
- [114].Ceradini DJ et al. 2004. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1 Nat. Med. 10 858–64 [DOI] [PubMed] [Google Scholar]
- [115].Ben-Yosef Y, Miller A, Shapiro S and Lahat N 2005. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death Am. J. Physiol. Cell Physiol. 289 C1321–31 [DOI] [PubMed] [Google Scholar]
- [116].Zimna A and Kurpisz M 2015. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies Biomed. Res. Int. 2015 549412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Diez H et al. 2007. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate Exp. Cell Res. 313 1–9 [DOI] [PubMed] [Google Scholar]
- [118].Liu Y, Cox SR, Morita T and Kourembanas S 1995. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer Circ. Res. 77 638–43 [DOI] [PubMed] [Google Scholar]
- [119].Ramakrishnan S, Anand V and Roy S 2014. Vascular endothelial growth factor signaling in hypoxia and inflammation J. Neuroimmune Pharmacol. 9 142–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Jiang M et al. 2008. In vivo enhancement of angiogenesis by adenoviral transfer of HIF-1alpha-modified endothelial progenitor cells (Ad-HIF-1alpha-modified EPC for angiogenesis) Int. J. Biochem. Cell Biol. 40 2284–95 [DOI] [PubMed] [Google Scholar]
- [121].Ma B et al. 2019. Short-term hypoxia promotes vascularization in co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells J. Biomed. Mater. Res. A 108 7–18 [DOI] [PubMed] [Google Scholar]
- [122].Shang T et al. 2019. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2 Stem Cell Res. Ther. 10 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ross AM, Jiang Z, Bastmeyer M and Lahann J 2012. Physical aspects of cell culture substrates: topography, roughness, and elasticity Small 8 336–55 [DOI] [PubMed] [Google Scholar]
- [124].Meyle J, Wolburg H and von Recum AF 1993. Surface micromorphology and cellular interactions J. Biomater. Appl. 7 362–74 [DOI] [PubMed] [Google Scholar]
- [125].Song S et al. 2015. The synergistic effect of micro-topography and biochemical culture environment to promote angiogenesis and osteogenic differentiation of human mesenchymal stem cells Acta Biomater. 18 100–11 [DOI] [PubMed] [Google Scholar]
- [126].Lai ES, Huang NF, Cooke JP and Fuller GG 2012. Aligned nanofibrillar collagen regulates endothelial organization and migration Regenerat. Med. 7 649–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Huang NF et al. 2013. The modulation of endothelial cell morphology, function, and survival using anisotropic nanofibrillar collagen scaffolds Biomaterials 34 4038–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gariboldi MI, Butler R, Best SM and Cameron RE 2019. Engineering vasculature: Architectural effects on microcapillary-like structure self-assembly PLoS One 14 e0210390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Engler AJ, Sen S, Sweeney HL and Discher DE 2006. Matrix elasticity directs stem cell lineage specification Cell 126 677–89 [DOI] [PubMed] [Google Scholar]
- [130].Jacob MP 2003. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions Biomed. Pharmacoth. 57 195–202 [DOI] [PubMed] [Google Scholar]
- [131].Davis GE and Senger DR 2005. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization Circ. Res. 97 1093–107 [DOI] [PubMed] [Google Scholar]
- [132].Derricks KE, Trinkaus-Randall V and Nugent MA 2015. Extracellular matrix stiffness modulates VEGF calcium signaling in endothelial cells: individual cell and population analysis Integrat. Biol. 7 1011–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sack KD, Teran M and Nugent MA 2016. Extracellular matrix stiffness controls VEGF signaling and processing in endothelial cells J. Cell. Physiol. 231 2026–39 [DOI] [PubMed] [Google Scholar]
- [134].Xue C et al. 2017. Substrate stiffness regulates arterial-venous differentiation of endothelial progenitor cells via the Ras/Mek pathway Biochim. Biophys. Acta 1864 1799–808 [DOI] [PubMed] [Google Scholar]
- [135].Schweller RM, Wu ZJ, Klitzman B and West JL 2017. Stiffness of protease sensitive and cell adhesive PEG hydrogels promotes neovascularization in vivo Ann. Biomed. Eng. 45 1387–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chen G, Dong C, Yang L and Lv Y 2015. 3D Scaffolds with different stiffness but the same microstructure for bone tissue engineering ACS Appl. Mater. Interfaces 7 15790–802 [DOI] [PubMed] [Google Scholar]
