Abstract
Ergothioneine, a natural longevity vitamin and antioxidant, is a thiol-histidine derivative. Recently, two types of biosynthetic pathways were reported. In the aerobic ergothioneine biosynthesis, a non-heme iron enzyme incorporates a sulfoxide to an sp2 C-H bond in trimethyl-histidine (hercynine) through oxidation reactions. In contrast, in the anaerobic ergothioneine biosynthetic pathway in a green sulfur bacterium, Chlorobium limicola, a rhodanese domain containing protein (EanB) directly replaces this unreactive hercynine C-H bond with a C-S bond. Herein, we demonstrate that polysulfide (HSSnSR) is the direct sulfur-source in EanB-catalysis. After identifying EanB’s substrates, X-ray crystallography of several intermediate states along with mass spectrometry results provide additional mechanistic details for this reaction. Further, quantum mechanics/molecular mechanics (QM/MM) calculations reveal that protonation of Nπ of hercynine by Tyr353 with the assistance of Thr414 is a key activation step for the hercynine sp2 C-H bond in this trans-sulfuration reaction.
Keywords: ergothioneine biosynthesis, polysulfide, C-H bond activation, transsulfuration, crystal structure
Graphical Abstract

INTRODUCTION
Sulfur-containing natural products are widely distributed in nature, yet their biosynthetic details are poorly understood.1-6 One such natural product is ergothioneine, a thiol-histidine derivative (4, Figure 1). Ergothioneine and glutathione (GSH) are among the most abundant cellular thiols in animals.7 Together, they maintain a proper redox environment under a wide range of conditions due to their difference in reduction potential (~200 mV).7,8 Animals enrich ergothioneine from their diet through an ergothioneine-specific transporter (OCTN1) to millimolar concentrations in various parts of the body.9 Ergothioneine is touted as a longevity vitamin,10 and many diseases may benefit from ergothioneine treatment, including rheumatoid arthritis, Crohn’s disease, diabetes, cardiovascular diseases, and neurodegeneration.8
Figure 1. Ergothioneine biosynthesis and our initial EanB characterization.
(a) Ergothioneine biosynthetic pathways: two aerobic (EgtB-EgtC-EgtE-catalysis in mycobacteria, and Egt1-Egt2-catalysis in fungi), and an anaerobic (EanB-catalysis in sulfur bacteria) pathways. (b) 1H-NMR of the IscS-EanB coupled reaction shows ergothioneine production. The ~7.6 ppm and ~ 6.9 ppm signals are from the hydrogen atoms of hercynine’s side-chain and the signal at ~ 6.7 ppm is from the ergothioneine’s side-chain hydrogen atom. (c) The reaction scheme of the IscS-EanB-ergothionase coupled assay, in which the formation of thiourocanic acid 5 is monitored at 311 nm by UV-visible spectroscopy. (d) A five-minute lag phase for the IscS-EanB-ergothionase coupled assay. The reaction mixture contained 2 μM IscS, 1 mM cysteine, 1 mM hercynine, 400 nM ergothionase (1000-fold of EanB activity), and EanB at various concentrations (2-10 μM) in 50 mM pH 8.0 potassium phosphate (KPi) buffer at 25 °C. The reaction was monitored at 311 nm (ε311nm = 1.8 × 105 M−1•cm−1).
In ergothioneine biosynthesis, the key step is the substitution of the hercynine’s ε-carbon sp2 C-H bond with a C-S bond (1 to 4 transformations, Figure 1a); a remarkable example of C-H bond activation in synthesis.11-13 In recent years, two types of ergothioneine biosynthetic pathways were discovered: the aerobic and anaerobic biosynthetic pathways. In the aerobic ergothioneine biosynthetic pathways in Mycobacterium smegmatis14 and Neurospora crassa15 (Figure 1a), a non-heme iron enzyme (EgtB or Egt1, Figure 1a) catalyzes the hercynine’s ε-carbon sp2 C-H bond activation using molecular oxygen as the oxidant. While in the anaerobic ergothioneine biosynthetic pathway in the green-sulfur bacterium Chlorobium limicola, a rhodanese (EanB-catalysis, Figure 1a) is proposed to directly replace the sp2 C-H bond by a C-S bond, using cysteine as the sulfur source and cysteine desulfurase (IscS) as the sulfur-transfer mediator (Figure 1b).16,17 In general, the rhodanese active site’s cysteine persulfide intermediate transfers its terminal sulfur atom to an activated acceptor,18-20 yet, in EanB-catalysis, the hercynine’s sp2 C-H bond is not activated.16,17 In contrast to the original proposal of cysteine along with IscS as the sulfur source (Figure 1b),16,17 herein, we report the unprecedented finding that polysulfide is the direct sulfur source for EanB-catalysis. In green-sulfur bacteria, the deposited sulfur granules contain several forms of polysulfides including S8, polysulfide, cysteine polysulfide, and glutathione polysulfide.21-23 In this report, we demonstrate that all of these different deposited polysulfides in sulfur bacteria could serve as EanB substrates, suggesting that this reaction is a biologically relevant transformation. Kinetic studies, mass spectrometry analyses, and X-ray crystal structures of the pre-reaction complex, the reaction intermediate, and the product complex of EanB-catalysis provide additional mechanistic details and insights into this reaction. Further, we propose a mechanistic model for this intriguing trans-sulfuration reaction based on experimental data as well as QM/MM metadynamic simulations. Given the wide-distribution of EanB homologs in nature, the discoveries in this report may serve as the starting point for characterizing many more similar reactions in the biosynthesis of sulfur-containing natural products.
RESULTS
Lag-phase in EanB-catalysis and the production of elemental sulfur (S8) in the IscS-EanB coupled reaction.
Recently, Seebeck and coworkers reported their biochemical studies of EanB-catalysis in the anaerobic ergothioneine biosynthesis in which cysteine was used as the direct sulfur source and an Escherichia coli cysteine desulfurase (IscS) was used as the sulfur transfer mediator (Figure 1b).17 The E. coli IscS was used to replace C. limicola IscS for three reasons. First, comparing to other cysteine desulfurases,24-27 IscS is a promiscuous enzyme participating in the biosynthesis of thio-cofactors such as Fe-S cluster, thiamin, and biotin.28-30 Second, E. coli IscS shares high sequence similarity to that of C. limicola IscS (Figure S1a). Finally, the structural model of the C. limicola IscS created by us using Phyre2 program is highly similar to the reported E. coli IscS crystal structure (Figure S1b).31
We first repeated the EanB reaction reported by Seebeck and co-workers.17 Indeed, the IscS-EanB coupled reaction produces ergothioneine (Figure 1b) as revealed by 1H-NMR assay. The ~7.6 ppm and ~ 6.9 ppm signals are from the hydrogen atoms of hercynine’s side-chain and the signal at ~ 6.7 ppm is from the ergothioneine’s side-chain hydrogen atom (Figure 1b). After demonstrating the EanB-activity, in order to accurately measure the EanB kinetic parameters, we developed a continuous assay (Figure 1c). In this assay, we included ergothionase,32 which degrades ergothioneine to thiolurocanic acid 5. Because thiolurocanic acid 5 has a strong absorption feature centered at 311 nm (ε311nm = 1.8 × 105 M−1•cm−1), we could use this coupled assay to accurately measure the kinetic parameters of EanB-catalysis. Interestingly, when we monitored the thiolurocanic acid formation at 311 nm continuously (the IscS-EanB-ergothionase coupled reaction to form thiolurocanic acid 5, Figure 1c), we noted two interesting observations: First, the formation of thiolurocanic acid (5) shows a five-minute lag phase (Figure 1d). Second, the reaction mixture becomes cloudy after four hours. We collected these precipitates and characterized them. The dominant component in the precipitate is elemental sulfur (S8), along with a very small amount of denatured EanB protein (Figure S1c-f). In literature, it has been reported that when IscS is used as the sulfur transfer mediator, if a proper sulfur acceptor is not present, IscS-catalysis will lead to the accumulation of elemental sulfur (S8).33,34 Given these literature IscS-examples, the results from our ergothionase coupled assay imply that the Cys-IscS pair may not be the biological sulfur source for EanB-catalysis.
Polysulfide as the direct sulfur source in EanB-catalysis.
Results from our IscS-EanB-ergothionase coupled assay (Figure 1d and Figure S1) do not support the hypothesis of an IscS-bound persulfide as the direct sulfur source for EanB-catalysis as previously proposed (Figure 1b).17 Therefore, the key question to be addressed in EanB-catalysis is: what are the EanB substrates, especially the identity of its direct sulfur source? To answer this question, we examined several potential sulfur donors for EanB-catalysis (Figure 2a), including thiosulfate (Na2S2O3), mercaptopyruvate (3-MP), bisulfide (NaHS), and polysulfide.18,35 Among these sulfur donors, only polysulfide supports robust ergothioneine production (trace v, Figure 2a), while octasulfur ring (S8) provides a barely detectable level of ergothioneine (trace vi, Figure 2a). Treatment of the octasulfur ring (S8) with dithiothreitol (DTT) to produce polysulfide, also affords a significantly enhanced level of ergothioneine (trace vii, Figure 2a). More importantly, no lag phase exists in the EanB-ergothionase coupled-assay when polysulfide is the direct sulfur source (Figure 2c vs Figure 1d). The steady-state kinetic parameters of wild type EanB (EanBWT) are: kcat of 0.68 ± 0.01 min−1, Km of 69.7 ± 8.7 μM for hercynine (1), and Km of 18.1 ± 1.2 μM for polysulfide (Figure S1g-h). Polysufides are a mixture of sulfanes with various chain lengths. Following literature protocols,36 we have characterized the composition of polysulfides in our reaction conditions. The dominant form of polysulfide in our assay conditions is S42− (Figure S2), therefore, the K2S4 concentration is used when we report the KM of polysulfide.
Figure 2. Polysulfide is the direct sulfur source in EanB-catalysis.