- [137].Juliar BA, Keating MT, Kong YP, Botvinick EL and Putnam AJ 2018. Sprouting angiogenesis induces significant mechanical heterogeneities and ECM stiffening across length scales in fibrin hydrogels Biomaterials 162 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].He YJ et al. 2018. Protease-sensitive hydrogel biomaterials with tunable modulus and adhesion ligand gradients for 3D vascular sprouting Biomacromolecules 19 4168–81 [DOI] [PubMed] [Google Scholar]
- [139].Davies PF 2009. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology Nat. Clin. Pract. Cardiovasc. Med. 6 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wragg JW et al. 2014. Shear stress regulated gene expression and angiogenesis in vascular endothelium Microcirculation 21 290–300 [DOI] [PubMed] [Google Scholar]
- [141].Cheng M. et al. Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067675. e67675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sivarapatna A et al. 2015. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor Biomaterials 53 621–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Giddens DP, Zarins CK and Glagov S 1993. The role of fluid mechanics in the localization and detection of atherosclerosis J. Biomech. Eng. 115 588–94 [DOI] [PubMed] [Google Scholar]
- [144].Arora S, Lam AJY, Cheung C, Yim EKF and Toh YC 2019. Determination of critical shear stress for maturation of human pluripotent stem cell-derived endothelial cells towards an arterial subtype Biotechnol. Bioeng. 116 1164–75 [DOI] [PubMed] [Google Scholar]
- [145].Wang H et al. 2005. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line Arter. Thromb. Vasc. Biol. 25 1817–23 [DOI] [PubMed] [Google Scholar]
- [146].Kim DH, Heo SJ, Kim SH, Shin JW and Park SH 2011. Shear stress magnitude is critical in regulating the differentiation of mesenchymal stem cells even with endothelial growth medium Biotechnol. Lett. 33 2351–9 [DOI] [PubMed] [Google Scholar]
- [147].Bai K, Huang Y, Jia X, Fan Y and Wang W 2010. Endothelium oriented differentiation of bone marrow mesenchymal stem cells under chemical and mechanical stimulations J. Biomech. 43 1176–81 [DOI] [PubMed] [Google Scholar]
- [148].Wang P et al. 2018. Shear stress promotes differentiation of stem cells from human exfoliated deciduous teeth into endothelial cells via the downstream pathway of VEGF-Notch signaling Int. J. Mol. Med. 42 1827–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nichol JW et al. 2010. Cell-laden microengineered gelatin methacrylate hydrogels Biomaterials 31 5536–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kolesky DB, Homan KA, Skylar-Scott MA and Lewis J A 2016. Three-dimensional bioprinting of thick vascularized tissues Proc. Natl Acad. Sci. USA 113 3179–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Miller JS et al. 2012. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues Nat. Mater. 11 768–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Moya ML, Hsu YH, Lee AP, Hughes CC and George SC 2013. In vitro perfused human capillary networks Tissue Eng. C 19 730–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Moya ML, Alonzo LF and George SC 2014. Microfluidic device to culture 3D in vitro human capillary networks Methods Mol. Biol. 1202 21–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wang X et al. 2016. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels Lab Chip 16 282–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Tan RP et al. 2018. Integration of induced pluripotent stem cell-derived endothelial cells with polycaprolactone/gelatin-based electrospun scaffolds for enhanced therapeutic angiogenesis Stem Cell Res. Ther. 9 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nakayama KH et al. 2015. Aligned-braided nanofibrillar scaffold with endothelial cells enhances arteriogenesis ACS Nano 9 6900–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wu W, DeConinck A and Lewis JA 2011. Omnidirectional printing of 3D microvascular networks Adv. Mater. 23 H178–83 [DOI] [PubMed] [Google Scholar]