(a) 1H-NMR assay of reaction mixtures using various potential sulfur-donors, including (i) IscS+cysteine, (ii) sodium thiosulfate (Na2S2O3), (iii) mercaptopyruvate (3-MP), (iv) sodium bisulfide (NaHS), (v) potassium polysulfide (K2Sx), (vi) elemental sulfur (S8), and (vii) dithiothreitol (DTT)-treated elemental sulfur. (b) The EanB-ergothionase coupled assay using polysulfide as the direct sulfur source. (c) Representative kinetic traces of EanB-polysulfide reactions. The reaction mixture contained 1 mM hercynine, 400 nM ergothionase (1000-fold of EanB activity), and 2 μM EanB and polysulfide at various concentrations (6-116 μM) in 50 mM pH 8.0 KPi buffer at 25 °C. The reaction was monitored at 311 nm (ε311nm = 1.8 × 105 M−1•cm−1). There is no lag phase when polysulfide is used as the direct sulfur source.
Cys412 is the only cysteine required for EanB catalysis.
There are five cysteine residues in EanB. In literature examples of sulfurtransferases, the sulfur-transfer process may involve multiple cysteine residues.4 To determine the importance of these five cysteine residues (Cys116, Cys184, Cys339, Cys370, Cys 412), we conducted two sets of experiments. In the first set of experiments, we generated the cysteine to serine mutation for each of the five cysteine residues. The catalytic activities of these mutants were characterized by both 1H-NMR and mass spectrometry. Among them, EanBC412S mutant is completely inactive. Even under single-turnover conditions, there is no ergothioneine production for the EanBC412S mutant (trace i in Figure 3a). Results from the first set of experiments imply that Cys412 might be the active site cysteine residue. In the second set of experiment, we demonstrated that Cys412 is the only required cysteine in EanB-catalysis by creating the EanBC412-only mutant and characterizing its activity. To create the EanBC412-only mutant, we replaced all of the other four cysteine resides (Cys116, Cys184, Cys339, and Cys370) with alanine. Therefore, in the EanBC412-only mutant, there is only one cysteine residue, which is Cys412. Consistent with the hypothesis of having Cys412 as the active site cysteine residue concluded from the first set of experiments, the EanBC412-only variant is active (trace ii, Figure 3a and Figure s4b) and its activity is only a few folds less than the wild type EanB. The steady state kinetic parameters are: kcat of 0.15 ± 0.01 min−1, Km of 65.9 ± 3.4 μM for hercynine, and Km of 10.4 ± 1.0 μM for polysulfide (Figure S3b-c). Results from these two sets of studies indicated that Cys412 is the active site’s cysteine in EanB and it is the only cysteine needed for catalysis.
Figure 3. Single-turnover studies to distinguish between the IscS-EanB and EanB-polysulfide reactions.
(a) 1H-NMR analysis of the EanB variants: The EanBC412S reaction (trace i) and EanBC412-only reaction (trace ii). (b) Flowchart of the EanBC412-only single-turnover reaction using persulfide containing IscS as the direct sulfur source. (c) MS/MS analysis of IscS (residues 319 - 340) for detecting the IscS Cys328 persulfide intermediate. (d) Flowchart of the EanBC412-only single-turnover reaction using K2Sx as the direct sulfur source. (e) MS/MS analysis of EanBC412-only (residues 407 - 417) for detecting the Cys412 persulfide intermediate. (f)-(g) Quantification of the amount of EanBC412-only protein (bar I or I’), EanBC412-only Cys412 persulfide (bar II or II’), ergothioneine produced using as-purified EanB (bar III or III’) and ergothioneine produced using IscS- or polysulfide-treated EanB (bar IV or V’). Additionally, quantification of EanBC412-only Cys412 persulfide after polysulfide treatment is shown in bar IV’.
Single-turnover studies to provide further evidence supporting polysulfide as the direct sulfur source in EanB-catalysis.
There are two potential mechanistic models to explain the observed ergothioneine production in the reported Cys-IscS-EanB system (Figure 1b). In the first model, EanB undergoes a conformation change to expose its Cys412 to the surface, which could then accept a sulfur atom from the persulfide intermediate on cysteine desulfurase (IscS). The second model is proposed based on results in our studies outlined in Figure 1 & 2. We proposed that polysulfide, instead of the IscS-persulfide, is the direct sulfur source in the EanB-catalysis (Figure 2a). In our Cys-IscS-EanB studies, we detected the production of a large amount of elemental sulfur (S8). The formation of S8 involves polysulfide intermediates,33,34 which, therefore, also explains the production of ergothioneine in the Cys-IscS-EanB system (Figure 1b) and the presence of a five-minute lag-phase in the kinetics of the Cys-IscS-EanB-ergothionase coupled assay (Figure 1d).
To provide further evidence to differentiate between the above two mechanistic options, we conducted two sets of single-turnover studies. In the first set, we prepared persulfide-containing IscS and used the IscS-persulfide as the sulfur source to conduct the EanB single-turnover study (Figure 3b). In the second set of experiments, we pre-incubated EanB with polysulfide and after excess polysulfide removal by gel-filtration, polysulfide-treated EanB was then used for the single turnover studies (Figure 3d). In addition, to simplify the data analysis, we used the EanBC412-only variant because Cys412 is the only cysteine residue needed for EanB-catalysis.
In the IscS-EanBC412-only single turnover study (Figure 3b, 3c, & 3f), we first incubated IscS with [β-13C]-cysteine in 1: 0.9 ratio anaerobically for 5 minute to produce the IscS-Cys328-persulfide (Figure S4a). The formation of the IscS-Cys328-persulfide intermediate is supported by analysis using mass spectrometry. After the IscS-cysteine reaction, the resulting IscS protein was derivatized by iodoacetamide (IAM). The presence of the IscS-Cys328-perfulfide was then characterized by analyzing its alkylated Cys328-based peptide-S-S-acetamide adduct using tandem mass spectrometry (Figure 3b and 3c). Indeed, tandem mass spectrometry analysis of IscS peptides obtained from trypsin-digested IscS supports the production of the IscS-Cys328-persulfide in the IscS-catalyzed cysteine desulfurase reaction (Figure 3c).
After the production of Cys328-persulfide containing IscS, it was then mixed with one equivalent of EanB, followed by the addition of an excess amount of hercynine (Figure 3b), The amount of ergothioneine produced was quantified via mass spectrometry (Figure S4c-f).15 The amount of the EanBC412-only Cys412 persulfide content was quantified using cyanolysis according to a literature procedure.16,37A typical example of the IscS- EanBC412-only single-turnover study result is shown in Figure 3f. In the as-purified EanBC412-only (155 μM, bar I), there are 44.4±3.6 μM of EanB having Cys412 persulfide already (bar II). The amount of ergothioneine produced by mixing the as-purified EanBC412-only with excess amount of hercynine is 42.0±1.5 μM (bar III). After mixing EanBC412-only with the Cys328 persulfide containing IscS, then treated with an excess amount of hercynine, the amount of ergothioneine produced is 41.1±5.5 μM (bar IV), which clearly indicates that the Cys328 persulfide containing IscS does not improve the amount of ergothioneine produced by the EanBC412-only protein under single-turnover conditions. Therefore, the result from this single turnover study does not support a direct transfer of the sulfur atom from the IscS-Cys328-perfulfide to EanB-Cys412 to form the EanB-Cys412-persulfide.
In the polysulfide-EanBC412-only single-turnover studies (Figure 3d), the treatment of EanBC412-only with polysulfide yields the Cys412-persulfide containing EanBC412-only protein. After the polysulfide treatment, the excess polysulfide was removed by gel filtration and a portion of the polysulfide treated EanBC412-only was alkylated with IAM and characterized by tandem mass spectrometry, which supports the successful production of the EanB-Cys412-persulfide (Figure 3e). The rest of the polysulfide treated EanBC412-only protein was then mixed with an excess amount of hercynine for the single-turnover study (Figure 3d). A typical example of the polysulfide single-turnover experimental result is shown in Figure 3g. After the treatment of EanBC412-only (bar I’) with K2Sx, the amount of Cys412 persulfide increased from 7.7±0.6 μM in the as purified EanBC412-only protein (bar II’) to 65.7±2.2 μM in the K2Sx treated EanBC412-only protein (bar IV’). In addition, when the K2Sx treated EanBC412-only protein was mixed with an excess amount of hercynine, 64.2±6.6 μM of ergothioneine was produced (bar V’), which is significantly higher than the amount of ergothioneine (5.3±1.1 μM) produced from the as-purified C412-only protein (bar III’). This experiment clearly indicates that polysulfide is the direct sulfur source in EanB-catalysis (Figure 3g).
EanB accepts other forms of polysulfides as the sulfur donors.
For decades, polysulfides are known to be present in biological systems and their potential chemical and biological roles are attracting interest.35,38 The EanB gene was identified from C. limicola, which is a green sulfur bacterium. Green sulfur bacteria accumulate sulfur and deposits sulfur globules with the short length of polysulfides (S32− and S42−) being the dominant species in periplasmic space.21-23 Recently, Prange et al. characterized bacterial sulfur granules using X-ray absorption spectroscopy23 and identified S8, polysulfide, cysteine polysulfide, and glutathione polysulfide in these sulfur granules. To determine whether other polysulfide forms could support EanB-catalysis, we synthesized glutathione polysulfide and cysteine polysulfide by mixing glutathione or cysteine with octasulfur ring (S8) (Figure S5 and S6).35 In this reaction, the free thiol groups from cysteine and glutathione nucleophilically attack S8 to produce glutathione and cysteine polysulfide (Figure S7a). Using glutathione polysulfide or cysteine polysulfide as the sulfur source, we also observed ergothioneine production (Figure S7b). As shown in the trace vi of Figure 2, when the octasulfur ring (S8) is used as the substrate in EanB-catalysis, there is a very low level of ergothioneine production. Some of EanB’ s non-essential cysteine residues are solvent exposed and they might react with octasulfur ring (S8) to produce a small amount of linear polysulfide to support EanB-catalysis. The green sulfur bacteria accumulate sulfur and deposit sulfur globules in periplasmic space. However, EanB is predicted as a cytosolic protein based on the sequence analysis from PSLpred and CELLO.39,40 Therefore, the cytosolic pool of polysulfide are the ones relevant to EanB-catalysis. Future studies will focus on characterizing this cytosolic pool of polysulfides.
Snapshots of several EanB-catalysis intermediate states.
After establishing polysulfide as the direct sulfur source for EanB-catalysis (Figure 2 & 3), we solved the EanB X-ray structures in several states using polysulfide as the substrate. The structures of EanB alone and its complex with hercynine were reported recently by Leisinger et al.16 and are consistent with our results (Table S1, Figure S8, and S9). In contrast to other literature rhodanese examples, whose active site’s cysteine residues are surface exposed (Figure S9a-b), the EanB active site Cys412 reside at the bottom of a ~13 Å deep tunnel (Figure S9c). This unique EanB structural feature is consistent with our discovery of having polysulfide as the direct sulfur source because polysulfide could access the deeply buried Cys412, while IscS-Cys328 may not.
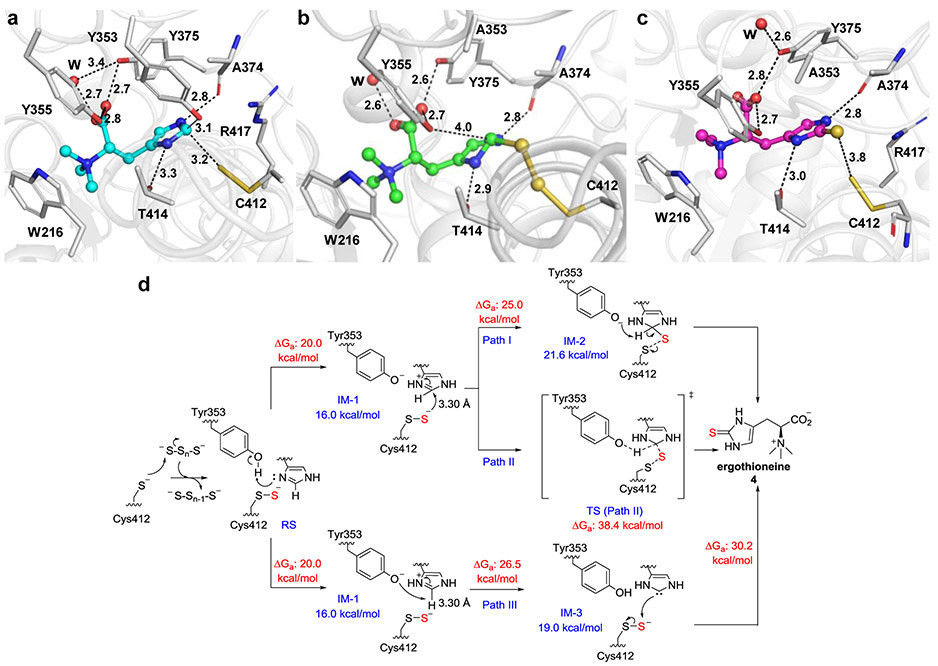
In continuation of our mechanistic studies, we captured three additional states of EanB-catalysis by X-ray crystallography (Figure 4a-4c). Upon co-crystallization of EanB with polysulfide and hercynine, we observed an EanB•hercynine binary complex with the Cys412 persulfide (Figure 4a & Figure S10a). In this structure, the distance between the Cys412 persulfide’s terminal sulfur and hercynine’s ε-carbon is 3.2 Å (Figure 4a), which is longer than a C-S bond (~1.82 Å). The dihedral angle between the Cys412-persulfide and the hercynine’s imidazole ring is −141.8°. Therefore, this structure is most likely the EanB•hercynine•persulfide intermediate before the sulfur-transfer reaction (the pre-reaction state). In the EanB pre-reaction state (Figure 4a), Tyr353 is 3.1 Å away from the hercynine’s ε-carbon, suggesting its potential role in acid/base catalysis, which was validated using the EanBY353A and EanBY353F variants. Even under single-turnover conditions, for both variants, ergothioneine production is below the 1H-NMR detection limit and < 0.3% according to quantitative mass spectrometry analysis (Figure S11a-c). Upon co-crystallizing of EanBY353A with polysulfide and hercynine, two more states were observed (Table S1): i) The EanBY353A•hercynine•trisulfide tetrahedral intermediate (Figure 4b); and ii) the EanBY353A•ergothioneine binary complex with the Cys412 persulfide (Figure 4c). In EanBY353A, the Tyr355’s hydroxyl group moves closer to the hercynine’s ε-carbon (4.0 Å), partially filling the space occupied by Tyr353 in EanBWT. In addition, Cys412 is in a trisulfide form in the EanBY353A mutant, instead of a persulfide in EanBWT, possibly due to additional space created by mutating Tyr353 to a smaller Ala residue (Figure 4b & Figure S10b). More importantly, the trisulfide directly links to the hercynine’s ε-carbon in a tetrahedral conformation and we propose that this state is the captured C-S bond containing tetrahedral intermediate. We also observed the EanBY353A•ergothioneine binary complex with the Cys412 persulfide (Figure 4c & Figure S10c). We attribute this result to the very low level of activity in this mutant and in the two-week crystallization process, some of the tetrahedral intermediate formed in Figure 4b proceeded to the product.
Figure 4. Crystal structures of three states in EanB-catalysis and our EanB-mechanistic models based on the QM/MM calculation.
(a)-(c) Catalytic states of EanB observed in crystallographic studies. (a) The EanB•hercynine binary complex with the Cys412 persulfide intermediate. Hercynine is shown in cyan stick and key interactions are shown in dash line with distances labeled (in Å). (b) EanBY353A•hercynine•Cys412-trisulfide in the tetrahedral intermediate state (shown in green stick). (c) The EanBY353A•ergothioneine binary complex (ergothioneine shown in a magenta stick). (d) Proposed mechanisms for EanB-catalysis based on the QM/MM calculation. After the protonation of Nπ of the imidazole ring of hercynine, C-S bond formation is followed by the deprotonation of Tyr353 (Path I) or a concerted mechanism in which the C-S bond is formed simultaneously with the transfer of H+ to the deprotonated Tyr353 (Path II). Path III shows the deprotonation of the protonated imidazole by Tyr353 to a carbene intermediate (IM-3), which is then followed by the C-S bond formation.
The activation of unreactive C-H bond in EanB-catalysis.
With the structural information, the next question we examined is: how is the unreactive C-H bond of hercynine activated to accept the sulfur atom from EanB’s Cys412 persulfide? We analyzed this reaction using metadynamics free energy simulations and efficient hybrid quantum mechanics/molecular mechanic methods (QM/MM). Our starting point is the crystal structure pre-reaction state (Figure 4a). The computed free energy surfaces (Figure S12) suggest that the initial step in the reaction involves the proton transfer from Tyr353 to the hercynine’s Nπ atom (Figure 4d). The role of Tyr353 for hercynine’s Nπ atom is also supported by hydrogen bonding analysis in EanB•hercynine binary complex with the Cys412 persulfide crystal structure (Figure S9f-g). The energetics of model reactions with different protonation states of substrate analogs support the proposal that protonation of hercynine is required for the nucleophilic persulfide attack (Figure S16 and Supporting discussion). The two-dimensional free energy surface in Figure S12b-c further supports this hypothesis, showing that the energetically favorable pathway starts from hercynine’s Nπ protonation by Tyr353, leading to a metastable intermediate IM-1; by contrast, sulfur transfer without this protonation is energetically uphill without any free energy basin that corresponds to a well-defined intermediate. In the EanB pre-reaction state (Figure 4a), the Thr414 and the hercynine’s Nπ atom participate in H-bonding (Figure S14). Specifically, the Thr414 plays a key role in orienting hercynine for productive proton transfer and in stabilizing the deprotonated Tyr353. IM-1 is ~16.0 kcal•mol−1 above the reactant state (RS) and this step (RS → IM-1, Figure 4d) has a free energy barrier (ΔGa) of ~20.0 kcal•mol−1. Once IM-1 is formed, nucleophilic attack by the Cys412 persulfide leads to the C-S bond formation, resulting in the tetrahedral intermediate IM-2 at the hercynine’s ε-carbon, which is ~21.6 kcal•mol−1 relative to the reactant state; the activation free energy barrier is ~25.0 kcal•mol−1 measured relative to the reactant. We created the EanBY353A, EanBY353F, and EanBT414V variants. Even under single-turnover conditions, ergothioneine production is below the 1H-NMR detection limit and < 3% according to quantitative mass spectrometry analysis (Figure S11a-d). These biochemical results are consistent with the proposed key roles of these residues in EanB-catalysis. Starting from IM-1 or IM-2, the sulfur transfer and the proton transfer back to Tyr353 may occur via one of several pathways as outlined in Figure 4d and Supporting discussion. Path II has much higher activation energy than Path I. In path III, after the hercynine’s imidazole is protonated by Tyr353, the deprotonated Tyr353 then deprotonates hercynine at the ε-position to produce a carbene intermediate. The fact that S-S bond cleavage has higher activation free energy in all three pathways suggests that proton transfer reactions are not rate-limiting step, in agreement with the solvent and substrate KIE data.16 These QM/MM studies suggest that the protonation of hercynine’s imidazole side-chain by Tyr353 is key activation step that initiates the S-S bond cleavage in EanB-catalysis.