- [158].Bertassoni LE et al. 2014. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs Lab Chip 14 2202–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Kolesky D B et al. 2014. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs Adv. Mater. 26 3124–30 [DOI] [PubMed] [Google Scholar]
- [160].Schuller-Ravoo S, Zant E, Feijen J and Grijpma DW 2014. Preparation of a designed poly(trimethylene carbonate) microvascular network by stereolithography Adv. Healthcare Mater. 3 2004–11 [DOI] [PubMed] [Google Scholar]
- [161].Kang TY, Hong JM, Jung JW, Kang HW and Cho DW 2016. Construction of large-volume tissue mimics with 3D functional vascular networks PLoS One 11 e0156529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wadnap S, Krishnamoorthy S, Zhang Z and Xu C 2019. Biofabrication of 3D cell-encapsulated tubular constructs using dynamic optical projection stereolithography J. Mater. Sci., Mater. Med. 30 36. [DOI] [PubMed] [Google Scholar]
- [163].Gao Q, He Y, Fu JZ, Liu A and Ma L 2015. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery Biomaterials 61 203–15 [DOI] [PubMed] [Google Scholar]
- [164].Jia W et al. 2016. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink Biomaterials 106 58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Korff T and Augustin HG 1998. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation J. Cell Biol. 143 1341–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Skiles ML, Sahai S, Rucker L and Blanchette JO 2013. Use of culture geometry to control hypoxia-induced vascular endothelial growth factor secretion from adipose-derived stem cells: optimizing a cell-based approach to drive vascular growth Tissue Eng. A 19 2330–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Heiss M et al. 2015. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro FASEB J. 29 3076–84 [DOI] [PubMed] [Google Scholar]
- [168].Walser R et al. 2013. Generation of co-culture spheroids as vascularisation units for bone tissue engineering Eur. Cells Mater. 26 222–33 [DOI] [PubMed] [Google Scholar]
- [169].Noguchi R et al. 2016. Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease J. Heart Lung Transpl. 35 137–45 [DOI] [PubMed] [Google Scholar]
- [170].Okudaira T, Amimoto N, Mizumoto H and Kajiwara T 2016. Formation of three-dimensional hepatic tissue by the bottom-up method using spheroids J. Biosci. Bioeng. 122 213–8 [DOI] [PubMed] [Google Scholar]
- [171].Alajati A et al. 2008. Spheroid-based engineering of a human vasculature in mice Nat. Methods 5 439–45 [DOI] [PubMed] [Google Scholar]
- [172].Gafni Y et al. 2006. Design of a filamentous polymeric scaffold for in vivo guided angiogenesis Tissue Eng. 12 3021–34 [DOI] [PubMed] [Google Scholar]
- [173].Zou D et al. 2012. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1alpha mediated BMSCs Biomaterials 33 2097–108 [DOI] [PubMed] [Google Scholar]
- [174].Chen RR, Silva EA, Yuen WW and Mooney DJ 2007. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation Pharmaceut. Res. 24 258–64 [DOI] [PubMed] [Google Scholar]
- [175].Zigdon-Giladi H, Khutaba A, Elimelech R, Machtei E E and Srouji S 2017. VEGF release from a polymeric nanofiber scaffold for improved angiogenesis J. Biomed. Mater. Res. A 105 2712–21 [DOI] [PubMed] [Google Scholar]
- [176].Wagner ER et al. 2018. VEGF-mediated angiogenesis and vascularization of a fumarate-crosslinked polycaprolactone (PCLF) scaffold Connect. Tissue Res. 59 542–9 [DOI] [PubMed] [Google Scholar]
- [177].Oshikawa M, Okada K, Kaneko N, Sawamoto K and Ajioka I 2017. Affinity-immobilization of VEGF on laminin porous sponge enhances angiogenesis in the ischemic brain Adv. Healthcare Mater. 6 1700183 [DOI] [PubMed] [Google Scholar]
- [178].Somo SI et al. 2015. Pore interconnectivity influences growth factor-mediated vascularization in sphere-templated hydrogels Tissue Eng. C 21 773–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rothuizen TC et al. 2016. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model Biomaterials 75 82–90 [DOI] [PubMed] [Google Scholar]
- [180].Yamanami M et al. 2013. Implantation study of small-caliber ‘biotube’ vascular grafts in a rat model J. Artif. Organs 16 59–65 [DOI] [PubMed] [Google Scholar]