DISCUSSION AND CONCLUSIONS
Despite the significance of sulfur-containing molecules in nature, many of their biosynthetic pathways are poorly understood.1-6 In literature, there are two types of C-S bond formation mechanisms: ionic and radical, and several reviews summarize the current knowledge on enzymatic C-S bond formation reactions.2,5,6,41 Recently, the biosynthetic information of a few more excellent examples of sulfur-containing natural products are described. One of these interesting classes of transformations is the conversion of amides to thioamides via the ionic type of reaction mechanism. Specifically, in 6-thioguanine biosynthesis, YcfA (a ATP-dependent sulfur-transferase) and YcfC (a cysteine desulfurase) catalyze thioamide formation.42 Recently, the closthiamide biosynthetic gene cluster has been identified and the biosynthetic pathway has been partially reconstituted in E. coli.43 Results from these genetic studies implied that the thioamide formation in closthiamide biosynthesis may follow a mechanism similar to that in 6-thioguanine biosynthesis. However, the sulfur source and the closthiamide biosynthetic details remain to be characterized. In contrast, the thioamidation of the α-subunit of methyl-coenzyme M reductase (McrA) by an ATP-dependent sulfur-transferase YcaO is slightly different.44 In YcaO-catalysis, sulfide is the direct sulfur source. Another recent example of the biosynthesis of sulfur-containing natural products is the installation of oxazolone and thioamide to produce methanobactin from its precursor peptide. This reaction is catalyzed the MbnB/MbnC herterodimer wherein the MbnB contains a non-heme iron active site. The Installation of oxazolone and thioamide in the MbnB/MbnC reaction is proposed to involve radical intermediates.45
Among these biosynthetic pathways for sulfur-containing natural products, ergothioneine biosynthesis is unique because both aerobic and anaerobic biosynthetic pathways have been discovered.6,14,15,17 One of the key features for the C-S bond formation is the replacement of an unactivated sp2 C-H bond by a C-S bond, which is a difficult chemical transformation (i.e., energetically unfavorable). In the aerobic ergothioneine biosynthetic pathways in M. smegmatis14 and N. crassa15 (Figure 1a), a non-heme iron enzyme (EgtB or Egt1, Figure 1a) catalyzes the sp2 C-H bond activation. EgtB/Egt1-catalyzed reactions are a four-electron oxidation process and molecular oxygen is the oxidant. Interestingly, in the anaerobic ergothioneine biosynthetic pathway in the green-sulfur bacterium C. limicola, a rhodanese (EanB, Figure 1a) directly replaces the sp2 C-H bond by a C-S bond. Rhodaneses perform key roles in the biosynthesis of sulfur-containing cofactors (e.g., thiamine or molybdenum cofactor), and in thiolation of nucleic acids.46 In these systems, thiocarboxylates (R-CO-SH) or protein-bound cysteine persulfides (R-S-SH) are the sulfur donors, resulting in persulfide-bound rhodaneses. Additionally, a complicated sulfur-transfer relay pathway provides the sulfur atom from cysteine, thiosulfate, or mercaptopyruvate.20,46 The persulfide sulfur of these rhodaneses then attacks electrophilic carbons such as those on phosphorylated C-terminus of sulfur carrier proteins or phosphorylated nucleobases to afford a covalent enzyme-substrate complex. A second active site cysteine cleaves the disulfide bond, resulting in the thiolate product. Unlike the other literature examples of rhodaneses, the trans-sulfuration reaction of EanB is unique in at least two aspects: 1) a small molecular polysulfide is the direct sulfur source; and, 2) the hercynine’s ε-carbon sp2 C-H bond is not activated for C-S bond formation.
Our biochemical results indicate that the small molecular polysulfide is the direct sulfur source for EanB-catalysis in ergothioneine biosynthesis. Pentasulfide has been observed in the crystal structure of a Radical SAM enzyme (MiaB).47 However, it is generally believed that the iron-sulfur cluster attached sulfide/polysulfide is the substrate in MiaB catalysis.47,48 Notably, sulfide does not support EanB-catalysis. Therefore, EanB is the first reported case of using polysulfide as the direct sulfur source in a natural product biosynthesis. This discovery also raises interesting questions on the link between sulfur related energy metabolism in these bacteria and the biosynthesis of sulfur-containing natural products. Recent characterization of sulfur granules deposited in sulfur bacteria revealed that these are various forms of polysulfides.21-23 In sulfur-based respiration, green sulfur bacteria utilize sulfide as the electron source and in these sulfur-oxidizing pathways, polysulfides are key intermediates.49 In sulfur bacteria, flavocytochrome c (FCC) and sulfide:quinone oxioreductase (SQR) are the primary enzymes for the oxidation of sulfide to produce polysulfide.35,50 A second system for polysulfide production is the degradation of cysteine and cystine by cystathionine β-lyase.35 This reaction leads to cysteine persulfide as the product. However, cysteine persulfide can disproportionate to produce polysulfide and this reaction is the key reaction for producing polysulfide in mammalian system. This reaction is also present in bacteria. Mutational study of SQR led to a growth defect when the mutant was cultured under high sulfide concentrations.51 Given by the abundance of different polysulfides deposited in green sulfur bacteria,23 and the ability of EanB to utilize these sulfur donors, it is tempting to propose that the trans-sulfuration reaction mediated by EanB is an example demonstrating the link between sulfur-related energy metabolism and biosynthesis of secondary metabolites in green sulfur bacteria. Our discovery warrants further investigation of this linkType equation here..
The structural studies also provide another line of evidence supporting the use of small-molecule polysulfides as the direct sulfur donor in EanB-catalysis. The EanB active site’s cysteine (Cys412) is deeply buried within the protein, and, therefore, not accessible by protein-bound thiocarboxylates or persulfide intermediates present in these previously reported sulfur-transfer relay systems. In contrast, a small molecular polysulfide is able to access this deep tunnel and act as the direct sulfur source for the EanB reaction. Moreover, structural studies coupled with the QM/MM analysis also provide valuable insight into the mechanism of C-H bond activation mediated by EanB. The computational studies high-light the importance of the protonation of hercynine’s Nπ atom by Tyr353 for nucleophilic persulfide attack. After the imidazole’s side-chain is activated, the reaction may proceed to produce a C-S bond via either a sequential (Path I) or concerted (Path II) mechanism (Figure 4d). Alternatively, the C-S bond can be formed via a carbene intermediate (Path III, Figure 4d). Intriguingly, the carbene pathway was proposed in previous studies and deemed unlikely.16,17 The crystallographic observation of a tetrahedral intermediate in EanBY353A variant implies that most likely, pathway I (i.e., the nucleophilic attack of the persulfide on the protonated hercynine) is in operation (Figure 4d). From our QM/MM analysis, the ΔGa for the sequential pathway (Path I) is ~25.0 kcal•mol−1, very close to that of the carbene-involved reaction (Path III, Figure 4d), suggesting that the carbene pathway may not be ruled out yet. Carbene intermediates have been proposed in thiamine diphosphate dependent enzymes,52 and orotidine 5′-phosphate decarboxylase.53
Overall, our study illuminates the use of polysulfide as the direct sulfur source for EanB-catalysis in biosynthesis of an emerging longevity vitamin, ergothioneine. Because polysulfides are the in vivo sulfur forms in green sulfur bacteria, EanB-catalysis also exemplifies how sulfur-containing secondary metabolite biosynthesis is coupled with bacteria physiology and sulfur-related energy metabolism.49,54,55 EanB homologs are found in many other sulfur bacteria, suggesting that other similar biosynthetic pathways await to be unveiled.17,56
EXPERIMENTAL SECTION
Materials:
Reagents were purchased from Sigma-Aldrich and Fisher Scientific unless otherwise specified. Hercynine was synthesized following reported procedure.57 Proton and carbon nuclear magnetic resonance spectra were recorded using Agilent 500 (500 MHz VNMRS). The kinetic parameter was determined using Cary Bio-100 spectrophotometer (Agilent).
Protein overexpression and purification.
EanB gene (accession number ACD90218.1) was codon-optimized for Escherichia coli overexpression by Genscript and sub-cloned into pET28a-(+) vector. E.coli BL21(DE3) was transformed with the EanB-pET28a plasmid and a single colony was inoculated into 50 mL LB media supplemented with 50 μg•mL−1 kanamycin at 37 °C overnight. Then, 10-mL of seed culture was used to inoculate 1 L of LB media supplemented with 50 μg•mL−1 kanamycin at 37 °C. When OD600nm reached 0.6-0.8, protein overexpression was induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) final concentration at 16 °C. The culture was supplemented with potassium phosphate (KPi) buffer pH 7.0 to 100 mM final concentration. After additional 12 hr, the cells were harvest by centrifugation.
The EanB purification was carried out under anaerobic environment (see Supporting Methods). The purified protein concentration was quantified by amino acid analysis (AAA Service Lab). The extinction co-efficient of the purified protein is 85815±1460 M−1cm−1 based amino acid analysis (correction factor based on concentration determined from Bradford analysis is 0.735).
The mutant constructs were generated using Q5 Site-directed Mutagenesis Kit following suggested protocol (New England Biolabs). The overexpression and purification of the variants were carried out following the protocol of the wild type EanB. Notably, due to the instability of EanBC412-only mutant, the lysis buffer for the purification of this mutant was changed to 100 mM Tris-HCl, 500 mM NaCl, and 10% glycerol, pH 8.0.
IscS gene (accession number YP_026169.1) was amplified from E. coli with forward primer 5’-GAGAGAATTCAAATTACCGATTTATCTCGACTACTCCG-3’ and reverse primer 5’-GAGACTCGAGATGATGAGCCCATTCGATGCTGTT-3’ (The EcoRI and XhoI digestion were indicated with underline). The gene was sub-cloned into pET28a-(+) vector. E.coli BL21(DE3) was transformed with the plasmid. The IscS protein was overexpressed using a protocol similar to that of EanB except that PLP was added as a supplement to 0.1 mM final concentration. Moreover, after addition of IPTG, cells were incubated at 16 °C for 8 hours before harvest by centrifugation. IscS was purified following protocol similar to that of EanB (see Supporting Methods).
The DNA sequence of Burkholderia sp. HME13 ergothionase (accession number BAM63550.1)32 was synthesized by Genscript, codon optimized for Escherichia coli overexpression. The synthetic gene was sub-cloned into pET28a-(+) vector. The ergothionase protein was overexpressed using a protocol similar to that of EanB. After induction by IPTG, cells were incubated at 16 °C for 12 hours before harvesting by centrifugation. Ergothionase was purified by following a protocol similar to that of EanB (see Supporting Methods).
1H NMR analysis of EanB activity.
The EanB-IscS was conducted anaerobically. A 1-mL reaction contained 2 μM EanB, 1 mM hercynine, 1 mM cysteine, and 4 μM IscS, in 50 mM KPi pH 8.0 buffer at room temperature for 8 hours. The reaction was lyophilized and analyzed by 1H NMR. To determine the sulfur source in EanB-catalysis, several potential sulfur donors were examined. A 1-mL reaction mixture containing 2 μM EanB, 1 mM hercynine, 1 mM of varous potential sulfur donors (K2Sx, S8, S8 with DTT, Na2S2O3, NaHS, mercaptopyruvate, S8 with cysteine, and S8 with GSH) in 50 mM pH 8.0 KPi buffer for 8 hours. The reaction was lyophilized and ergothioneine formation was analyzed using 1H NMR assay. All 1H-NMR spectra were calibrated by the water peak at 4.65 ppm.
EanB-ergothionase coupled assay with IscS.
An EanB continuous activity assay was developed by converting ergothioneine to thiolurocanic acid using ergothionase (HME13) from Burkholderia sp.32 The reaction was monitored at 311 nm (ε311nm = 1.8 × 105 M−1•cm−1). A 1-mL reaction contained 2 μM IscS, 1 mM cysteine, 1 mM hercynine, 400 nM ergothionase (1000-fold of EanB activity), and various EanB concentration (2-10 μM) in 50 mM pH 8.0 KPi buffer at 25 °C. Specifically, the buffer, substrates, ergothionase, and IscS were added in order and mixed by stirring bar in the buffer for 10 seconds and the reaction was initiated by the addition of EanB. A control reaction containing all except ergothionase, was set up to serve as the control reaction for the baseline correction. The absorption from the control reaction was subtracted from that of the reaction to yield final reaction trace. Each reaction was repeated in triplicates and the activity measurement was repeated at least three more times.
Analysis of precipitate from EanB-IscS reaction mixture.
To characterize the precipitates from the EanB-IscS reaction, a 1.2-mL reaction containing 2 μM EanB, 2 μM IscS, 1 mM hercynine, and 1 mM cysteine, was set up anaerobically in 50 mM pH 8.0KPi buffer. At hour 6 and hour 12, the precipitate was collected by centrifuging at 10,000 rpm for 1 min at 4 °C and washed to remove residual soluble protein using 1 mL of 50 mM KPi, pH 8.0. Then, the precipitate was resuspended in 20 μL of 100 mM Na2S solution and incubated at room temperature for 0.5 h. Subsequently, the sample was diluted with MeOH to 0.2 mL final volume and derivatized with 20 μL of methyl triflate. The dimethylated species were subjected to HPLC analysis (90% MeOH, 10% H2O, 0.5 mL•min−1) using C18 column (4.6 × 250 mm, 5 μm, YMC-triart) with 10 μL injection volume.
In addition to sulfur-based analysis, the precipitates were analyzed by SDS-PAGE to visualize protein in the samples. At the beginning of the reaction, the concentration of protein was quantified to be ~0.35 mg•mL−1. At hour 6 and hour 12, the precipitate was collected by centrifuging at 10,000 rpm for 1 min at 4 °C and resuspended in 30 μL of SDS-loading dye. A 100-μL aliquot of the supernatant was taken and diluted with 100 μL SDS-loading dye and 10 μL of each sample was loaded for SDS-PAGE analysis. Accounting for the dilution factor of SDS-PAGE sample preparation, the proteins in precipitates at hour 6 and hour 12 were quantified as ~2 μg and ~4 μg by SDS-PAGE quantification with in-house protein standard, which represented only 1% and 2% of total soluble protein in supernatant, respectively.
Steady-state kinetic analysis of EanB reaction with polysulfides.
The kinetic parameters of EanB-catalysis were determined using EanB-ergothionase coupled assay. For hercynine concentration dependent study, a 1-mL reaction contained 1.8 μM EanB, 400 nM ergothionase (1000-fold of EanB activity), 0.116 mM K2Sx, and varying concentration of hercynine (0.01 – 1 mM) in 50 mM KPi buffer, pH 8.0 at 25°C. Buffer, substrates and ergothionase were added in order and mixed by stir bar for 2 min to equilibrate the polysulfide disproportionation reaction in buffer so as to confirm there is no background absorbance from polysulfide disproportionation reaction and trace among of protein. The reaction was initiated by the addition of EanB. The baseline correction was performed following the EanB-IscS ergothionase assay. The reaction was monitored at 311 nm. Each reaction was repeated in triplicates. For polysulfide concentration dependent study, a 1-mL reaction mixture containing 1.8 μM EanB, 400 nM ergothionase (1000-fold of EanB activity), 0.5 mM hercynine, and varying concentration of K2Sx (6 - 289 μM) in 50 mM KPi buffer, pH 8.0 was set up at 25°C. Each reaction was repeated in triplicates and the data was fitted by GraphPad Prism. Polysulfide is a mixture of S32− to S92− species, while the dominant species of polysulfide under our assay condition was determined as S42− (see Supporting Methods). The reported KM was calculated using concentration of S42−.
Similar to the wild type, the kinetic parameters of EanBC412-only was characterized using ergothionase coupled assay. For hercynine concentration dependent study, a 1-mL reaction contained 7.6 μM EanB, 400 nM ergothionase (1000-fold of EanB activity), 0.116 mM K2Sx, and varying concentration of hercynine (0.02 – 2 mM) in 50 mM KPi buffer, pH 8.0 at 25°C. For polysulfide concentration dependent study, a 1-mL reaction mixture containing 7.6 μM EanB, 400 nM ergothionase (1000-fold of EanB activity), 0.5 mM hercynine, and varying concentration of K2Sx (6 - 289 μM) in 50 mM KPi buffer, pH 8.0 was set up at 25 °C. Each reaction was repeated in triplicates.
Protein crystallization.
Crystals of the wild-type of EanB were grown at 18 °C in anaerobic box, using the sitting-drop vapor diffusion method in 2 μL drops, containing 1:1 mixture of 10 mg•mL−1 protein solution (in 25 mM Tris-HCl, pH8.0, 150 mM NaCl) and the reservoir consisting of 0.1 M MOPS/HEPES, pH 7.5, 0.03 M of each ethylene glycol, 10% PEG 20k, 20% PEG 550 MME. Needle-like crystals were observed after two weeks. EanB•hercynine binary complex was crystallized at 20 °C using sitting drop vapor diffusion method under aerobic condition. Purified EanB protein was incubated with 1 mM hercynine solved in water on ice for 30 min. 1 μL of this protein-substrate mixture was mixed with 1 μL reservoir and the complex crystals appeared after one week in the condition of 0.1 MMES/imidazole pH 6.5, 0.02 M of each alcohol, 10% PEG20k, 20% PEG 550 MME. All EanB-hercynine-polysulfide complex were crystallized using sitting drop vapor diffusion method at 18 °C in anaerobic box. The purified EanB proteins were incubated with 1 mM hercynine (prepared in water) and 2 mM potassium polysulfide (prepared in water) for 30 min and followed by centrifugation, mixing in a 1:1 ratio with the reservoir solution in a final volume of 2 μL.
The complex crystals of EanB•ergothioneine intermediate were grown in 0.1 M Bis-Tris pH 6.5, 28% PEG 2000MME. The complex crystals of EanBY353A•ergothioneine with Cys412 in its persulfide form were grown in 0.1 M imidazole/MES buffer system, pH 6.5, 0.09 M halogens (NaF, NaBr, NaI), 30% mix 1 (PEG500MME_PEG20k). The crystals of EanBY353A•ergothioneine tetrahedral intermediate were grown in condition of 0.1 M imidazole, sodium cacodylate, MES, Bis-Tris, pH 6.5, 0.09 M halogens (NaF,NaBr,NaI), 37.5% MPD_P1k_PEG3350. All crystals were flash-frozen in liquid nitrogen after being dipped into a solution containing 10-15% glycerol or only reservoir.
Data collection and structures determination.
The diffraction data of apo EanB was collected at the wavelength of 0.97918 Å on SSRF beamline SSRF-BL18U1 Shanghai (China). The data of EanB with hercynine, EanB with hercynine and polysulfide complex were collected at the wavelength of 0.97930 Å on SSRF beamline SSRF-BL18U1 Shanghai (China). The complex data of EanBY353A with hercynine and polysulfide was collected at the wavelength of 0.97791 Å on SSRF beamline SSRF-BL19U1 Shanghai (China). All data collection was performed at 100 K. All data, collected on SSRF-BL18U1 and 19U1, sets were indexed, integrated, and scaled using the HKL3000 package,58 collected on SSRF-BL17U1, sets were indexed, integrated, and scaled using the HKL2000 package.59 The structures of apo EanB were solved by molecular replacement method using the program PHASER60 and the structure of 3IPP as the search model, rounds of automated refinement were performed with PHENIX61 and the models were extended and rebuilt manually with COOT,62 the structure have been refined to 2.17 Å. The complex structure of EanB with hercynine, with hercynine and polysulfide, mutant EanB with hercynine and polysulfide were solved by the same method and using apo EanB structure as the search model. The statistics for data collection and crystallographic refinement are summarized in Table S1.
EanBC412-only-IscS single-turnover reaction.
The following experiment was conducted under anaerobic environment unless noted otherwise. For IscS single-turnover reaction, a 2-mL reaction containing 1.3 mM IscS and 1.2 mM [β13C]-cysteine (0.9-fold of IscS) in 100 mM Tris-HCl, pH 8.0, was set up at room temperature for 5 min. A 400-μL portion was taken and quench by HCl. Precipitated protein was removed by centrifugation. The supernatant was neutralized with NH4HCO3 and analyzed by 13C NMR to ensure the formation of protein-bound persulfide of IscS by checking the formation of [β-13C]-alanine. Then, excess cysteine in the remaining portion was removed by G25 size-exclusion chromatography (30 mm × 600 mm) at 4 °C. Then, a portion of cysteine-treated IscS was then alkylated in a 200-μL reaction containing 0.22 mM cysteine-treated IscS and 2.6 mM iodoacetamide in 100 mM Tris-HCl, 200 mM NaCl, pH 8.0 for 1 hour and the mixture was shielded from light.