- [181].May KM, Vogt A, Bachas LG and Anderson KW 2005. Vascular endothelial growth factor as a biomarker for the early detection of cancer using a whole cell-based biosensor Anal. Bioanal. Chem. 382 1010–6 [DOI] [PubMed] [Google Scholar]
- [182].Mokry J and Nemecek S 1998. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells Folia Biol. 44 155–61 [PubMed] [Google Scholar]
- [183].Lewin M et al. 1999. In vivo assessment of vascular endothelial growth factor-induced angiogenesis Int. J. Cancer 83 798–802 [DOI] [PubMed] [Google Scholar]
- [184].Xu C. et al. Long circulating reduced graphene oxide-iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale. 2016;8 doi: 10.1039/c5nr09193d. 12683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Antonelli A, Pacifico S, Sfara C, Tamma M and Magnani M 2018. Ferucarbotran-loaded red blood cells as long circulating MRI contrast agents: first in vivo results in mice Nanomedicine 13 675–87 [DOI] [PubMed] [Google Scholar]
- [186].Zhu Y et al. 2019. LED-based photoacoustic imaging for monitoring angiogenesis in fibrin scaffolds Tissue Eng. C 25 523–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Demene C et al. 2019. 3D longitudinal imaging of tumor angiogenesis in mice in vivo using ultrafast doppler tomography Ultrasound Med. Biol. 45 1284–96 [DOI] [PubMed] [Google Scholar]
- [188].Hong G et al. 2014. Near-infrared II fluorescence for imaging hindlimb vessel regeneration with dynamic tissue perfusion measurement Circulation 7 517–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Stegen S, van Gastel N and Carmeliet G 2015. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration Bone 70 19–27 [DOI] [PubMed] [Google Scholar]
- [190].Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA and Niedzwiedzki T 2017. The role of vasculature in bone development, regeneration and proper systemic functioning Angiogenesis 20 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Liu Y et al. 2013. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering Tissue Eng. A 19 893–904 [DOI] [PubMed] [Google Scholar]
- [192].Hardy D. et al. Comparative study of injury models for studying muscle regeneration in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147198. e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Christov C et al. 2007. Muscle satellite cells and endothelial cells: close neighbors and privileged partners Mol. Biol. Cell 18 1397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Grenier G et al. 2007. Resident endothelial precursors in muscle, adipose, and dermis contribute to postnatal vasculogenesis Stem Cells 25 3101–10 [DOI] [PubMed] [Google Scholar]
- [195].Nakayama KH et al. 2019. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration Commun. Biol. 2 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ruan L, Wang B, ZhuGe Q and Jin K 2015. Coupling of neurogenesis and angiogenesis after ischemic stroke Brain Res. 1623 166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Madelaine R et al. 2017. MicroRNA-9 couples brain neurogenesis and angiogenesis Cell Rep. 20 1533–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chen CK et al. 2018. TRPV4 activation contributes functional recovery from ischemic stroke via angiogenesis and neurogenesis Mol. Neurobiol. 55 4127–35 [DOI] [PubMed] [Google Scholar]
- [199].Rosell A. et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073244. e73244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Iskander A et al. 2013. Intravenous administration of human umbilical cord blood-derived AC133 + endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings Stem Cells Transl. Med. 2 703–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Baranski JD et al. 2013. Geometric control of vascular networks to enhance engineered tissue integration and function Proc. Natl Acad. Sci. USA 110 7586–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Cochain C, Channon K M and Silvestre J S 2013. Angiogenesis in the infarcted myocardium Antioxid. Redox Signal. 18 1100–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Chang ZT et al. 2013. Application of peripheral-blood-derived endothelial progenitor cell for treating ischemia-reperfusion injury and infarction: a preclinical study in rat models J. Cardiothor. Surg. 8 33. [DOI] [PMC free article] [PubMed] [Google Scholar]