After confirming the activity of IscS, EanBC412-only was incubated with the remaining portion of cysteine-treated IscS in a 1:1 ratio at 155 μM final concentration in 4.5-mL reaction of 100 mM Tris-HCl, 200 mM NaCl, pH 8.0. Then, a 200-μL alkylation reaction containing 100 μM cysteine-treated IscS, 100 μM EanBC412-only, and 1.6 mM iodoacetamide in 100 mM Tris-HCl, 200 mM NaCl, pH 8.0 for 1 hour. The remaining of EanBC412-only-IscS was used for single-turnover reaction. A 4.2-mL reaction containing 155 μM EanBC412-only, 155 μM IscS, and 775 μM hercynine (5-fold of EanB) in 100 mM Tris-HCl, 200 mM NaCl, pH 8.0, was set up for 15 min. Then, a 200-μL alkylation reaction was set up as previously described prior to hercynine addition. A 500-μL aliquot of reaction mixture was taken and mixed with 100 μL 6 M HCl. The protein precipitates were removed by centrifugation and the supernatant was taken for ergothioneine quantification by mass spectrometry. Additionally, the activity of IscS after size exclusion column was confirmed through multiple-turnover assay, which contained 10 μM EanBC412-only, 1 mM hercynine, 1 mM cysteine, 4 μM IscS in 50 mM KPi, pH 8.0 for 8 hr. The reaction was lyophilized and analyzed 1H NMR assay. Additionally, to determine the production of ergothioneine from persulfide of as-purified protein, a 50-μL reaction containing 396 μM EanBC412-only and 1.98 mM hercynine in 100 mM Tris-HCl, 200 mM NaCl, pH 8.0, was set up for 15 min. Then, the ergothioneine formation was quantified using mass spectrometry. This experiment was repeated twice. The figures presented here are representative of the results from this experiment.34
EanBC412-only-polysulfide single-turnover reaction.
This study was carried out following that of wild type EanB reaction. The experiment was performed anaerobically. An alkylation reaction of as-purified EanBC412-only was set up. This 100-μL reaction containing 0.2 mM EanBC412-only and 0.8 mM iodoacetamide in 150 mM Tris-HCl, pH 8.0, was set up at room temperature shielded from light for 1 hour. Then, a 2.7-mL reaction containing 353 μM EanBC412-only and 5.3 mM K2Sx in 150 mM Tris-HCl, pH 8.0, was set up at room temperature for 5 min. Then, excess polysulfides were removed using G-25 size-exclusion chromatography. The fractions containing protein were pooled. Then, a 120-μL alkylation reaction containing 65 μM K2Sx-treated EanBC412-only and 260 μM iodoacetamide in 150 mM Tris-HCl, pH 8.0, was set up at room temperature for 1 hour and the mixture was shielded from light. Then, a 7.2-mL reaction containing 65 μM K2Sx-treated EanBC412-only and 325 μM hercynine (5-fold of enzyme) in 150 mM Tris-HCl, pH 8.0 at room temperature for 15 min. A 120-μL reaction containing 65 μM EanBC412-only and 260 μM of iodoacetamide as described previously. The alkylated samples were used for tandem mass spectrometry analysis as discussed previously. A 500-μL aliquot of reaction mixture was taken and mixed with 100 μL of 6 M HCl. The protein precipitates were removed by centrifugation and the supernatant was taken for ergothioneine quantification by mass spectrometry (See Supporting Methods). Additionally, the activity of EanBC412-only was confirmed through multiple-turnover assay, which contained 10 μM K2Sx-treated EanBC412-only, 1 mM hercynine, 1 mM K2Sx, in 50 mM KPi, pH 8.0 for 8 hours. The reaction was lyophilized and analyzed by 1H NMR spectroscopy. Additionally, to determine the production of ergothioneine from persulfide of as-purified protein, a 50-μL reaction containing 396 μM EanBcC412-only and 1.98 mM hercynine in 100 mM Tris-HCl, 200 mM NaCl, pH 8.0, was set up for 15 min. Then, the ergothioneine formation was quantified using mass spectrometry. The persulfide content of EanB after treated with polysulfide was determined through cyanolysis assay (see Supporting Methods). This experiment was then repeated twice. The figures presented here are representative of the results from this experiment.
Computational Methods.
All simulations were performed using CHARMM63. The crystal structure of EanB in complex with hercynine and Cys412 polysulfide was used. Hydrogens were added to the structure by using the HBUILD module of CHARMM. A 25 Å spherical water droplet centered on the terminal sulfur atom of Cys412 persulfide was added to solvate the enzyme system. Non-crystallographic waters within 2.5 Å of any crystallographic atom were deleted. The system was separated into three regions following the QM/MM-GSBP protocol (Figure S12)64,65: a QM region with the active site treated with an efficient quantum mechanical method at the DFTB3 level of theory64,66,67, along with the 3OB-3-1 parameters.68,69 The QM region consisted of 134 atoms, including key reacting residues, the hercynine substrate and some residues in α-helix 18 (i.e. GlU345, Tyr353, Tyr411, Cys412, Gly413, Thr414, Gly415, Trp416, Arg417 and Gly418). QM link atoms, using the DIV scheme70, were placed between the alpha and beta carbons of QM residues. No water molecules were included in the QM region. Non-QM atoms within a 28 Å sphere surrounding the active site (7961 atoms) were treated with the CHARMM36 force field71,72. Water molecules were described with a modified TIP3P model73. All atoms (1971 atoms) outside of the 28 Å sphere were frozen. The inner sphere was treated with classical Newtonian dynamics, except for a buffer region of 3 Å thickness from the edge of the sphere, which was treated with Langevin dynamics. Protein atoms in the buffer region were harmonically constrained with force constants determined from the average of crystallographic B factors.74 After a short geometry optimization, the system was heated from 48 K and equilibrated at 298 K over the course of 100 ps. Then, the system was further equilibrated at 298 K for 250 ps.
Possible catalytic mechanisms were explored with metadynamics simulations75,76 using the PLUMED package77 interfaced to CHARMM. The collective variables (CVs) for metadynamics were the antisymmetric stretch involving proton transfers between Tyr353 and the hercynine substrate (N for the first step, and ε-C for the subsequent steps), and the antisymmetric stretch that describes the nucleophilic attack of sulfur to ε-carbon of the substrate. In the simulation, SHAKE78 was applied to bonds involving hydrogens that do not participate in proton transfer. The multiple walker metadynamics method was utilized with typically 500 walkers per simulation. Gaussian potentials were placed every 100 fs; the Gaussian height was set to be 0.25 kcal•mol−1, and the Gaussian width for the antisymmetric stretch was set as 0.1 Å. The convergence was examined by monitoring the free energy of key species as a function of the number of Gaussians included (Figure S13); representative snapshots for these key species are included in Figure S14 and S15. The two-dimensional potential of mean force PMF shown in Figure S12 is based on a metadynamics simulation with 500 walkers. The corresponding simulation was performed for 50 ns (100 ps for each walker). and 100 ns (200 ps for each walker) for Figure S12b and S12c, respectively. The similar QM/MM methodology has been successfully applied to a number of enzyme systems in recent studies.79-81
To evaluate the accuracy of DFTB3 for the reactions of interest here, several small model systems were used as shown in Figure S16. All QM calculations for these model systems were performed using the Gaussian 16 program82. Single point energy calculations at DFTB3 minimized structures were performed using the Becke, three-parameter, Lee-Yang-Parr exchange-correlation functional (B3LYP)83 with the addition of Grimme’s third version semi-empirical dispersion correction (D3)84. The 6-31G(d,p) basis set85,86 and 6-311++G(d,p) basis set87,88 were used in the calculation. In addition, the G3B3 method89 was used in proton affinity calculations. Due to significant errors in proton affinity, the C-H repulsive potential in DFTB3/3OB was reparametrized based on electronic energies obtained at the MP2/aug-cc-pVQZ level of theory,90-92 as shown in Table S2. To further confirm the exothermicity of the reaction and the stability of possible intermediates, geometry optimizations were also performed with conductor-like polarizable continuum solvation model93,94 (dielectric constant as 4.0 to model the solvation effect of the protein environment) by using a truncated model based on QM/MM calculations (136 atoms, as shown in Figure S16c). In addition, the potential energy surface (PES) for carbene and persulfide reaction was examined both at DFTB3 and B3LYP-D3/6-311++G(d,p) levels of theory (as shown in Figure S17).
Supplementary Material
ACKNOWLEDGMENT
This work is partially supported by the MOST (2019YFA09005000 and 2018YFA0901900), CAS (XDB20000000) and SMSTC (19XD1404800) (J.Z.), the National Science Foundation (CHE-2004109) and National Institute of Health (AT010878 to P.L. and GM-106443 to Q. C.), National Natural Science Foundation of China project (31500667, C.P.) and the Chinese Academy of Science Key Technology Talent Program to C.P. We thank Zhihong Li and Chen Su of the Mass Spectrometry System at the National Facility for Protein Science in Shanghai (NFPS), Zhangjiang Lab, SARI, China for providing technical support and assistance in data collection and analysis. The authors thank the staff of beamlines BL18U1 and BL19U1 of Shanghai Synchrotron Radiation Facility for access and help with the X-ray data collection. The authors also thank P. Li and J. Gan for help with data collection and structure refinement, and Profs. Sean Elliott and Deborah Perlstein for feedback on the manuscript.
Footnotes
The authors declare no competing financial interest.
Supporting Information
More detailed experimental procedures, protein purification and characterization, additional structural information and expanded discussion of our computation work are included in the Supporting information. This information is available free of charge on the ACS Publications website.
REFERENCES
- (1).Fontecave M; Ollagnier-de-Choudens S; Mulliez E, Biological radical sulfur insertion reactions. Chem. Rev 2003, 103, 2149–66. [DOI] [PubMed] [Google Scholar]
- (2).Lin CI; McCarty RM; Liu HW, The biosynthesis of nitrogen-, sulfur-, and high-carbon chain-containing sugars. Chem. Soc. Rev 2013, 42, 4377–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kessler D, Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev 2006, 30, 825–840. [DOI] [PubMed] [Google Scholar]
- (4).Mueller EG, Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat. Chem. Biol 2006, 2, 185–194. [DOI] [PubMed] [Google Scholar]
- (5).Dunbar KL; Scharf DH; Litomska A; Hertweck C, Enzymatic carbon-sulfur bond formation in natural product biosynthesis. Chem. Rev 2017, 117, 5521–5577. [DOI] [PubMed] [Google Scholar]
- (6).Naowarojna N; Cheng R; Chen L; Quill M; Xu M; Zhao C; Liu P, Mini-review: ergothioneine and ovothiol biosyntheses, an unprecedented trans-sulfur strategy in natural product biosynthesis. Biochemistry 2018, 57, 3309–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fahey RC, Novel thiols of prokaryotes. Annu. Rev. Microbiol 2001, 55, 333–356. [DOI] [PubMed] [Google Scholar]
- (8).Cheah IK; Halliwell B, Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta, Mol. Basis Dis 2012, 1822, 784–793. [DOI] [PubMed] [Google Scholar]
- (9).Grundemann D; Harlfinger S; Golz S; Geerts A; Lazar A; Berkels R; Jung N; Rubbert A; Schomig E, Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. U.S.A 2005, 102, 5256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ames BN, Prolonging healthy aging: longevity vitamins and proteins. Proc. Natl. Acad. Sci. U.S.A 2018, 115, 10836–10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Davies HML; Du Bois J; Yu JQ, C-H functionalization in organic synthesis. Chem. Soc. Rev 2011, 40, 1855–1856. [DOI] [PubMed] [Google Scholar]
- (12).Gutekunst WR; Baran PS, C-H functionalization logic in total synthesis. Chem. Soc. Rev 2011, 40, 1976–1991. [DOI] [PubMed] [Google Scholar]
- (13).Yamaguchi J; Yamaguchi AD; Itami K, C-H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed 2012, 51, 8960–9009. [DOI] [PubMed] [Google Scholar]
- (14).Seebeck FP, In vitro reconstitution of mycobacterial ergothioneine biosynthesis. J. Am. Chem. Soc 2010, 132, 6632–6633. [DOI] [PubMed] [Google Scholar]
- (15).Hu W; Song H; Her AS; Bak DW; Naowarojna N; Elliott SJ; Qin L; Chen XP; Liu PH, Bioinformatic and biochemical characterizations of C-S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway. Org. Lett 2014, 16, 5382–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Leisinger F; Burn R; Meury M; Lukat P; Seebeck FP, Structural and mechanistic basis for anaerobic ergothioneine biosynthesis. J. Am. Chem. Soc 2019, 141, 6906–6914. [DOI] [PubMed] [Google Scholar]
- (17).Burn R; Misson L; Meury M; Seebeck FP, Anaerobic origin of ergothioneine. Angew. Chem. Int. Ed 2017, 56, 12508–12511. [DOI] [PubMed] [Google Scholar]
- (18).Cipollone R; Ascenzi P; Visca P, Common themes and variations in the rhodanese superfamily. IUBMB Life 2007, 59, 51–59. [DOI] [PubMed] [Google Scholar]
- (19).Jurgenson CT; Begley TP; Ealick SE, The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem 2009, 78, 569–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sasaki E; Zhang X; Sun HG; Lu MYJ; Liu TL; Ou A; Li JY; Chen YH; Ealick SE; Liu HW, Co-opting sulphur-carrier proteins from primary metabolic pathways for 2-thiosugar biosynthesis. Nature 2014, 510, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Gregersen LH; Bryant DA; Frigaard N-U, Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front. Microbiol 2011, 2, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Frigaard N-U; Dahl C, Sullur metabolism in phototrophic sulfur bacteria. Adv. Microb. Physiol 2008, 54, 103–200. [DOI] [PubMed] [Google Scholar]
- (23).Prange A; Chauvistre R; Modrow H; Hormes J; Truper HG; Dahl C, Quantitative speciation of sulfur in bacterial sulfur globules: X-ray absorption spectroscopy reveals at least three different species of sulfur. Microbiology 2002, 148, 267–276. [DOI] [PubMed] [Google Scholar]
- (24).Mihara H; Esaki N, Bacterial cysteine desulfurases: their function and mechanisms. Appl. Microbiol. Biotechnol 2002, 60, 12–23. [DOI] [PubMed] [Google Scholar]
- (25).Selbach B; Earles E; Dos Santos PC, Kinetic Analysis of the Bisubstrate Cysteine Desulfurase SufS from Bacillus subtilis. Biochemistry 2010, 49, 8794–8802. [DOI] [PubMed] [Google Scholar]
- (26).Albrecht AG; Netz DJA; Miethke M; Pierik AJ; Burghaus O; Peuckert F; Lill R; Marahiel MA, SufU Is an Essential Iron-Sullur Cluster Scaffold Protein in Bacillus subtilis. J. Bacteriol 2010, 192, 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Loiseau L; Ollagnier-de Choudens S; Lascoux D; Forest E; Fontecave M; Barras F, Analysis of the Heteromeric CsdA-CsdE Cysteine Desulfurase, Assisting Fe-S Cluster Biogenesis in Escherichia coli. J. Biol. Chem 2005, 280, 26760–26769. [DOI] [PubMed] [Google Scholar]
- (28).Lauhon CT; Kambampati R, The iscS Gene in Escherichia coli Is Required for the Biosynthesis of 4-Thiouridine, Thiamin, and NAD. J. Biol. Chem 2000, 275, 20096–20103. [DOI] [PubMed] [Google Scholar]
- (29).Zheng L; Cash VL; Flint DH; Dean DR, Assembly of Iron-Sulfur Clusters: identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter Vinelandii. J. Biol. Chem 1998, 273, 13264–13272. [DOI] [PubMed] [Google Scholar]
- (30).Hidese R; Mihara H; Esaki N, Bacterial cysteine desulfurases: versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl. Microbiol. Biotechnol 2011, 91, 47–61. [DOI] [PubMed] [Google Scholar]
- (31).Kelley LA; Mezulis S; Yates CM; Wass MN; Sternberg MJE, The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc 2015, 10, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Muramatsu H; Matsuo H; Okada N; Ueda M; Yamamoto H; Kato S.-i.; Nagata S, Characterization of ergothionase from Burkholderia sp. HME13 and its application to enzymatic quantification of ergothioneine. Appl. Microbiol. Biotechnol 2013, 97, 5389–5400. [DOI] [PubMed] [Google Scholar]
- (33).Black KA; Dos Santos PC, Shared-intermediates in the biosynthesis of thio-cofactors: mechanism and functions of cysteine desulfurases and sulfur acceptors. Biochim. Biophys. Acta, Mol. Cell. Res 2015, 1853, 1470–1480. [DOI] [PubMed] [Google Scholar]
- (34).Zheng LM; White RH; Cash VL; Jack RF; Dean DR, Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A 1993, 90, 2754–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Filipovic MR; Zivanovic J; Alvarez B; Banerjee R, Chemical biology of H2S signaling through persulfidation. Chem. Rev 2018, 118, 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kamyshny A; Goifman A; Gun J; Rizkov D; Lev O, Equilibrium distribution of polysulfide ions in aqueous solutions at 25 °C: a new approach for the study of polysulfides equilibria. Environ. Sci. Technol 2004, 38, 6633–6644. [DOI] [PubMed] [Google Scholar]
- (37).Wood JL, Sulfane sulfur. Methods Enzymol. 1987, 143, 25–29. [DOI] [PubMed] [Google Scholar]
- (38).Kharma A; Grman M; Misak A; Dominguez-Alvarez E; Nasim MJ; Ondrias K; Chovanec M; Jacob C, Inorganic polysulfides and related reactive sulfur-selenium species from the perspective of chemistry. Molecules 2019, 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bhasin M; Garg A; Raghava GP, PSLpred: prediction of subcellular localization of bacterial proteins. Bioinformatics 2005, 21, 2522–4. [DOI] [PubMed] [Google Scholar]
- (40).Yu CS; Chen YC; Lu CH; Hwang JK, Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinf 2006, 64, 643–651. [DOI] [PubMed] [Google Scholar]
- (41).Burkhart BJ; Schwalen CJ; Mann G; Naismith JH; Mitchell DA, YcaO-dependent posttranslational amide activation: biosynthesis, structure, and function. Chem. Rev 2017, 117, 5389–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Litomska A; Ishida K; Dunbar KL; Boettger M; Coyne S; Hertweck C, Enzymatic thioamide formation in a bacterial antimetabolite pathway. Angew. Chem. Int. Ed 2018, 57, 11574–11578. [DOI] [PubMed] [Google Scholar]
- (43).Dunbar KL; Büttner H; Molloy EM; Dell M; Kumpfmüller J; Hertweck C, Genome editing reveals novel thiotemplated assembly of polythioamide antibiotics in anaerobic bacteria. Angew. Chem. Int. Ed 2018, 130, 14276–14280. [DOI] [PubMed] [Google Scholar]
- (44).Dong S-H; Liu A; Mahanta N; Mitchell DA; Nair SK, Mechanistic basis for ribosomal peptide backbone modifications. ACS Cent. Sci 2019, 5, 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kenney GE; Dassama LM; Pandelia M-E; Gizzi AS; Martinie RJ; Gao P; DeHart CJ; Schachner LF; Skinner OS; Ro SY, The biosynthesis of methanobactin. Science 2018, 359, 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wright CM; Christman GD; Snellinger AM; Johnston MV; Mueller EG, Direct evidence for enzyme persulfide and disulfide intermediates during 4-thiouridine biosynthesis. Chem. Commun 2006, 3104–3106. [DOI] [PubMed] [Google Scholar]
- (47).Forouhar F; Arragain S; Atta M; Gambarelli S; Mouesca JM; Hussain M; Xiao R; Kieffer-Jaquinod S; Seetharaman J; Acton TB; Montelione GT; Mulliez E; Hunt JF; Fontecave M, Two Fe-S clusters catalyze sulfur insertion by radical-SAM methylthiotransferases. Nat. Chem. Biol 2013, 9, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Landgraf BJ; Arcinas AJ; Lee KH; Booker SJ, Identification of an intermediate methyl carrier in the radical S-adenosylmethionine methylthiotransferases RimO and MiaB. J. Am. Chem. Soc 2013, 135, 15404–15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Findlay AJ, Microbial impact on polysulfide dynamics in the environment. FEMS Microbiol. Lett 2016, 363, 1–12. [DOI] [PubMed] [Google Scholar]
- (50).Griesbeck C; Hauska G; Schüz M, Biological sulfide oxidation: sulfide-quinone reductase (SQR), the primary reaction. Recent Res. Devel. Microbiology 2000, 4, 179–203. [Google Scholar]
- (51).Chan L-K; Morgan-Kiss RM; Hanson TE, Functional analysis of three sulfide: quinone oxidoreductase homologs in Chlorobaculum tepidum. J. Bacteriol 2009, 191, 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Meyer D; Neumann P; Ficner R; Tittmann K, Observation of a stable carbene at the active site of a thiamin enzyme. Nat. Chem. Biol 2013, 9, 488–490. [DOI] [PubMed] [Google Scholar]
- (53).Miller BG; Wolfenden R, Catalytic proficiency: the unusual case of OMP decarboxylase. Annu. Rev. Biochem 2002, 71, 847–885. [DOI] [PubMed] [Google Scholar]
- (54).Falkenby LG; Szymanska M; Holkenbrink C; Habicht KS; Andersen JS; Miller M; Frigaard NU, Quantitative proteomics of Chlorobaculum tepidum: insights into the sulfur metabolism of a phototrophic green sulfur bacterium. FEMS Microbiol. Lett 2011, 323, 142–150. [DOI] [PubMed] [Google Scholar]
- (55).Jormakka M; Yokoyama K; Yano T; Tamakoshi M; Akimoto S; Shimamura T; Curmi P; Iwata S, Molecular mechanism of energy conservation in polysulfide respiration. Nat. Struct. Mol. Biol 2008, 15, 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ruszczycky MW; Liu HW, The surprising history of an antioxidant. Nature 2017, 551, 37–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Song H; Leninger M; Lee N; Liu R, Regioselectivity of the oxidative C-S bond formation in ergothioneine and ovothiol biosyntheses. Org. Lett 2013, 15, 4854–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Minor W; Cymborowski M; Otwinowski Z; Chruszcz M, HKL-3000: the integration of data reduction and structure solution - from diffraction images to an initial model in minutes. Acta Crystallogr., Sect. D: Biol. Crystallogr 2006, 62, 859–866. [DOI] [PubMed] [Google Scholar]
- (59).Otwinowski Z; Minor W, Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [DOI] [PubMed] [Google Scholar]
- (60).McCoy AJ; Grosse-Kunstleve RW; Adams PD; Winn MD; Storoni LC; Read RJ, Phaser crystallographic software. J. Appl. Crystallogr 2007, 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Adams PD; Afonine PV; Bunkóczi G; Chen VB; Davis IW; Echols N; Headd JJ; Hung L-W; Kapral GJ; Grosse-Kunstleve RW, PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr., Sect. D: Biol. Crystallogr 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Emsley P; Cowtan K, Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr 2004, 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- (63).Brooks BR; Brooks CL III; Mackerell AD Jr; Nilsson L; Petrella RJ; Roux B; Won Y; Archontis G; Bartels C; Boresch S, CHARMM: the biomolecular simulation program. J. Comput. Chem 2009, 30, 1545–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Cui Q; Elstner M; Kaxiras E; Frauenheim T; Karplus M, A QM/MM implementation of the self-consistent charge density functional tight binding (SCC-DFTB) method. J. Phys. Chem. B 2001, 105, 569–585. [Google Scholar]
- (65).Schaefer P; Riccardi D; Cui Q, Reliable treatment of electrostatics in combined QM/MM simulation of macromolecules. Chem. Phys 2005, 123, 014905. [DOI] [PubMed] [Google Scholar]
- (66).Elstner M; Porezag D; Jungnickel G; Elsner J; Haugk M; Frauenheim T; Suhai S; Seifert G, Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B: Condens. Matter 1998, 58, 7260–7268. [Google Scholar]
- (67).Gaus M; Cui Q; Elstner M, DFTB3: extension of the self-consistent-charge density-functional tight-binding method (SCC-DFTB). J. Chem. Theory Comput 2011, 7, 931–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Gaus M; Lu X; Elstner M; Cui Q, Parameterization of DFTB3/3OB for sulfur and phosphorus for chemical and biological applications. J. Chem. Theory Comput 2014, 10, 1518–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Lu X; Gaus M; Elstner M; Cui Q, Parametrization of DFTB3/3OB for magnesium and zinc for chemical and biological applications. J. Phys. Chem. B 2014, 119, 1062–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).König P; Hoffmann M; Frauenheim T; Cui Q, A critical evaluation of different QM/MM frontier treatments with SCC-DFTB as the QM method. J. Phys. Chem. B 2005, 109, 9082–9095. [DOI] [PubMed] [Google Scholar]
- (71).Mackerell AD Jr; Feig M; Brooks CL III, Extending the treatment of backbone energetics in protein force fields: limitations of gas - phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem 2004, 25, 1400–1415. [DOI] [PubMed] [Google Scholar]
- (72).MacKerell AD Jr; Bashford D; Bellott M; Dunbrack RL Jr; Evanseck JD; Field MJ; Fischer S; Gao J; Guo H; Ha S, All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [DOI] [PubMed] [Google Scholar]
- (73).Jorgensen WL; Chandrasekhar J; Madura JD; Impey RW; Klein ML, Comparison of simple potential functions for simulating liquid water. J. Chem. Phys 1983, 79, 926–935. [Google Scholar]
- (74).Brooks CL III; Karplus M, Solvent effects on protein motion and protein effects on solvent motion: dynamics of the active site region of lysozyme. J. Mol. Biol 1989, 208, 159–181. [DOI] [PubMed] [Google Scholar]
- (75).Laio A; Gervasio FL, Metadynamics: a method to simulate rare events and reconstruct the free energy in biophysics, chemistry and material science. Rep. Prog. Phys 2008, 71, 126601. [Google Scholar]
- (76).Laio A; Parrinello M, Escaping free-energy minima. Proc. Natl. Acad. Sci. U.S.A 2002, 99, 12562–12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Bonomi M; Branduardi D; Bussi G; Camilloni C; Provasi D; Raiteri P; Donadio D; Marinelli F; Pietrucci F; Broglia RA, PLUMED: a portable plugin for free-energy calculations with molecular dynamics. Comput. Phys. Commun 2009, 180, 1961–1972. [Google Scholar]
- (78).Ryckaert J-P; Ciccotti G; Berendsen HJ, Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys 1977, 23, 327–341. [Google Scholar]
- (79).Roston D; Demapan D; Cui Q, Leaving Group Ability Observably Affects Transition State Structure in a Single Enzyme Active Site. J. Am. Chem. Soc 2016, 138, 7386–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Roston D; Cui Q, Substrate and transition state binding in alkaline phosphatase analyzed by computation of oxygen isotope effects. J. Am. Chem. Soc 2016, 138, 11946–11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Roston D; Demapan D; Cui Q, Extensive free-energy simulations identify water as the base in nucleotide addition by DNA polymerase. Proc. Natl. Acad. Sci. U.S.A 2019, 116, 25048–25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Gaussian 16, Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Petersson GA; Nakatsuji H; Li X; Caricato M; Marenich AV; Bloino J; Janesko BG; Gomperts R; Mennucci B; Hratchian HP; Ortiz JV; Izmaylov AF; Sonnenberg JL; Williams; Ding F; Lipparini F; Egidi F; Goings J; Peng B; Petrone A; Henderson T; Ranasinghe D; Zakrzewski VG; Gao J; Rega N; Zheng G; Liang W; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Throssell K; Montgomery JA Jr.; Peralta JE; Ogliaro F; Bearpark MJ; Heyd JJ; Brothers EN; Kudin KN; Staroverov VN; Keith TA; Kobayashi R; Normand J; Raghavachari K; Rendell AP; Burant JC; Iyengar SS; Tomasi J; Cossi M; Millam JM; Klene M; Adamo C; Cammi R; Ochterski JW; Martin RL; Morokuma K; Farkas O; Foresman JB; Fox DJ Gaussian, Inc., Wallingford, CT, 2016 [Google Scholar]
- (83).Becke A, Density - functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648–5652. [Google Scholar]
- (84).Grimme S; Antony J; Ehrlich S; Krieg H, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys 2010, 132, 154104. [DOI] [PubMed] [Google Scholar]
- (85).Hehre WJ; Ditchfield R; Pople JA, Self - consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys 1972, 56, 2257–2261. [Google Scholar]
- (86).Francl MM; Pietro WJ; Hehre WJ; Binkley JS; Gordon MS; DeFrees DJ; Pople JA, Self - consistent molecular orbital methods. XXIII. A polarization - type basis set for second - row elements. J. Chem. Phys 1982, 77, 3654–3665. [Google Scholar]
- (87).Clark T; Chandrasekhar J; Spitznagel GW; Schleyer PVR, Efficient diffuse function - augmented basis sets for anion calculations. III. The 3 – 21+ G basis set for first - row elements, Li - F. J. Comput. Chem 1983, 4, 294–301. [Google Scholar]
- (88).Krishnan R; Binkley JS; Seeger R; Pople JA, Self - consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys 1980, 72, 650–654. [Google Scholar]
- (89).Baboul AG; Curtiss LA; Redfern PC; Raghavachari K, Gaussian-3 theory using density functional geometries and zero-point energies. J. Chem. Phys 1999, 110, 7650–7657. [Google Scholar]
- (90).Kendall RA; Dunning TH Jr; Harrison RJ, Electron affinities of the first - row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys 1992, 96, 6796–6806. [Google Scholar]
- (91).Physical reviewMøller C; Plesset MS, Note on an approximation treatment for many-electron systems. Phys. Rev 1934, 46, 618. [Google Scholar]
- (92).Dunning TH Jr, Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys 1989, 90, 1007–1023. [Google Scholar]
- (93).Barone V; Cossi M, Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar]
- (94).Cossi M; Rega N; Scalmani G; Barone V, Energies, structures, and electronic properties of molecules in solution with the C - PCM solvation model. J. Comput. Chem 2003, 24, 669–681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.